Abstract
Background
Radiotherapy-induced oral mucositis (RIOM) and chemotherapy-induced oral mucositis (CIOM) are common complications in cancer patients, leading to negative clinical manifestations, reduced quality of life, and unsatisfactory treatment outcomes.
Objective
The present study aimed to identify potential molecular mechanisms and candidate drugs by data mining.
Methods
We obtained a preliminary list of genes associated with RIOM and CIOM. In-depth information on these genes was explored by functional and enrichment analyses. Then, the drug–gene interaction database was used to determine the interaction of the final enriched gene list with known drugs and analyze the drug candidates.
Results and conclusion
This study identified 21 hub genes that may play an important role in RIOM and CIOM, respectively. Through our data mining, bioinformatics survey, and candidate drug selection, TNF, IL-6, and TLR9 could play an important role in disease progression and treatment. In addition, eight candidate drugs (olokizumab, chloroquine, hydroxychloroquine, adalimumab, etanercept, golimumab, infliximab, and thalidomide) were selected by the drug–gene interaction literature search additionally, as candidates for treating RIOM and CIOM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiotherapy-induced oral mucositis (RIOM) and chemotherapy-induced oral mucositis (CIOM) are serious toxic effects of radiotherapy and chemotherapy in cancer patients [1]. As an inflammatory reaction of the oral mucosa, they lead to clinical manifestations such as erythema, hemorrhage, ulceration, and pain [2]. The pathogenesis of RIOM and CIOM is quite complex, and it is believed to be multifactorial, involving the interaction of a broad range of cellular, tissue, and oral environmental factors and many dynamic biological processes and molecular pathways [1, 3]. Currently, the pathogenesis of RIOM and CIOM is mainly based on the five-stage theory proposed by Sonis et al. [2]. In the initial stage, chemotherapy or radiotherapy causes cell or DNA damage, and many reactive oxygen species are generated in the cytoplasm [4], followed by an initial injury that up-regulates and activates the generation of messengers. Next, it enters the signal amplification phase, where pro-inflammatory cytokines amplify the damage [5]. When the damage reaches a certain level, ulceration with inflammation appears, and the epithelial barrier is disrupted [6]. Finally, in the healing phase, the basal epithelial cells migrate, proliferate, and repair the ulcer [7]. Therefore, they not only cause pain to patients but also cause dysphagia and reduced intake by patients, leading to weight loss and malnutrition, reduced quality of life, and anti-cancer treatment effects [8, 9].
Over the past several decades, extensive investigations have been conducted on the prevalence, pathogenesis, and treatment modalities of RIOM and CIOM. However, few treatment options are available, and their efficacy is still limited. Globally, only two drugs have been approved for intervention [10]. Benzydamine HCl is an anti-inflammatory rinse that has shown efficacy in mitigating RIOM in patients being treated with radiation only for cancers of the head and neck; however, it is ineffective for severe RIOM in patients treated with a typical standard of care for concomitant chemoradiotherapy regimens [11]. Intravenously administered palifermin can significantly reduce the incidence and severity of RIOM and CIOM. However, palifermin is only effective in 4% of the at-risk population and can produce undesired side effects such as goblet cell hyperplasia [12].
Drug repurposing is a strategy for identifying new uses for approved or investigational drugs beyond the scope of the original medical indication [13]. Compared with traditional drug development, drug repurposing can pose a lower risk, less investment, and shorter time alternative, and may reveal new targets and pathways for disease treatment [14]. A prominent example of successful drug repurposing is sildenafil (Viagra), which was developed to treat high blood pressure but was later discovered to be effective in treating erectile dysfunction. In addition, thalidomide was initially used as a sedative but was later used to treat erythema nodosum leprosy and multiple myeloma [14]. A considerable number of drugs have successfully undergone drug repurposing, providing some inspiration for discovering new drugs to treat RIOM and CIOM.
Following the repurposing paradigm, this study investigated new drug treatment options for RIOM and CIOM using computational methods to mine a continuously expanding wealth of knowledge associated with publicly available biological data [12]. Text mining of large volumes of biomedical literature has been established as an effective tool to reveal new associations between genes and diseases. In particular, it can help identify the most interesting candidate genes for a disease for further experimental analysis [15]. Furthermore, new evidence on the potential to repurpose existing drugs can be obtained by combining text mining of available literature, analyzing various databases, and using search tools and other computational resources (e.g., Metascape, STRING, DGIdb) [16]. Therefore, we used text mining, pathway analysis, database analysis, computer resources, etc., to identify existing drugs that have the potential to be repurposed to treat RIOM and CIOM.
Methods
Data mining
In order to extract related genes by data mining, the literature on RIOM and CIOM was first searched in PubMed (https://pubmed.ncbi.nlm.nih.gov/) [17]. The computer search strings were the following: RIOM: (((radiotherapy) OR (radiation therapy) OR (radiation) OR (radiochemotherapy)) AND ((induced))) AND ((oral mucositis) OR (stomatitis)). CIOM: (((chemotherapy) OR (chemoradiotherapy)) AND ((induced))) AND ((oral mucositis) OR (stomatitis)). The abstracts of the identified studies were downloaded, and data mining was conducted with the R package “pubmed.mineR” (version 1.0.19) [18]. The RIOM and CIOM genes were extracted from each identified study and used for the next steps.
GO and KEGG pathway enrichment analyses
The genes of RIOM and CIOM were used for gene ontology (GO) enrichment analysis to explore the biological process, cellular components, and molecular functions of these genes [19]. Furthermore, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was conducted to explore the signaling pathways these genes participated in [20]. GO and KEGG enrichment analyses were conducted by the Metascape database (https://metascape.org/), which can provide comprehensive analysis and interpretation for OMICs-based data [21]. False discovery rate (FDR) was used to assess the statistical significance of the results, and FDR < 0.05 was considered statistically significant [22].
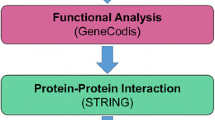
Protein-to-protein interaction (PPI) network
PPI network analysis was conducted to explore the interactions among these genes and further identify hub genes in this network. The web-based database String (http://string-db.org/) was used to perform the PPI analysis among the identified genes [23]. This database integrated PPI evidence from the experiment, database association, co-expression, and PubMed data mining. The PPI data were exported from the String database and imported into Cystoscope software (version 3.9.1) for depicting the PPI network [24]. The confidence parameter used to infer the interaction was set at high (70%). The hub genes (genes that play a significant role in the interaction network) in this PPI network were identified by algorithms including maximal clique centrality (MCC) [25], the density of maximum neighborhood component (DMNC) [26], maximum neighborhood component (MNC) [27], edge percolated component (EPC) [28], and degree [29]. These algorithms were integrated into “Cytohubba,” a plug-in of Cystoscope software [30]. The final list of hub genes was the sum of hub genes identified by the five algorithms (MCC, DMNC, MNC, EPC, and Degree).
Drug–gene interaction
The hub genes were used as the potential treatment targets for exploring drugs or small organic compounds in the DGIdb database (https://dgidb.genome.wustl.edu/), which aggregates drug–gene interaction data from 27 sources, including DrugBank, PharmGKB, ChEMBL, NCBI Entrez, Ensembl, PubChem, various clinical trial databases, and literature available through NCBI PubMed [31]. To ensure the predicated drugs had desirable therapeutic effects on R/CIOM, the drug–gene interactions were evaluated in the context of the gene’s association with mucositis and the drug’s correlation with the gene. The linked references were also explored to extract the related metadata, including the administration and approved use. Drugs or small organic compounds were included in the final drug list if they reached the criteria of targeting at least one of the identified hub genes through appropriate interaction. Furthermore, the clinical trials of these drugs for oral mucositis were screened using ClinicalTrials.gov (https://clinicaltrials.gov) to evaluate their treatment efficiency [32]. Figure 1 presents the overall design of our study.
Results
Genes identified through data mining
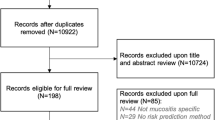
A literature search in PubMed provided 1413 studies of RIOM and 4528 studies of CIOM. Then, the abstracts of these studies were downloaded and used for data mining. Data mining identified 109 genes in RIOM and 209 genes in CIOM (Fig. 2). The lists of RIOM genes are presented in Supplementary file 1 (sheet 1) and CIOM genes in Supplementary file 1 (sheet 2).
Results of GO and KEGG pathway enrichment analyses
GO and KEGG pathway enrichment analyses were conducted to clarify the molecular functions of these genes and the signaling pathway they participated in. The top five significant annotation terms were identified (Table 1). In RIOM, the top 5 terms of GO enrichment analysis were “response to molecule of bacterial origin,” “ERK1 and ERK2 cascade,” “positive regulation of DNA metabolic process,” “positive regulation of peptidyl-tyrosine phosphorylation,” and “response to lipopolysaccharide.” The top 5 terms of KEGG pathway enrichment analysis were “FoxO signaling pathway,” “toll-like receptor signaling pathway,” “NF-kappa B signaling pathway,” “MAPK signaling pathway,” and “PI3K-Akt signaling pathway.” In CIOM, the top 5 GO terms enriched were “response to molecule of bacterial origin,” “response to lipopolysaccharide,” “cellular response to lipopolysaccharide,” “positive regulation of cytokine production,” and “cellular response to biotic stimulus.” The top 5 KEGG terms enriched were “toll-like receptor signaling pathway,” “NF-kappa B signaling pathway,” “Chemokine signaling pathway,” “TNF signaling pathway,” and “NOD-like receptor signaling pathway.” In the meanwhile, all of the GO and KEGG terms of RIOM were presented in Supplementary file 1 (sheet 3 and sheet 4) and terms of CIOM Supplementary file 1 (sheet 5 and sheet 6).
Results of PPI network analysis
First, the PPI network analysis was conducted, combining the top 5 terms in GO enrichment analysis and the top 5 signaling pathways in KEGG pathway enrichment analysis. Then, the interaction data were imported into Cystoscope software. Second, hub genes in this network were identified using the algorithms in Cytohubba. Furthermore, the final list of hub genes was the sum of hub genes identified by the five algorithms (MCC, DMNC, MNC, EPC, and Degree). The results of each algorithm for RIOM were presented in Supplementary file 1 (sheet 7) and CIOM in Supplementary file 1 (sheet 8). Third, the high-confidence PPI networks of hub genes in RIOM (Fig. 3A) and CIOM (Fig. 3B) were exhibited, respectively. Connecting lines with different colors represents different types of interaction, and the confidence parameter used to infer the interaction was set at a high level (70%).
Results of exploring drug–gene interaction
Table 2 presents the candidate drugs for treating RIOM and CIOM predicted by drug–gene interactions. To ensure that the predicated drugs had desirable therapeutic effects, the drug–gene interactions were evaluated in the context of the gene’s association with mucositis and the drug’s correlation with the gene. The linked references were also explored to extract the related metadata, including the administration and approved use. Drugs or small organic compounds were included in the final drug list if they reached the criteria of targeting at least one of the identified hub genes through appropriate interaction. Using these hub genes identified from the PPI network, we reached candidate drugs that met our inclusion criteria. The targets of these potential drugs are interleukin 6 (IL-6), toll-like receptor 9 (TLR9), and tumor necrosis factor (TNF). Furthermore, eight approved drugs (olokizumab, chloroquine, hydroxychloroquine, adalimumab, etanercept, golimumab, infliximab, and thalidomide) were identified through drug–gene interactions. Table 2 presents their related clinical evidence.
Discussion
This study explored the potentially important genes and pathways in RIOM and CIOM and identified the candidate drugs for treatment. First, we obtained a preliminary list of genes associated with RIOM and CIOM using text mining. Then, to validate the association of our genes and generate a targeted set of targets, we integrated in-depth information on these genes in a functional analysis and enrichment manner. Next, the extracted genes were analyzed for their function and gene ontology using Metascape, resulting in an enriched gene list of high-priority targets. Finally, the drug–gene interaction database was used to determine the interaction of the final enriched gene list with known drugs and analyze the drug candidates. We analyzed three potential candidate genes (IL-6, TLR9, and TNF) and eight approved drugs (olokizumab, chloroquine, hydroxychloroquine, adalimumab, etanercept, golimumab, infliximab, and thalidomide) were identified, which could play an important role in the treatment of RIOM and CIOM.
TNF plays a vital role in OM. Adalimumab, etanercept, golimumab, infliximab, and thalidomide are inhibitors of TNF, playing important anti-inflammatory and immunosuppressive roles. Adalimumab was effective in treating aphthous stomatitis in three case reports [33,34,35], and a randomized clinical trial demonstrated its effect on Crohn’s disease (NCT01562951). Golimumab was also effective in treating ulcerative colitis, supported by a case report and two non-randomized clinical trials. According to a case report, infliximab relieved the pembrolizumab-induced steroid refractory oral mucositis, but further evidence is still necessary [36]. However, in preclinical trials, infliximab could not improve mucositis, which might restrict its potential in further research [37, 38]. Finally, thalidomide has been supported by a multicenter, open-label, randomized controlled trial. Patients with locally advanced nasopharyngeal carcinoma undergoing concurrent chemoradiotherapy were recruited and treated with thalidomide. The results indicated that thalidomide prolonged the latency period, reduced the incidence of oral mucositis, and did not affect the short-term efficacy of concurrent chemoradiotherapy [39].
IL-6 is a pro-inflammation cytokine in oral mucositis [40]. IL-6 induces the breakdown of vascular endothelial calmodulin on endothelial cells either directly or by inducing VEGF, leading to vascular hyper-permeability and tissue damage [41]. IL-6 could also induce tissue factor expression on the surface of monocytes and trigger the coagulation cascade, leading to thrombin activation and clot formation [42]. Olokizumab, as a newly designed inhibitor of IL-6, was suggested as a candidate. However, further clinical evidence is still required.
The role of TLR9 in RIOM and CIOM is still not well documented. TLR9 is mainly expressed on the membrane of endosomes and binds nucleic acids, including DNA and RNA from both bacteria and viruses. In addition, it is expressed in oral mucosal epithelial cells and multiple mucosal immune cells, with an important role in innate and adaptive immunity [43]. After chemotherapy, TLR9 is more likely to recognize a part of the intestinal microbiota, send downstream signals, produce pro-inflammatory mediators, and mediate the development of gastrointestinal mucositis. Therefore, TLR9 antagonists may reduce anti-tumor drug-induced intestinal injuries. Several studies have also found that deletion of the TLR9 receptor gene improves animal survival and reduces intestinal injury and bacteremia. Also, it reduces the expression of inflammatory markers, such as NF-κB, IL-1, IL-18, and COX-2 [44]. Wong et al. noted that TLR9 knockout preserved mucosal structure, bacterial translocation, and IL-1β expression compared to wild-type mice injected with saline or irinotecan to induce intestinal mucositis [45]. Therefore, TLR9 could be a promising target in the treatment. Case reports have reported that hydroxychloroquine is a TLR9 antagonist and might alleviate RIOM and CIOM [46, 47]. Nevertheless, further clinical evidence is required.
Since radiotherapy and chemotherapy will also lead to epithelial injury besides mucositis, the similarity and differences between R/CIOM and radio/chemotherapy-induced epithelial injury should be noticed. On the issue of similarity, radiation and chemo drugs can damage liquid and DNA, leading to destruction of epithelial cell. In addition, in the mucosa, radiation-induced loss of stem cells from the basal layer interferes with the replacement of cells in the superficial mucosal layers when they are lost through normal physiological sloughing [48]. Changes of the process of neovascularization have also been observed in both R/CIOM and radio/chemotherapy-induced epithelial injury. Neovascularization requires signaling through the vascular endothelial growth factor (VEGF) family. After exposure to 10Gy irradiation, the synthesis of angiogenic factor VEGF in the blood of rat tumor carriers was significantly hindered [49]. Preclinical studies supported this by showing that irradiated rat bladder epithelium administrated with VEGF resulted in a marked reduction in fibrotic tissue and enhanced tissue angiogenesis, which suggested the potential value of VEGF in radio/chemotherapy-induced epithelial injury [50]. However, there also exist differences between R/CIOM and radio/chemotherapy-induced epithelial injury. It has been reported that transforming growth factor β (TGF-β), a peptide which has a fundamental role in controlling proliferation of many cell types, is intricately involved in the development of chronic radiation dermatitis. It activates fibroblasts to secrete extracellular matrix protein, leading to epithelium fibrosis. In the irradiated tissue of pigs, TGF-β plays an important role in promoting and regulating the late fibrotic process [51]. However, mice oral mucosa lacks Smad3, a downstream mediator of TGF-b, which demonstrates decreased tissue damage and fibrosis after irradiation, as well as accelerated healing [52]. IL-10 is capable of inhibiting the inflammatory response and reducing the activity of macrophages in radio/chemotherapy-induced epithelial injury, but it has been reported not expressed in RIOM [53]. When it comes to RIOM and CIOM, keratinocyte growth factor-1 (KGF-1) has been demonstrated as a valuable target for treatment. Palifermin, a kind of recombinant KGF-1 use, has been approved by FDA to decrease the incidence and severity of RIOM and CIOM [54]. Recent studies also reported that Wnt/β-catenin signaling activator has a protective effect on post irradiation tissues and promotes regeneration of colonic epithelium after chemical damage [55, 56]. Furthermore, LiCl has been indicated to promote the renewal of tongue mucosa, thus diminishing oral mucositis and taste dysfunction of irradiated mice by activating Wnt/β-catenin signaling pathway [57]. By verifying the special mechanisms of RIOM and CIOM, further studies could explore more valuable targets and more effective drugs.
The importance of the kinetics of genomic expression should also be noticed in treating RIOM and CIOM. The overexpression of TNF and IL-6 usually occurs after the inflammation cascade is active, which means the progress of OM enters the signal amplification phase, and pro-inflammatory cytokines amplify the damage [58]. From a therapeutic perspective, this time may be too late to inhibit TNF and IL-6. Therefore, applying phosphodiesterase-5 (PDE5) agents could be a good reference. By inhibiting PDE5, these drugs prevent the degradation of cGMP, which can activate protein kinase G [59]. Therefore, it could be promising to develop drugs that could inhibit the injury cascade beforehand.
This study had some limitations and should be interpreted with caution. First, the genomic data we used was widely and roughly associated with RIOM and CIOM, which reduces the accuracy of outputs. Second, there was heterogeneity in the data we analyzed, including different anti-cancer treatments, vacations in OM trajectory, and the time of sample collection, which would affect the signals. Third, the data mining was conducted only in PubMed, possibly including limited genes and their function or ways in a specific pathway. Fourth, not all existing drug–gene interactions were clear and included in the database. Potential drugs might have been ignored because their drug–gene interactions have not yet been fully demonstrated or could have had detrimental effects. Finally, further high-quality clinical research is required to confirm and verify our findings.
Conclusion
Through our data mining, bioinformatics survey, and candidate drug selection, TNF, IL-6, and TLR9 could play important roles in the progression and treatment of R/CIOM. In addition, eight candidate drugs (olokizumab, chloroquine, hydroxychloroquine, adalimumab, etanercept, golimumab, infliximab, and thalidomide) were selected by drug–gene interaction literature search as candidates for treating RIOM and CIOM.
References
Scully C, Epstein J, Sonis S (2003) Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy: part 1, pathogenesis and prophylaxis of mucositis. Head Neck 25(12):1057–1070
Cinausero M, Aprile G, Ermacora P, Basile D, Vitale MG, Fanotto V, et al. New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury. Front Pharmacol 2017;8:354.
Sonis ST (2007) Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol 5(9 Suppl 4):3–11
Sonis ST (2010) New thoughts on the initiation of mucositis. Oral Dis 16(7):597–600
Logan RM, Gibson RJ, Bowen JM, Stringer AM, Sonis ST, Keefe DM (2008) Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: implications for the pathobiology of mucositis. Cancer Chemother Pharmacol 62(1):33–41
Pulito C, Cristaudo A, Porta C, Zapperi S, Blandino G, Morrone A et al (2020) Oral mucositis: the hidden side of cancer therapy. J Exp Clin Cancer Res 39(1):210
Al-Dasooqi N, Sonis ST, Bowen JM, Bateman E, Blijlevens N, Gibson RJ et al (2013) Emerging evidence on the pathobiology of mucositis. Support Care Cancer 21(7):2075–2083
McCullough RW (2017) US oncology-wide incidence, duration, costs and deaths from chemoradiation mucositis and antimucositis therapy benefits. Future Oncol 13(30):2823–2852
Gruber S, Bozsaky E, Roitinger E, Schwarz K, Schmidt M, Dörr W (2017) Early inflammatory changes in radiation-induced oral mucositis: effect of pentoxifylline in a mouse model. Strahlenther Onkol 193(6):499–507
Villa A, Sonis ST (2020) An update on pharmacotherapies in active development for the management of cancer regimen-associated oral mucositis. Expert Opin Pharmacother 21(5):541–548
Nicolatou-Galitis O, Sarri T, Bowen J, Di Palma M, Kouloulias VE, Niscola P et al (2013) Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients. Support Care Cancer 21(11):3179–3189
Kirk J, Shah N, Noll B, Stevens CB, Lawler M, Mougeot FB et al (2018) Text mining-based in silico drug discovery in oral mucositis caused by high-dose cancer therapy. Support Care Cancer 26(8):2695–2705
Ashburn TT, Thor KB (2004) Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 3(8):673–683
Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A et al (2019) Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 18(1):41–58
Yu S, Tranchevent LC, De Moor B, Moreau Y (2010) Gene prioritization and clustering by multi-view text mining. BMC Bioinformatics 11:28
Berg EL (2014) Systems biology in drug discovery and development. Drug Discov Today 19(2):113–125
Kang P, Kalloniatis M, Doig GS (2021) Using updated PubMed: new features and functions to enhance literature searches. Jama 326(6):479–480
Rani J, Shah AB, Ramachandran S (2015) pubmed.mineR: an R package with text-mining algorithms to analyse PubMed abstracts. J Biosci 40(4):671–682
Resource TGO (2019) 20 years and still GOing strong. Nucleic Acids Res 47(D1):D330–D3d8
Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K (2017) KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45(D1):D353–Dd61
Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O et al (2019) Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 10(1):1523
Chen X, Robinson DG, Storey JD (2021) The functional false discovery rate with applications to genomics. Biostatistics 22(1):68–81
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J et al (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47(D1):D607–Dd13
Otasek D, Morris JH, Bouças J, Pico AR, Demchak B (2019) Cytoscape Automation: empowering workflow-based network analysis. Genome Biol 20(1):185
Meghanathan N (2015) Maximal clique size versus centrality: a correlation analysis for complex real-world network graphs. In: Nagar A, Mohapatra D, Chaki N (eds) Proceedings of 3rd International Conference on Advanced Computing, Networking, and Informatics (ICACNI). Smart Innovation, Systems and Technologies. India, pp 95–101. https://doi.org/10.1007/978-81-322-2529-4_9
Latecki LJ, Sobel M, Lakaemper R (2009) Piecewise linear models with guaranteed closeness to the data. IEEE Trans Pattern Anal Mach Intell 31(8):1525–1531
Martins P (2012) Cliques with maximum/minimum edge neighborhood and neighborhood density. Comput Oper Res 39(3):594–608
Serrano MA, Rios PDL (2007) Interfaces and the edge percolation map of random directed networks. Phys Rev E 76(5)
Chung F, Horn P, Lu LY (2009) The giant component in a random subgraph of a given graph. In: 6th International Workshop on Algorithms and Models for the Web-Graph. Lecture Notes in Computer Science. Barcelona, Spain, p 38
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY (2014) cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol 8((Suppl 4)):S11
Freshour SL, Kiwala S, Cotto KC, Coffman AC, McMichael JF, Song JJ et al (2021) Integration of the Drug-Gene Interaction Database (DGIdb 4.0) with open crowdsource efforts. Nucleic Acids Res 49(D1):D1144–D1d51
Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database--update and key issues. N Engl J Med 2011;364(9):852-860.
Vujevich J, Zirwas M (2005) Treatment of severe, recalcitrant, major aphthous stomatitis with adalimumab. Cutis 76(2):129–132
Sánchez-Cano D, Callejas-Rubio JL, Ruiz-Villaverde R, Ortego-Centeno N (2009) Recalcitrant, recurrent aphthous stomatitis successfully treated with adalimumab. J Eur Acad Dermatol Venereol 23(2):206
de Perosanz-Lobo D, Latour I, Ortega-Quijano D, Fernández-Guarino M, Torrelo A (2019) Severe recurrent aphthous stomatitis treated with adalimumab: a case report in a teenage patient. Pediatr Dermatol 36(6):986–987
Dang H, Sun J, Wang G, Renner G, Layfield L, Hilli J (2020) Management of pembrolizumab-induced steroid refractory mucositis with infliximab: a case report. World J Clin Cases 8(18):4100–4108
Hemperly A, Vande CN (2018) Clinical pharmacokinetics and pharmacodynamics of infliximab in the treatment of inflammatory bowel disease. Clin Pharmacokinet 57(8):929–942
Vegh Z, Kurti Z, Lakatos PL (2017) Real-life efficacy, immunogenicity and safety of biosimilar infliximab. Dig Dis 35(1-2):101–106
Liang L, Liu Z, Zhu H, Wang H, Wei Y, Ning X et al (2022) Efficacy and safety of thalidomide in preventing oral mucositis in patients with nasopharyngeal carcinoma undergoing concurrent chemoradiotherapy: A multicenter, open-label, randomized controlled trial. Cancer 128(7):1467–1474
Khan S, Wardill HR, Bowen JM (2018) Role of toll-like receptor 4 (TLR4)-mediated interleukin-6 (IL-6) production in chemotherapy-induced mucositis. Cancer Chemother Pharmacol 82(1):31–37
Deng S, Wu D, Li L, Li J, Xu Y (2021) TBHQ attenuates ferroptosis against 5-fluorouracil-induced intestinal epithelial cell injury and intestinal mucositis via activation of Nrf2. Cell Mol Biol Lett 26(1):48
Candel-Martí ME, Flichy-Fernández AJ, Alegre-Domingo T, Ata-Ali J, Peñarrocha-Diago MA (2011) Interleukins IL-6, IL-8, IL-10, IL-12 and periimplant disease. An update. Med Oral Patol Oral Cir Bucal 16(4):e518–e521
Ji L, Hao S, Wang J, Zou J, Wang Y. Roles of toll-like receptors in radiotherapy- and chemotherapy-induced oral mucositis: a concise review. Front Cell Infect Microbiol 2022;12:831387.
Ribeiro RA, Wanderley CW, Wong DV, Mota JM, Leite CA, Souza MH et al (2016) Irinotecan- and 5-fluorouracil-induced intestinal mucositis: insights into pathogenesis and therapeutic perspectives. Cancer Chemother Pharmacol 78(5):881–893
Wong DV, Lima-Júnior RC, Carvalho CB, Borges VF, Wanderley CW, Bem AX, et al. The adaptor protein Myd88 is a key signaling molecule in the pathogenesis of irinotecan-induced intestinal mucositis. PLoS One 2015;10(10):e0139985.
Hussein H, Brown R (2021) Hydroxychloroquine and the treatment of Sjogren syndrome, chronic ulcerative stomatitis, and oral lichen planus in the age of COVID-19. Oral Surg Oral Med Oral Pathol Oral Radiol 131(1):e9–e13
Stoopler ET, Kulkarni R, Alawi F, Sollecito TP (2021) Novel combination therapy of hydroxychloroquine and topical tacrolimus for chronic ulcerative stomatitis. Int J Dermatol 60(4):e162–e1e3
Hymes SR, Strom EA, Fife C (2006) Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol 54(1):28–46
Mitryayeva NA, Grebinyk LV, Uzlenkova NE (2019) influence of combined action of X-radiation and cyclooxygenase-2 - meloxivet inhibitor on VEGF and PGE-2 content in blood of rat-tumor carriers. Probl Radiac Med Radiobiol 24:261–269
Soler R, Vianello A, Füllhase C, Wang Z, Atala A, Soker S et al (2011) Vascular therapy for radiation cystitis. Neurourol Urodyn 30(3):428–434
Lefaix JL, Delanian S, Vozenin MC, Leplat JJ, Tricaud Y, Martin M (1999) Striking regression of subcutaneous fibrosis induced by high doses of gamma rays using a combination of pentoxifylline and alpha-tocopherol: an experimental study. Int J Radiat Oncol Biol Phys 43(4):839–847
Flanders KC, Major CD, Arabshahi A, Aburime EE, Okada MH, Fujii M et al (2003) Interference with transforming growth factor-beta/ Smad3 signaling results in accelerated healing of wounds in previously irradiated skin. Am J Pathol 163(6):2247–2257
Groeger S, Meyle J (2019) Oral Mucosal Epithelial Cells Front Immunol 10:208
Coutsouvelis J, Corallo C, Spencer A, Avery S, Dooley M, Kirkpatrick CM (2022) A meta-analysis of palifermin efficacy for the management of oral mucositis in patients with solid tumours and haematological malignancy. Crit Rev Oncol Hematol 172:103606
Huo K, Sun Y, Li H, Du X, Wang X, Karlsson N et al (2012) Lithium reduced neural progenitor apoptosis in the hippocampus and ameliorated functional deficits after irradiation to the immature mouse brain. Mol Cell Neurosci 51(1-2):32–42
Raup-Konsavage WM, Cooper TK, Yochum GS (2016) A Role for MYC in lithium-stimulated repair of the colonic epithelium after DSS-induced damage in mice. Dig Dis Sci 61(2):410–422
Zhu J, Zhang H, Li J, Zheng X, Jia X, Xie Q et al (2021) LiCl promotes recovery of radiation-induced oral mucositis and dysgeusia. J Dent Res 100(7):754–763
Sonis ST (2021) Treatment for oral mucositis-current options and an update of small molecules under development. Curr Treat Options in Oncol 22(3):25
Ahmed WS, Geethakumari AM, Biswas KH (2021) Phosphodiesterase 5 (PDE5): structure-function regulation and therapeutic applications of inhibitors. Biomed Pharmacother 134:111128
Funding
This study was supported by Natural Science Foundation of Sichuan Province (Youth Science Foundation Project) (2022NSFSC1482 to YW) and Science & Technology Department of Sichuan province (2023YFS0349 to JW). None of the funders participated in this study.
Author information
Authors and Affiliations
Contributions
Y. W. and S. H. contributed to the overall conceptualization and design of the study. S. H., Y. J., and Y. Y. conducted the literature search, carried out the data extraction and quality assessment, analyzed the data. S. H. and Y. W. drafted the manuscript. J.W. and J.Z. revised the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study is a data mining study which did not include any experiments on live vertebrates. Therefore, it did not need approval, accordance, and (for human subjects) informed consent. All data of this study was from available public databases which did not need ethical approval.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
Supplementary file 1 The supplied data of our study for the convenience of readers. Sheet 1: Lists of genes found by text-ming in RIOM. Sheet 2: Lists of genes found by text-ming in CIOM. Sheet 3: All terms of GO enrichment analysis in RIOM. Sheet 4: All terms of GO enrichment analysis in CIOM. Sheet 5: All signaling pathways of KEGG pathway enrichment analysis in RIOM. Sheet 6: All signaling pathways of KEGG pathway enrichment analysis in CIOM. Sheet 7: The list of hub-genes identified by each algorithms (MCC, DMNC, MNC, EPC and Degree) in RIOM. Sheet 8: The list of hub-genes identified by each algorithms (MCC, DMNC, MNC, EPC and Degree) in CIOM.
ESM 2
Supplementary file 2 The computer operation strings of our study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hao, S., Jin, Y., Yu, Y. et al. Identification of potential molecular mechanisms and candidate drugs for radiotherapy- and chemotherapy-induced mucositis. Support Care Cancer 31, 223 (2023). https://doi.org/10.1007/s00520-023-07686-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-07686-7