Abstract
Aim
To assess the relationship between psychological distress and quality of life (QoL), cancer-related fatigue (CRF), and chemotherapy efficacy in advanced gastric cancer patients.
Methods
Advanced gastric cancer patients (39 with psychological distress and 35 without psychological distress) completed the Distress Thermometer (DT), QoL, and CRF test before receiving chemotherapy and assessed the efficacy after completing 2 courses of chemotherapy.
Results
Psychological distress was a significant factor in the efficacy of chemotherapy in advanced gastric cancer patients (χ2 = 6.324; p = 0.042). Compared to advanced gastric cancer patients with no psychological distress, advanced gastric cancer patients with psychological distress had a poorer QoL (50.41 ± 6.17 vs. 60.01 ± 7.94, t = − 5.882, p < 0.01) and more pronounced CRF (5.75 ± 1.16 vs. 3.22 ± 0.75, t = 11.231, p < 0.01) while receiving chemotherapy. FACT-G (p = 0.0035, r = − 0.4568), as well as PFS (p < 0.0001, r = 0.6599), correlated significantly with efficacy for patients in the psychological distress group. The FACT-G (p = 0.0134, r = − 0.4139) of patients in the no psychological distress group correlated significantly with efficacy.
Conclusion
Psychological distress has a negative impact on QoL, CRF, and efficacy and may be a potential risk for the efficacy of palliative chemotherapy in advanced gastric cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the fifth most common cancer worldwide and the absolute number of cases has remained stable or even increased due to the increase in the world’s population and life expectancy [1]. There is therefore an interest in the QoL and prognosis of GC survivors. Psychological distress has been defined as “a multifactorial unpleasant emotional experience of a psychological, social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment” [2]. The International Society of Psychological Oncology has classified psychological distress as the sixth vital sign, with up to 30% of GC patients experiencing psychological distress [3]. Most GC patients face varying degrees of psychological distress from the time the disease is first diagnosed until it progresses beyond cure and is associated with worse outcomes [4]. Studies have shown that 70.6% of advanced cancer patients exhibit high levels of psychological distress, significantly higher than those with early-stage cancer [5]. Therefore, the importance of psychological distress in advanced GC patients for the decision of treatment will continue to grow.
QoL is quite a subjective concept, which is accepted as a subjective multidimensional concept aiming to function satisfactorily in four core domains (physical, psychological, social, and functional well-being) [6, 7]. Several attempts have been made in the past to give a precise definition of QoL, but no consensus has been reached [8]. Accepting this impossibility, a practical solution is to describe the four main health dimensions of QoL mentioned above [9]. In order to have a comprehensive understanding of QoL in GC patients, taking into account the disease characteristics, treatment side effects, and emotional issues specific to gastric cancer, the main scales applicable to assessing QoL in gastric cancer patients are the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30, and the Functional Assessment of Cancer Therapy-General (FACT-G). Previous studies have shown a negative association between both physical and psychological symptoms and QoL in cancer patients [10]. Studies based on breast cancer have found that psychological distress is associated with a reduction in health-related QoL [11]. And a review of patients with advanced genitourinary cancers found that the great psychological distress faced by patients can lead to impaired QoL [12]. Gastric cancer itself, as well as various treatment modalities, may affect a patient’s QoL. Among the psychological domains that affect QOL, psychological distress appears to have a greater impact [13]. It is important to study the factors that affect QoL so that clinicians can identify patients who may be more susceptible to these conditions and manage them appropriately for more effective remission and increased QOL. CRF is defined as “a distressing, persistent, subjective feeling of fatigue or exhaustion associated with cancer or cancer treatment that is out of proportion to recent activity and significantly interferes with normal function” [14]. CRF is multifaceted and may have physical and emotional manifestations, including general weakness, decreased concentration, reduced motivation or interest in engaging in daily activities, and emotional instability [15]. During palliative care, CRF is one of the most common symptoms of advanced cancer. As CRF is difficult to relieve with basic rest or sleep, it often affects the patient’s QoL, and in severe cases, even the patient’s compliance with treatment or the regularity of the course of treatment, thus affecting the outcome.
With improvements in the treatment room approach, chemotherapy-based systemic therapy has improved the survival of advanced GC patients [16, 17]. Identifying the risk factors that influence the efficacy of chemotherapy in advanced GC patients remains one of the current research priorities. However, clarifying the impact of patients’ psychological distress on CRF, QoL, and even therapeutic effectiveness is equally important to clarify and improve patient prognosis. Especially for patients with advanced gastric cancer who may have more psychological distress, understanding the impact of psychological distress on therapeutic effectiveness in the palliative chemotherapy state may be one way to improve therapeutic effectiveness as well as survival. This study aimed to find out whether the level of psychological distress in patients with stage 4 gastric cancer in palliative chemotherapy correlates with CRF, QoL, and efficacy.
Materials and methods
Test design
This cohort study explored the effects of psychological distress on chemotherapy efficacy, CRF, and QoL among advanced stomach cancer patients receiving palliative chemotherapy. We collected baseline data from all participants at the time of study enrollment, including metastases tumor size, distress thermometer (DT), FACT-G, and Piper Fatigue Scale (PFS). Patients scoring ≥ 4 points on the DT were classified as having distress, whereas patients scoring ≤ 3 points were classified as having no distress [18]. Evaluation of efficacy after completion of 2 courses of dividend chemotherapy. This research was approved by the Research Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (Number of Ethical Approval: 2,012,088). All participants provided oral informed consent to participate in this study.
Participants
All patients are selected according to the following criteria: (1) pathologically confirmed stage 4 gastric cancer with a target lesion for which efficacy can be assessed; (2) patients with a Karnofsky Performance Status (KPS) score ≥ 80; (3) patients who were at least 18 years old at the time of diagnosis, had a primary school education or above, and had sufficient audiovisual abilities to complete the questionnaire tests and intervention procedures; (4) patients with a life expectancy greater than 3 months. Patients were excluded if they met the following criteria: (1) patients with symptomatic brain metastases; (2) patients currently being treated for mental disorders; (3) advanced cachexia; (4) patients with other diseases that impact QoL, such as severe heart failure or disability.
Program
Figure 1 shows the research flowchart. Patients were orally introduced to the experiment by oncologists, and their informed consent was obtained. Patients were recruited from July 2022 to December 2022 and were hospitalized in the Oncology Department of the Hefei Cancer Hospital, Chinese Academy of Sciences, was assessed (n = 74). The questionnaires included DT, FACT-G, PFS, and tumor size data were also collected. Patients were followed until they completed 2 courses of chemotherapy, and tumor sizes were collected during follow-up. An oncologist evaluated the treatment efficacy and collected all baseline demographic and clinical data, and a psychologist administered all questionnaires, both the oncologist and psychologist were blinded to other study details.
Measures
All questionnaires were completed with paper and pencil at baseline. Self-reported demographic and medical characteristics were collected at baseline. Participants were screened for psychological distress using the DT. The DT is a single-item tool using a 0 (no distress) to 10 (extreme distress)-point Likert scale resembling a thermometer. The patient rates his/her level of distress over the past week. In this study, patients with DT scores ≥ 4 were classified as having distress.
Treatment
All patients enrolled received 2 courses of chemotherapy-based systemic treatment, some in combination with targeted drugs and/or immunotherapy.
Primary outcome
The Response Evaluation Criteria in Solid Tumors (RECIST) criteria were used to evaluate the therapeutic effectiveness. The RECIST criteria characterize the response of target lesions into four categories: (1) complete response (CR), in which all target lesions disappear; (2) partial response (PR), associated with a 30% reduction in the total length or diameter of baseline lesions; (3) stable disease (SD), in which the total length or diameter of baseline lesions decreases without reaching the PR value or increases without reaching the PD value; and (4) progressive disease (PD), associated with a 20% increase in the total length or diameter of baseline lesions or the detection of new lesions. The objective response rate (ORR) was defined as the sum of PR and CR cases. The disease control rate (DCR) was defined as the sum of PR, CR, and SD cases.
Secondary outcome
The FACT-G is one of the most widely used patient-reported outcome measures in cancer research [19]. This questionnaire is designed for self-assessment. The FACT-G survey scores physical well-being (PWB; 7 items, score range 0–28), social/family well-being (SWB; 7 items, score range 0–28), emotional well-being (EWB; 6 items, score range 0–24), and functional well-being (FWB; 7 items, score range 0–28). All questions in the FACT-G use a 5-point rating scale (0 = not at all; 1 = a little bit; 2 = somewhat; 3 = quite a bit; and 4 = very much). The FACT-G total score is computed as the sum of the four subscale scores provided that the overall item response is at least 80% (i.e., at least 22 of the 27 items were answered) and has a possible range of 0–108 points.
The PFS has been validated in the Chinese population as a self-rating scale designed to assess CRF in cancer survivors [20] and covers four dimensions of fatigue: behavior/daily life (6 items), cognition (6 items), affect/emotional meaning (5 items), and feeling/body (5 items). Each item was rated on a scale from 0 to 10, with a score of 0 being “not fatigued.”
Statistical analysis
SPSS, version 22.0, software (http://spss.en.softonic.com/; IL, USA) was applied to conduct the statistical analysis. Waterfall plots were drawn with GraphPad Prism 5 (Graph Pad Software, Inc., CA, USA). The clinical baseline data were divided into the psychological distress and no psychological distress groups, adopting independent-sample t-tests for normally distributed data. The variables of sex, years of education, methods of treatment, KPS scores, and therapeutic effect were analyzed by the chi-square (χ2) test in the two groups. Correlations between FACT-G and therapeutic effect, PFS, and therapeutic effect were analyzed using Pearson’s correlation coefficient. All tests were two-tailed, and the significance level was set to 0.05.
Results
The clinical baseline data
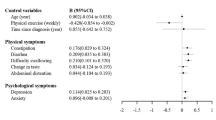
A total of 74 advanced GC patients were confirmed to meet the criteria and were divided into two groups. The psychological distress group included 39 patients, and the no psychological distress group included 35 patients. As shown in Table 1, there were no significant differences in clinical characteristics, including age (t = 0.069; p = 0.945), sex (χ2 = 3.422; p = 0.668), an education level (χ2 = 0.537; p = 0.764), methods of treatment (χ2 = 1.616; p = 0.446), and performance status (χ2 = 2.440; p = 0.118).
Primary outcome
The clinical curative effect is shown in Table 2 and Fig. 2. None of the patients achieved CR in the two groups. In the psychological distress group, 8 patients had PR, 15 had SD, and 16 had PD. In contrast, in the no psychological distress group, 16 patients had PR, 12 patients had SD, and 7 patients had PD. The objective response rate and disease control rate of the psychological distress group were 20.51% and 58.97%, and those of the no psychological distress group were 45.71% and 80.00%, respectively. A significant difference in efficacy was found between the two groups (χ2 = 6.324; p = 0.042). Waterfall charts displaying the curative effects for both groups are shown in Fig. 2.
Comparison of the therapeutic effect in advanced GC patients with or without psychological distress. A The therapeutic effectiveness in psychological distress group. B The therapeutic effectiveness in psychological distress group. PD, progressive disease; PR, partial response; SD, stable disease; ORR, objective response rate; DCR, disease control rate
Secondary outcome
Table 3 shows the FACT-G scores of the two groups of patients. The total FACT-G score was significantly lower in the psychological distress group than in the no psychological distress group (50.41 ± 6.17 vs. 60.01 ± 7.94, t = − 5.882, p < 0.01). Specifically, physical well-being (12.56 ± 3.76 vs. 15.77 ± 2.30, t = − 4.473, p < 0.01), social/family well-being (16.23 ± 3.31 vs. 17.31 ± 3.11, t = − 1.445, p = 0.153), and functional well-being (9.84 ± 4.23 vs 15.74 ± 5.05, t = − 5.462, p < 0.01) were both lower than the no psychological distress group, but the difference in social/family well-be in scores was not statistically significant. Although the scores for emotional well-being (11.77 ± 2.91 vs. 11.26 ± 2.30, t = 0.835, p = 0.407) were slightly higher than those in the no psychological distress, there was no statistically significant difference.
Table 4 shows the PFS scores for the two groups. The total PFS score was significantly higher in the psychological distress group than in the no psychological distress group (5.75 ± 1.16 vs. 3.22 ± 0.75, t = 11.231, p < 0.01). The scores for all four items of PFS were higher in the psychological distress group than in the no psychological distress group and all were statistically different. The specific scores were as follows: behavioral/daily life CRF (6.50 ± 1.53 vs. 2.71 ± 0.92, t = 13.028, p < 0.01), emotional/affective CRF (5.95 ± 1.47 vs. 2.90 ± 0.93, t = 10.765, p < 0.01), sensory/physical CRF (5.49 ± 1.13 vs 3.80 ± 1.12, t = 6.442, p < 0.01), and cognitive CRF (5.08 ± 1.17 vs 3.48 ± 1.00, t = 6.279, p < 0.01).
As shown in Fig. 3, FACT-G (p = 0.0035, r = − 0.4568) and PFS (p < 0.0001, r = 0.6599) correlated significantly with efficacy for patients in the psychological distress group. The FACT-G (p = 0.0134, r = − 0.4139) of patients in the no psychological distress group correlated significantly with efficacy.
Correlation of tumor size change with scale scores. A Correlation of tumor size change in the psychological distress group with FACT-G and PFS scores, respectively (p = 0.0035, r = − 0.4568; p < 0.0001, r = 0.6599). B Correlation of tumor size change in the no psychological distress group with FACT-G and PFS scores, respectively (p = 0.0134, r = − 0.4139; p = 0.1006, r = 0.2821). FACT-G, Functional Assessment of Cancer Therapy-General; PFS, Piper Fatigue Scale
Discussion
The present research investigated whether psychological distress was one of the risk factors influencing the prognosis of advanced GC patients who underwent chemotherapy. The authors found that advanced GC patients with psychological distress have a poorer QoL and more severe fatigue, and the objective response rate of advanced GC patients with psychological distress was worse than those of patients without psychological distress. The efficacy of patients in both groups was significantly correlated with QoL, and CRF in the psychological distress group was correlated with efficacy. It was found that the negative effects of psychological distress could be recognized in advanced GC patients with chemotherapy.
Palliative systemic treatment is an option to extend overall survival and although new systemic treatment modalities have improved survival in advanced GC, the 5-year survival rate is still below 30% [21, 22]. In clinical practice, the factors affecting the prognosis of advanced GC patients are complex, including differences in treatment methods, pathological staging of tumors, and the psychological status of patients. Lijuan Han found that anxiety and depression were among the factors affecting disease-free survival and overall survival in patients with gastric cancer after surgery [23]. Results from a Korean population-based survey of patients with stage IV gastric cancer showed that overall survival with psychological distress was lower than that of patients without psychological distress. And our latest study also found that survival of brain metastasis patients undergoing WBRT was negatively influenced by psychological distress [24]. These results were similar in that patient with advanced gastric cancer with psychological distress had poorer overall outcomes than patients without psychological distress when receiving chemotherapy-based systemic therapy [4].
With the change in the medical model from pure biomedicine to bio-psycho-social medicine, psychological distress has gradually become a concern involving the physical diseases themselves and the side effects of treatment [25]. Patients with advanced or metastatic gastric cancer often suffer from dysphagia, anorexia, nausea, and vomiting, and these problems can lead to psychological distress. Much of the psychological distress of cancer patients is associated with a decline in functioning and QoL, which can be exacerbated by a decline in QoL [26]. CRF has a devastating physical, mental, economic, and social impact, and it can be severe enough to seriously affect QoL during and after treatment [27]. Our findings found that fatigue was worse in patients with advanced GC with psychological distress. Several studies have found that CRF in cancer patients shows a significant positive correlation with psychological distress [28, 29]. And a previous review analysis based on predictors of distress in breast cancer suggested that CRF is one of the controllable symptoms associated with distress [30]. Therefore, we suggest that CRF on the table of patients with advanced GC receiving systemic therapy should be taken seriously and effective interventions should be made to alleviate the resulting psychological distress. The screening and assessment of psychological distress are important, and pertinent intervention measures should be implemented, which could improve the health outcomes of cancer patients [31].
The DT has demonstrated sufficient reliability as an NCCN-recommended tool for assessing psychological distress to assist clinicians in the identification and assessment of the degree of psychological distress in patients [18]. New or increased psychological distress in patients with advanced GC during treatment may be a consequence of fatigue from treatment or fear of death from advanced tumors. The impact of psychological distress on QoL, CRF, and therapeutic effectiveness during chemotherapy-based systemic therapy in patients with advanced gastric cancer has not been previously reported. This study confirms for the first time the negative impact of psychological distress on patients with advanced GC cancer receiving chemotherapy-based systemic therapy as a risk factor for treatment therapeutic effectiveness. The possible hypotheses on how psychological distress affects the therapeutic effectiveness of GC cancer patients are as follows. First, the presence of psychological distress is a risk factor for treatment non-adherence. A meta-analysis showed that non-adherence was higher in depressed patients compared to non-depressed patients [32]. Poorer patient adherence significantly reduces treatment efficacy. Second, chronic stress from psychological distress can lead to a body that can be in constant overdrive, leading to possible deleterious effects on the modulation of stress response and organ systems [33]. There is growing support that chronic stress-mediated activation of the hypothalamic–pituitary–adrenal axis (HPA), sympathetic nervous system (SNS), and microbiota-gut-brain (MGB) axis directly modulates the cancer profile of chronic exposure to multiple stress hormones such as glucocorticoids and catecholamines [34]. Third, immune dysfunction and changes in signaling pathways may lead to tumor progression. β-Adrenergic signaling has been found to reduce the number of cytotoxic T lymphocytes or their antitumor function, resulting in reduced cancer survival [35]. Finally, psychological distress may lead to tumorigenesis by stimulating an inflammatory response. Psychological distress was found to be associated with several inflammatory markers (white blood cell, C-reactive protein) [36] and increased expression of inflammatory factors (IL-6, IL-8, TNF-α, MCP-1, VEGF, MMP-2, MMP-9) enhanced tumor metastatic invasion [37]. Notably, TNF-α, IL-1, and IL-6 also mediated the development of CRF [38], which can and will negatively affect the QoL of gastric cancer patients [39]. Therefore, the biological mechanisms by which psychological distress affects the efficacy of chemotherapy in GC patients in this study can be further investigated from these aspects.
The modalities of ways to improve psychological distress include both non-pharmacologic and pharmacologic, but currently much attention is focused on interventions through non-pharmacologic approaches. A variety of non-pharmacological interventions regarding psychological distress, including methods based on positive thinking interventions [40, 41], talk/communication/cognitive behavioral therapy (CBT)-based methods [42], and psychological and psychosocial therapies [43], have shown improvement in heart distress due to a variety of cancers, including GC. In addition, studies based on breast cancer have shown that music interventions [44] as well as yoga [45] have a significant role in alleviating psychological distress as well as improving quality of life. Among pharmacologic therapies, much attention is being paid to the use of adrenergic receptors as actionable target targets, given the possible biological mechanisms mentioned in the previous section. Propranolol and the β2-adrenergic receptor (ADRB2)-specific antagonist ICI118,551 have been identified as having antitumor properties in GC by inhibiting the stress-regulated activation of the ERK1/2-JNK-MAPK pathway [46]. It is worthwhile to pay attention to the improvement of the efficacy of advanced gastric cancer by ameliorating psychological distress.
The limitations of this study should also be mentioned. Firstly, it was a single-center, small-sample study, and the sample was not selected to completely avoid the influence of some confounding factors. Second, no long-term follow-up study was conducted, and further follow-up is still needed regarding the effect of psychological distress on progression-free survival and overall survival of patients with advanced GC receiving chemotherapy. Thirdly, this study only found a negative effect of psychological distress on the efficacy of chemotherapy in advanced GC, and its further biological mechanisms are still unclear and need to be further investigated.
Conclusion
In summary, our results provide direct proof that psychological distress in GC patients affects the curative effect of systemic antineoplastic therapy. Moreover, we found that psychological distress is one of the factors affecting the QoL and CRF during systemic treatment in patients with advanced GC. This provides a theoretical basis for the intervention and improvement of the effect of efficacy through psychological factors in advanced GC patients.
Data Availability
The data that support the findings of this study are available from the corresponding author, Huaidong Cheng, upon reasonable request. All authors agree to provide data to the journal for review if needed.
References
Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S (2017) Gastric adenocarcinoma Nat Rev Dis Primers 3:17036
Holland JC, Alici Y (2010) Management of distress in cancer patients. J Support Oncol 8(1):4–12
Chung J, Ju G, Yang J, Jeong J, Jeong Y, Choi MK, Kwon J, Lee KH, Kim ST, Han HS (2018) Prevalence of and factors associated with anxiety and depression in Korean patients with newly diagnosed advanced gastrointestinal cancer. Korean J Intern Med 33(3):585–594
Kim GM, Kim SJ, Song SK, Kim HR, Kang BD, Noh SH, Chung HC, Kim KR, Rha SY (2017) Prevalence and prognostic implications of psychological distress in patients with gastric cancer. BMC Cancer 17(1):283
Abu-Odah H, Molassiotis A, Zhao IY, Su JJ, Allsop MJ (2022) Psychological distress and associated factors among Palestinian advanced cancer patients: a cross-sectional study. Front Psychol 13:1061327
Fallowfield L (2002) Quality of life: a new perspective for cancer patients. Nat Rev Cancer 2(11):873–879
Blazeby JM, Vickery CW (2001) Quality of life in patients with cancers of the upper gastrointestinal tract. Expert Rev Anticancer Ther 1(2):269–276
Post MW (2014) Definitions of quality of life: what has happened and how to move on. Top Spinal Cord Inj Rehabil 20(3):167–180
Schütte K, Schulz C, Middelberg-Bisping K (2021) Impact of gastric cancer treatment on quality of life of patients. Best Pract Res Clin Gastroenterol 50–51:101727
Deshields TL, Potter P, Olsen S, Liu J (2014) The persistence of symptom burden: symptom experience and quality of life of cancer patients across one year. Support Care Cancer 22(4):1089–1096
Phoosuwan N, Lundberg PC (2022) Psychological distress and health-related quality of life among women with breast cancer: a descriptive cross-sectional study. Support Care Cancer 30(4):3177–3186
Bergerot CD, Philip EJ, Bergerot PG, Pal SK (2020) Distress and quality of life among patients with advanced genitourinary cancers. Eur Urol Focus 6(6):1150–1154
Brown LF, Kroenke K, Theobald DE, Wu J, Tu W (2010) The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psychooncology 19(7):734–741
Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, Weis J, Jordan K, Ripamonti CI (2020) Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol 31(6):713–723
Bower JE (2014) Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 11(10):597–609
Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Mansoor W, Braghiroli MI, Karaseva N, Caglevic C, Villanueva L, Goekkurt E, Satake H, Enzinger P, Alsina M, Benson A, Chao J, Ko AH, Wainberg ZA, Kher U, Shah S, Kang SP, Tabernero J (2020) Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 6(10):1571–1580
Park S, Nam CM, Kim SG, Mun JE, Rha SY, Chung HC (2021) Comparative efficacy and tolerability of third-line treatments for advanced gastric cancer: a systematic review with Bayesian network meta-analysis. Eur J Cancer 144:49–60
Ownby KK (2019) Use of the distress thermometer in clinical practice. J Adv Pract Oncol 10(2):175–179
Yost KJ, Thompson CA, Eton DT, Allmer C, Ehlers SL, Habermann TM, Shanafelt TD, Maurer MJ, Slager SL, Link BK, Cerhan JR (2013) The Functional Assessment of Cancer Therapy - General (FACT-G) is valid for monitoring quality of life in patients with non-Hodgkin lymphoma. Leuk Lymphoma 54(2):290–297
Zhang Q, Li F, Zhang H, Yu X, Cong Y (2018) Effects of nurse-led home-based exercise & cognitive behavioral therapy on reducing cancer-related fatigue in patients with ovarian cancer during and after chemotherapy: a randomized controlled trial. Int J Nurs Stud 78:52–60
Dalhammar K, Malmström M, Schelin M, Falkenback D, Kristensson J (2020) The impact of initial treatment strategy and survival time on quality of end-of-life care among patients with oesophageal and gastric cancer: a population-based cohort study. PLoS ONE 15(6):e0235045
Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, Ho J, Unverzagt S (2017) Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 8(8):CD004064
Han L (2020) Prevalence, risk factors and prognostic role of anxiety and depression in surgical gastric cancer patients. Transl Cancer Res 9(3):1371–1383
Li W, Gan C, Zuo H, Yin X, Jing Y, Pang L, Yu S, Tang L, Yao S, Cheng H (2023) Psychological distress as risk factor for the efficacy of whole-brain radiotherapy in brain metastasis patients. Future Oncol 19(1):49–60
Ettlin DA, Napimoga MH, Meira ECM, Clemente-Napimoga JT (2021) Orofacial musculoskeletal pain: an evidence-based bio-psycho-social matrix model. Neurosci Biobehav Rev 128:12–20
Miles A, McClements PL, Steele RJ, Redeker C, Sevdalis N, Wardle J (2017) Perceived diagnostic delay and cancer-related distress: a cross-sectional study of patients with colorectal cancer. Psychooncology 26(1):29–36
Lesage P, Portenoy RK (2002) Management of fatigue in the cancer patient. Oncology (Williston Park) 16(3):373–8, 381 (discussion 381-2, 385-6, 388-9)
Stone P, Richards M, A’Hern R, Hardy J (2000) A study to investigate the prevalence, severity and correlates of fatigue among patients with cancer in comparison with a control group of volunteers without cancer. Ann Oncol 11(5):561–567
Ahlberg K, Ekman T, Wallgren A, Gaston-Johansson F (2004) Fatigue, psychological distress, coping and quality of life in patients with uterine cancer. J Adv Nurs 45(2):205–213
Syrowatka A, Motulsky A, Kurteva S, Hanley JA, Dixon WG, Meguerditchian AN, Tamblyn R (2017) Predictors of distress in female breast cancer survivors: a systematic review. Breast Cancer Res Treat 165(2):229–245
Chad-Friedman E, Coleman S, Traeger LN, Pirl WF, Goldman R, Atlas SJ, Park ER (2017) Psychological distress associated with cancer screening: a systematic review. Cancer 123(20):3882–3894
DiMatteo MR, Lepper HS, Croghan TW (2000) Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 160(14):2101–2107
Moreno-Smith M, Lutgendorf SK, Sood AK (2010) Impact of stress on cancer metastasis. Future Oncol 6(12):1863–1881
Dai S, Mo Y, Wang Y, Xiang B, Liao Q, Zhou M, Li X, Li Y, Xiong W, Li G, Guo C, Zeng Z (2020) Chronic stress promotes cancer development. Front Oncol 10:1492
Nissen MD, Sloan EK, Mattarollo SR (2018) β-Adrenergic signaling impairs antitumor CD8(+) T-cell responses to B-cell lymphoma immunotherapy. Cancer Immunol Res 6(1):98–109
Baek JH, Lee H, Myung W, Kim H, Choi YH, Kim DK, Hong KS, Choi H (2019) The association between inflammatory markers and general psychological distress symptoms. Gen Hosp Psychiatry 56:9–12
Baritaki S, de Bree E, Chatzaki E, Pothoulakis C (2019) Chronic stress, inflammation, and colon cancer: a crh system-driven molecular crosstalk. J Clin Med 8(10):1669
Molfino A, Formiconi A, Rossi Fanelli F, Muscaritoli M (2014) Ghrelin: from discovery to cancer cachexia therapy. Curr Opin Clin Nutr Metab Care 17(5):471–6
Fu L, Feng X, Jin Y, Lu Z, Li R, Xu W, Chang VT, Hu Y, Ye X (2022) Symptom clusters and quality of life in gastric cancer patients receiving chemotherapy. J Pain Symptom Manage 63(2):230–243
Compen F, Bisseling E, Schellekens M, Donders R, Carlson L, van der Lee M, Speckens A (2018) Face-to-face and internet-based mindfulness-based cognitive therapy compared with treatment as usual in reducing psychological distress in patients with cancer: a multicenter randomized controlled trial. J Clin Oncol 36(23):2413–2421
Lei Chui P, Wai S, Lai LL, See MH, Tan SB (2021) Mindful breathing: effects of a five-minute practice on perceived stress and mindfulness among patients with cancer. Clin J Oncol Nurs 25(2):174–180
Hejazi F, Bahrami M, Keshvari M, Alavi M (2017) The effect of a communicational program on psychological distress in the elderly suffering from cancer. Iran J Nurs Midwifery Res 22(3):201–207
Clark PG (2010) Decreasing psychological distress in cancer inpatients using FLEX Care®: a pilot study. Soc Work Health Care 49(9):872–890
Rossetti A, Chadha M, Torres BN, Lee JK, Hylton D, Loewy JV, Harrison LB (2017) The impact of music therapy on anxiety in cancer patients undergoing simulation for radiation therapy. Int J Radiat Oncol Biol Phys 99(1):103–110
Lundt A, Jentschke E (2019) Long-term changes of symptoms of anxiety, depression, and fatigue in cancer patients 6 months after the end of yoga therapy. Integr Cancer Ther 18:1534735418822096
Zhang X, Zhang Y, He Z, Yin K, Li B, Zhang L, Xu Z (2019) Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis 10(11):788
Funding
This work was supported by the grants from the National Natural Science Foundation of China (No. 81872504).
Author information
Authors and Affiliations
Contributions
Conceptualization: Huaidong Cheng, Chen Gan, and Yongkang Zhang; methodology: Wen Li and Jian Xu; resources: Yongkang Zhang and Lulian Pang; writing—original draft preparation: Yongkang Zhang and Chen Gan; writing—review and editing: Chen Gan and Wen Li; and funding acquisition: Huaidong Cheng; Yongkang Zhang and Chen Gan contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Research Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (Number of Ethical Approval: 2012088).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Gan, C., Xu, J. et al. Psychological distress as a risk factor for the efficacy of chemotherapy in advanced gastric cancer patients. Support Care Cancer 31, 669 (2023). https://doi.org/10.1007/s00520-023-08143-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08143-1