Abstract
Purpose
The status and associated factors of the health-related quality of life of non-small-cell lung cancer (NSCLC) patients under targeted anti-cancer therapy have not been investigated. Self-management and coping style have been proven to be closely related to patients’ health-related quality of life. Based on these observations, this study was designed to firstly assess the status of health-related quality of life, and then explore the relationships among coping styles, self-management, and health-related quality of life of NSCLC patients with skin adverse drug reactions under targeted therapy.
Methods
We performed a cross-sectional study including 536 NSCLC patients with skin adverse drug reactions under targeted therapy in cancer clinics of three hospitals in China between May 2020 and May 2021. Structured questionnaires, including the Functional Assessment of Cancer Therapy-Epidermal Growth Factor Receptor Inhibitor 18, Cancer Patient Self-management Evaluation Scale, and Medical Coping Modes Questionnaire, were used to collect data. Relationships among coping style, self-management, and health-related quality of life were identified by Pearson correlation analysis and a multiple linear regression algorithm.
Results
The total score of health-related quality of life was 46 ± 12.84 in 536 NSCLC patients with skin adverse drug reactions undergoing targeted therapy. Health-related quality of life was positively correlated with self-management (r = 0.785, P < 0.01) and facing (r = 0.807, P < 0.01) and negatively correlated with yield (r = − 0.718, P < 0.01), avoidance (r = − 0.711, P < 0.01), and the severity of skin adverse reactions (r = − 0.722, P = 0.000). Via multiple linear regression analysis, we identified some significant factors associated with health-related quality of life, including age, education level, combination of medicine, Charlson Comorbidity Index, stages of disease, facing, yield, symptom management, daily activity management, psychological and emotional management, self-efficacy, and self-management (P < 0.05).
Conclusions
NSCLC patients with skin adverse drug reactions undergoing targeted therapy generally had a compromised health-related quality of life. The critical factors that were associated with the status of health-related quality of life were age, education level, comorbidity, the combinatorial application of drugs, stage of disease, self-management, and coping styles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, the incidence of lung cancer accounts for 11.4% of all cancers and the death caused by lung cancer accounts for 18.0% of all cancers. Lung cancer is still the main cause of cancer-related death [1]. In 2020, there were approximately 820,000 new lung cancer cases and 710,000 lung cancer-related deaths in China, accounting for 17.9% of all new cancers and 23.8% of the total cancer deaths [2]. Among various types of lung cancer, non-small-cell lung cancer (NSCLC) accounts for 85% of the total cases [3]. Significantly, 75% of NSCLC patients are in advanced stages when diagnosed with a 5-year survival rate of approximately 15% [4]. After chemotherapy, the average survival time of NSCLC patients was only 8.5 months, and the 5-year survival rate was only 4 ~ 17% [5]. As a result, NSCLC-related treatment and clinical care have received great attention in recent years.
The identification of molecular targets of particular cancer cells has enabled individualized anti-cancer treatment, thus providing a framework for developing targeted drugs that can interfere with the growth, proliferation, and apoptosis of cancer cells [6, 7]. Several studies had shown that targeted therapies could inhibit symptoms and improve the overall survival (OS), progression-free survival, and response rate (RR) with significant advantages in better tolerance and quality of life (QoL) of patients [8,9,10]. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) had been confirmed as a first-line treatment for advanced NSCLC patients who are mutation-positive [11], representing more than 50% of NSCLC patients in the Asia–Pacific region [12]. Partly because of its oral administration, EGFR-TKIs have broken the limitation of hospitalization which reduced patients’ burden and the waste of medical resources [13], highlighting the importance of studying this group of drugs in clinical settings.
However, because EGFR signaling plays a critical role in epidermal physiology, the association of skin toxicity with EGFR inhibition is not an unexpected phenomenon. A recent investigation reported that 80% of NSCLC patients had skin adverse drug reactions (ADRs) within 3 months after EGFR-TKI treatment. The manifestations of skin ADRs associated with EGFR-TKIs include acneiform eruptions (even papule pustular rash), pruritus, dryness of skin, hand-foot syndrome, paronychia and nail loss, hair changes, and hypersensitivity [14]. Severe skin ADRs hurt patients both physically and psychologically [15]. The pruritus, irritation, and sensitivity of skin could disrupt the sleep cycle and daily activities of patients [16]. Moreover, the xerosis, shriveling, and darkening of skin could damage patients’ self-image and self-esteem which may increase patients’ pressure in social participation or affect their intimate and interpersonal relationships [17]. These issues could be further enhanced by anxiety, depression, and inferiority caused by the persistent helplessness to ADRs [18].
Obviously, skin toxicities can have a profound impact on patients’ QoL owing to symptoms and potential esthetic sequelae. Several studies indicated that poor QoL could reduce medication adherence in patients receiving EGFR-TKI-based targeted therapy. One study showed that approximately 32% of the patients stopped taking drugs due to poor QoL caused by various toxic skin reactions, which compromised the treatment effect and even led to cancer recurrence or progression [19]. Another study indicated patients with low QoL were pessimistic about treatment, which led to treatment interruption [20]. As suggested by a randomized controlled trial, the level of QoL was positively correlated with RR and OS [9]. Therefore, the QoL of NSCLC cancer patients with skin ADRS under targeted therapy needs to be considered.
However, QoL is a broad concept that considers multiple aspects and is influenced by various factors, such as the diagnosis and treatment of cancer, which have a greater impact on QoL than skin ADRs. A study showed that health-related quality of life (HRQOL) was affected by symptoms and discomfort caused by skin ADRs of EGFR-TKIs [21]. Thus, we focused more on the impact of skin ADRs on HRQOL. HRQOL in this study refers to the extent to which a person’s expected physical, emotional, and social well-being is affected by skin ADRs. Therapy-Epidermal Growth Factor Receptor Inhibitor 18 (FACT-EGFRI-18) is a specialized scale to evaluate the HRQOL of cancer patients with specific skin toxicity caused by EGFR-TKI treatment. In this study, FACT-EGFRI-18 was used to assess the HRQOL of NSCLC patients in three dimensions: physiological status, social and family status, and functional status [22].
Several factors had been previously identified were associated with the HRQOL of patients. As shown in a recent study, a positive coping style enabled patients to actively communicate disease with health providers and solve difficulties during symptom management [23]. In another study, it was reported that the negative effects of treatment and the distress induced by symptoms could be reduced through the adjustment of coping strategies [24]. Indeed, a reasonable coping style might enable patients to better coordinate the relationship between the degree of ADRs and treatment and promote patients’ HRQOL. Meanwhile, self-management was another factor correlated with functional status in cancer patients with targeted therapies [25]. Self-management recommended by clinical guidelines including preventive measures and appropriate interventions may help to prevent and control severe skin reactions for improved HRQOL [26]. A previous study demonstrated that good self-management could effectively regulate the troubles caused by treatment-related ADRs in lung cancer patients [27]. Additionally, a meta-analysis indicated that patients with better self-management ability showed less anxiety and better adaptability under stressful environments and tended to learn knowledge and skills to improve HRQOL positively [28]. However, although factors such as coping style and self-management might significantly influence the HRQOL of patients with skin ADRs under targeted therapy, no comprehensive investigations in this area have been reported.
This study aimed to assess the HRQOL of NSCLC patients with skin ADRs undergoing targeted therapy and determine whether and how coping style and self-management are associated with HRQOL. Then, based on the collected data, we endeavored to develop pertinent promotion strategies to reduce the severity of ADRs for improved HRQOL. The hypotheses were as follows: (1) the severity of skin ADRs is associated with the HRQOL of NSCLC patients undergoing targeted therapy; (2) coping style is associated with the HRQOL; and (3) self-management is associated with HRQOL.
Methods
Design and setting
This work employed a cross-sectional study from May 2020 to May 2021. Outpatient NSCLC patients with skin ADRs under EGFR-TKI treatment were recruited from three tertiary hospitals in Henan Province.
Study population
Patients’ electronic medical records were reviewed to identify eligible participants. A total of 536 NSCLC patients with skin ADRs undergoing EGFR-TKI treatment were recruited by a convenience sampling method from three hospitals in Henan, China. The NCCN guidelines indicate that EGFR-TKIs are mostly used in the treatment of advanced NSCLC patients; however, EGFR-TKIs can also be used as a complementary treatment in early-stage NSCLC patients [29]. Therefore, we recruited patients in stages I-IV. Inclusion criteria for participation in the study were as follows: (1) the pathological diagnosis was NSCLC; (2) EGFR-positive NSCLC patients; (3) patients received EGFR-TKI (including erlotinib, gefitinib, ektinib, afatinib, dactinib, ositinib) treatment for more than 4 weeks; (4) patients had any skin ADR according to the common terminology criteria for adverse events version 5.0 (CTCAE 5.0); (5) patients were 18 ~ 80 years old; and (6) patients provided informed consent. The exclusion criteria were as follows: (1) patients who were seriously ill and unable to complete the research; (2) patients who were unable to complete the questionnaire independently or with the assistance of investigators; and (3) patients who had impaired cognitive function and could not communicate normally.
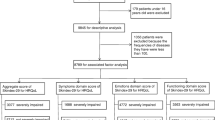
The sample size was calculated based on the following formula: N = (UαS/δ)2 with a desired significance level of 0.05 and a power of 0.80. Uα is the U value (1.96) corresponding to the inspection level of α, S is the standard deviation, and δ is the allowable error [30]. The S/δ (8.2) was obtained from the pretest we conducted on 10 patients. Therefore, the estimated minimum sample size was 258. The screening process of the study participants is shown in Fig. 1.
Procedure and data collection
Skin ADRs were confirmed and evaluated according to CTCAE 5.0. In total, 536 NSCLC patients were invited to participate in this study and informed of the detailed study design. The potential participants were interviewed face to face by nurses when they attended the clinic. The principal investigator (PI) explained the purpose, content, and investigation procedures as well as the principle of anonymity of the study. Informed consent forms were signed by all participants. Questionnaires were distributed to the participants during their clinical consultation or after the clinical visit in the waiting room.
After obtaining the consent of relevant departments, we consulted the medical records of patients to obtain disease-related information. Trained postgraduate students were recruited to distribute questionnaires and give explanations to the patients’ questions. For the illiterate participants, the researchers read the question items verbatim. Then responses were recorded on the questionnaire. The questionnaires were collected immediately after completion, checked for any missing information, and followed up with the participants.
Measures
Demographic characteristics
This is a self-designed questionnaire to collect information of sociodemographic variables, such as gender, age, level of education, family monthly income, marital status, occupation, residence, health insurance, disease stages, duration of treatment, and combined drug application and comorbidities (calculated as the number of chronic diseases included in the Charlson Comorbidity Index (CCI)).
FACT-EGFRI-18
The FACT-EGFRI-18 was used to investigate HRQOL [22]. The scale was divided into three dimensions: physiological status including 7 items (e.g., my skin or scalp itches), social and family status including 6 items (e.g., I avoid going to public places because of the appearance of my skin), and functional status including 5 items (e.g., skin ADRs make my daily life difficult) to investigate the HRQOL of patients in the last 7 days. According to the degree of impact on patients’ HRQOL, a Likert 5-level scoring method was adopted ranging from “no impact” (0 points) to “a lot impact” (4 points). The positively phrased items were scored 0 ~ 4 points, and the negatively phrased items were scored 4 ~ 0 points. The total score was obtained by summing the scores of each item. The total score range was 0 ~ 72. The higher the score, the better the HRQOL. Cronbach’s α coefficient of the 3 dimensions ranged from 0.704 to 0.949, and Cronbach’s α coefficient of the total scale was 0.919 in this study. This scale was used in another study on patients with skin ADRs caused by EGFR-TKIs with a total Cronbach’s α of 0.867, and the Cronbach’s α coefficients of the 3 dimensions were 0.673, 0.731, and 0.816 [31].
Self-management scale for cancer patients
Self-management was evaluated by the Cancer Patient Self-management Evaluation Scale developed by Cheng Lingling in 2017 [32]. This scale was developed based on Chinese cancer patients covering six core dimensions that cancer patients need to master in self-management: daily life management including 11 items (e.g., I can manage my daily life), symptom management including 7 items (e.g., I can cope with the adverse effects of treatment), psychological and emotional management including 9 items (e.g., I can control negative emotions), communication with medical staff including 4 items (e.g., I can choose the suitable treatment guided by doctors), information management including 3 items (e.g., I can actively acquire disease-related knowledge), and self-management efficacy including 10 items (e.g., I have great confidence in the treatment of my disease). Likert 5-level scoring ranged from “no” (1 point) to “always” (5 points) was adopted in this scale. The higher the score, the better the self-management ability of patients. Cronbach’s α coefficients of the 6 dimensions ranged from 0.698 to 0.933, and Cronbach’s α coefficient of the total scale was 0.889. This scale was used in a previous study to evaluate the self-management behavior of post-treatment survivors of lung cancer. Cronbach’s α coefficient of the total scale was 0.916, the test–retest reliability was 0.856, and the content validity of each item was 0.834 ~ 1 [33].
Medical coping modes questionnaire
The Medical Coping Modes Questionnaire (MCMQ) was used to measure the patients’ coping styles [34]. At present, the MCMQ has been widely used to assess coping styles in patients with cancer and various chronic diseases with good reliability and validity [35]. It included 3 dimensions: face including 8 items (e.g., Do you often want to talk about your disease with relatives and friends), avoid including 7 items (e.g., When thinking about your disease, will you do something else to distract your attention), and yield including 5 items (e.g., Do you often feel there is nothing you can do about the disease). A 4-point Likert scoring method was used for each item to measure the degree of agreement with each statement (1 = never, 2 = seldom, 3 = sometimes, 4 = always). The higher the cumulative score, the more the coping style. Cronbach’s α coefficients of the 3 dimensions were 0.84, 0.83, and 0.92 in this study. This scale was used in a study on coping style and HRQOL of patients with lung cancer. The test–retest reliability was 0.882, and Cronbach’s α coefficients of the 3 dimensions were 0.69, 0.60, and 0.76 [36].
Ethics statement
This study was approved by the Ethics Committee of our university, and administrative permissions were obtained from directors of oncology departments. All methods were carried out in accordance with relevant guidelines and regulations. Information about the study was provided to the participants, and we obtained written informed consent from all participants prior to their participation in the study.
Statistical analysis
A total of 536 patients with complete data were finally included in the analysis. All data were analyzed by the statistical software SPSS 21.0. Quantitative data were analyzed using descriptive statistical methods. In the descriptive analyses, means and standard deviations were calculated for continuous data, while frequencies and percentages were calculated for categorical variables. Pearson correlation analysis was applied to determine the correlation among patients’ self-management, coping styles, and HRQOL. Pearson correlation coefficients were categorized as negligible (0.3 ~ 0.5), low (0.3 ~ 0.5), moderate (0.5 ~ 0.7), high (0.7 ~ 0.9), and very high (0.9 ~ 1) [37]. Multiple linear regression analysis was performed to explore factors (age, sex, marital status, educational level, residence, medical insurance, family income per month, CCI, duration of medicine, combination of medicine, stages of disease, coping styles, and self-management) independently related to the total HRQOL score evaluated by the FACT-EGFRI-18 in patients with skin ADRs undergoing targeted therapy. Stepwise variable selection with the forward selection and backward elimination methods was used to filter the independent variables. The forward selection brought all related independent variables into the regression model individually; in this way, all possible independent variables can be included. However, the independent variables included in the final model may have collinearity with less important variables. Therefore, we applied backward elimination to eliminate the independent variables with less contribution to the model. It should be noted that after backward elimination, the eliminated variables can no longer enter the regression equation, which may lead to the exclusion of important variables. To avoid losing important variables, we set the P value of backward elimination as 0.1. During multiple analyses, Benjamini–Hochberg correction was used to minimize the risk of type I error. All statistical tests used were two-tailed, and P values < 0.05 were considered statistically significant.
Results
Demographical characteristics
In this study, a total of 615 questionnaires were distributed, of which 536 were completed for a response rate of 93.7%. The reasons why the questionnaires were not completed included that patients’ disease progressed or patients needed to receive other treatment which interrupted the completion of the questionnaire, or some patients lost interest in continuing the study. There were no significant differences in the characteristics of patients who completed the questionnaire and those who did not complete the questionnaire.
A total of 536 participants were enrolled in the study, with more men (53.2%) than women (46.8%). The ages of these patients ranged from 41 to 79 years. The mean age of the respondents was 63.6 years (SD = 8.32 years), with 59.9% being over 60 years old. Other key characteristics of the sample were also included, as shown in Table 1.
Skin ADRs of NSCLC patients under targeted therapy
The skin ADR scores were rated from 1 to 5 according to CTCAE 5.0. The mean score for skin ADRs was 1.96 (SD = 0.87). Participants may have had more than one skin ADR, and only the most serious ADRs were scored. The type and degree of each skin ADR are shown in Table 2.
HRQOL of NSCLC patients with skin ADRs undergoing targeted therapy
The mean total HRQOL score was 46 (12.84). The lowest and highest scores were 27 and 70, respectively. Details of HRQOL are shown in Table 3.
Correlation analysis between the severity of skin ADRs and HRQOL
HRQOL was negatively correlated with the severity of skin ADRs (P = 0.000) (Table 4).
Self-management and coping styles
The mean value of the total score was (129.31 ± 30.71) for the patient’s self-management, and the details of self-management and the coping styles are shown in Table 5.
Correlation analysis among self-management, coping styles, and HRQOL
Pearson’s correlations among the main variables indicated that HRQOL was significantly positively correlated with symptom management (r = 0.746, P < 0.01), daily activity management (r = 0.712, P < 0.01), psychological and emotional management (r = 0.667, P < 0.01), information management (r = 0.685, P < 0.01), self-management efficacy (r = 0.643, P < 0.01), and self-management (r = 0.785, P < 0.01). However, there was no significant correlation between communication with medical staff and HRQOL (P > 0.05). Facing was positively correlated with HRQOL (r = 0.807, P < 0.01), but yield and avoidance were significantly negatively correlated with HRQOL (r = − 0.718, − 0.711, P < 0.01).
Relationship between coping style, self-management, and HRQOL (controlling for confounding factor)
Disease stage has a great impact on patients’ HRQOL, and it is necessary to control this confounding factor to analyze the relationship between coping style, self-management, and HRQOL by analysis of covariance. Stratified by disease stage, the results are shown in Table 6. Multivariate analysis showed that facing, yield, avoidance, and self-management were significantly associated with HRQOL after controlling disease stage (F = 4.126, 3.266, 1.756, 4.032; P = 0.000, 0.001, 0.029, 0.000).
Factors associated with patients’ HRQOL
Multiple linear regression analysis was performed to examine HRQOL-related factors in NSCLC patients with skin ADRs undergoing targeted therapy. All variables, including demographic characteristics, self-management, and coping strategies, were entered into the regression model. The model explained 72% of the explained variance (R2) in total HRQOL scores. As shown in Table 7, the significant factors independently associated with HRQOL were as follows: age [(95% CI: 2.093 ~ 5.172); P = 0.000], education level [(95% CI: 1.089 ~ 2.226); P = 0.034], combination of medicine [(95% CI: 1.070 ~ 3.880); P = 0.001], CCI [(95% CI: 1.145 ~ 2.039); P = 0.031], disease of stages [(95% CI: 3.145 ~ 5.039); P = 0.000], facing [(95% CI: 1.681 ~ 6.185); P = 0.000], yield [(95% CI: − 1.234 ~ − 3.336); P = 0.001], symptom management [(95% CI: 3.145 ~ 6.039); P = 0.000], daily activity management [(95% CI: 2.145 ~ 3.439); P = 0.028], psychological and emotional management [(95% CI: − 0.307 ~ − 0.027); P = 0.020], self-management efficacy [(95% CI: 2.219 ~ 3.197); P = 0.024] and the total score of self-management [(95% CI: 1.826 ~ 8.875); P = 0.000]. Individuals with comorbidities, combination of medicine, older age, lower education level, and worse disease stages were more likely to have worse HRQOL. Patients with better self-management and a positive coping style had a significantly higher possibility of high HRQOL. Table 8 shows the independent variable assignment in multiple linear regression analysis.
Discussion
NSCLC patients undergoing targeted therapy usually have poor HRQOL due to skin ADRs [18]. Analyzing the HRQOL status and identifying the factors correlated with HRQOL are critical for designing targeted skin ADR management strategies to improve patients’ HRQOL. In the current cross-sectional study, we explored HRQOL, self-management, and coping styles as well as the relationship among them in NSCLC patients with skin ADRs under targeted therapy. Based on these results, the potential factors associated with HRQOL were further investigated. As shown in our study, age, education level, comorbidities, combination of medicine, stages of disease, facing, yield, symptom management, daily activity management, psychological/emotional management, and self-efficacy of self-management were crucial indicators related to patients’ HRQOL.
This study suggested a positive correlation between age and HRQOL, with elderly patients showing poor HRQOL. One possible explanation is that a higher CCI in elderly patients was confirmed in this study which deteriorated their physical function. Moreover, older people were more prone to age-related organ damage and poor tolerance, which may reduce HRQOL [38]. As confirmed by previous studies, patients with comorbidities and performing combined medicine applications normally had more complicated conditions, and the interactions of diseases and drugs might lead to aggravation of skin ADRs and worse HRQOL [39]. Our previous retrospective study indicated that the level of education affected HRQOL, which was further confirmed in this study [40]. Indeed, poorly educated patients generally lacked the awareness to actively gain information to improve their HRQOL. As a result, they tended to be difficult to accept and understand self-management. Additionally, late-stage diseases are correlated with more severe symptoms and declined HRQOL. This study discussed one aspect of HRQOL (skin-related QoL). For patients with skin ADRs, advanced NSCLC patients may have worse HRQOL than patients of early disease stage due to more serious skin ADRs caused by malnutrition, combined medication, and continuous medication [41, 42].
In this study, the average degree of skin ADRs of patients was 1.96, and most patients had mild skin ADRs. The skin ADRs in the study included rash, hand-foot syndrome, pruritus, xerosis, paronychia, nail loss, and hair loss. The incidence of rash was as high as 82% and the incidence of severe rash was 23.1 (≥ grade 3). These results are similar to those of previous studies [43]. The mean score of 46 (the full score is 72) for the HRQOL of the 536 participants investigated in this study indicated that the HRQOL for NSCLC patients with skin ADRs undergoing targeted therapy still needs to be improved. Due to the limited studies worldwide evaluating the HRQOL of cancer patients undergoing targeted therapies, we were unable to comprehensively compare the HRQOL of patients in similar populations in different countries, while in the validation study of the FACT-EGFRI-18 which recruited the same population as this study, the mean score of HRQOL (51) reported was higher than this study. However, the sample size of the validation study was only 42, which limited the representativeness of the study [31]. In another study of lung cancer patients undergoing chemotherapy, the mean HRQOL score was 45.8 (the full score was 100), which was worse than that reported in this study [44]. One possible reason might be that the patients selected in that study were advanced patients who may have poor physical function and malnutrition, which could affect HRQOL. Another reason might be that the side effects of chemotherapy could further reduce the HRQOL.
A previous study divided cancer patients receiving targeted therapy into two groups according to whether they had skin ADRs, and there was no significant difference in other characteristics between the two groups. The results showed that the HRQOL of patients with skin ADRs was lower than that of patients without skin ADRs [40]. In this study, we found a negative correlation between HRQOL and the severity of skin ADRs. These two studies confirmed that skin ADRs affected the HRQOL of NSCLC patients undergoing targeted therapy and that patients with more severe skin ADRs had lower HRQOL.
This study demonstrated a relatively low self-management score (129.31, SD = 30.71) among patients with targeted cancer therapy compared with another study (169.7, SD = 23.88) that analyzed self-management in lung cancer patients with chemotherapy [45]. The plausible reasons may be that the self-management of patients with chemotherapy has been focused on extensively. In addition, many studies have provided effective measures to improve the self-management of chemotherapy patients, which have been applied and emphasized in clinical and continuous care practice. At the same time, we discovered that patients in this study needed more positive coping strategies. This study showed less facing (18.29) and more yield (13.34) than another study (23.57, 9.74) focused on advanced lung cancer [46]. This finding can be explained by the fact that patients in this study were hoping for targeted therapy for the better effects and milder adverse reactions; however, they were very disappointing and painful after a period of treatment because of serious skin ADRs. Therefore, they were pessimistic and lost confidence in treatment, which led to negative coping styles.
This study also analyzed the relationship between self-management, coping style, and HRQOL. The results showed that HRQOL was positively correlated with all dimensions of self-management, the total score of self-management, and positive coping styles (facing). Meanwhile, HRQOL was negatively correlated with yield and avoidance. Considering that the disease stage may have a great impact on the HRQOL, we conducted a multivariate analysis based on stratified disease stage to further verify the relationship between the three variables. After controlling for confounding factors, we still found that coping style and self-management were significantly associated with the HRQOL.
As an important predictor of HRQOL in clinical practice, self-management ability represents the knowledge, attitude, and skills of patients in maintaining high HRQOL [47]. According to a previous study, patients with good self-management could effectively deal with the adverse effects of lung cancer treatment [48]. Meanwhile, patients with good self-management paid more attention to their health status and adhered better to the advice given by medical staff. In this study, we found that the patient’s management of symptoms, daily activities, self-efficacy, psychology, and emotion could significantly affect HRQOL. Patients who conducted good symptom management were aware of insisting on assessing, monitoring, and recording symptoms of adverse reactions. Once symptoms occur, they can reduce physical and psychological damage through pharmacological and non-pharmacological interventions [49]. For self-management, we should guide patients to strengthen the identification and the dynamic evaluation of skin ADRs. At the same time, health providers should train patients to develop medication management and adhere to good health behavior. In addition, the self-management of emotion, self-image, and stress should also be considered.
Adequate coping strategies are the premise of good HRQOL. Positive coping styles enhanced the ability and confidence of individuals to relieve pressure, while negative coping styles strengthened negative psychology and reduced resilience. Patients with good coping skills, such as facing, were able to communicate and solve problems actively. Adjustment to positive coping styles could reduce the negative effects of treatment and symptom distress [50]. This study showed that when patients yielded more, their HRQOL was worse; in contrast, when patients faced more, their HRQOL was better. A study used mobile health promotion strategies to encourage cancer patients with oral chemotherapy to cope with difficulties positively, and the results showed that the HRQOL was improved [23]. Yield weakened patients’ health responsibility, which might further make patients reluctant to do self-management. During treatment, the yield might reduce communication between the patients and others, which hinders them from receiving high-quality and professional guidance. Moreover, if patients yielded, their consciousness of maintaining good HRQOL was weak, and they might fail to self-examine which would cause recurrence of adverse events [51].
To our knowledge, this was the first study to describe the HRQOL of cancer patients with targeted therapy in China. The results of this study are enlightening: it is imperative to improve patients’ self-management ability and adopt positive coping styles that contribute to maintain good HRQOL. Therefore, in critical practice, medical staff should dynamically evaluate patients’ self-management and coping style during the whole process of targeted therapy. Patients’ self-management awareness should be improved first, and then, targeted self-management promotion strategies should be formulated according to the defects and needs of self-management of patients assessed by medical staff. At the same time, medical staff should conduct health education, inform patients that skin ADRs can be controlled to improve patients' confidence, and encourage patients to cope positively. Furthermore, healthcare providers should develop personalized coping plans and guide patients on how to solve problems and overcome difficulties effectively. Additionally, we should take measures to promote communication between patients and others and encourage patients to express their real thoughts to avoid yield. Moreover, providing social support, family support, and peer support was important.
Strengths and limitations
Skin ADRs caused by targeted therapy had a great impact on HRQOL which is an important indicator to determine the patients' prognosis and therapeutic effect [40]. It is necessary to know the level of HRQOL of cancer patients with skin ADRs undergoing targeted treatment and the possible factors associated with HRQOL. This study evaluated the HRQOL of cancer patients with skin ADRs under targeted treatment, which previous studies did not focus on, and the sample size was large. In addition, this study explored the factors that might affect the HRQOL of patients and identified some changeable factors, which laid the foundation for the development of targeted interventions.
However, there were also several limitations. First, this study only evaluated one aspect of HRQOL (skin-related well-being). Future research can explore other aspects of HRQOL through appropriate methods. Second, the sample was selected from three hospitals in one area without systematic sampling methods, which indicates the possibility of selection bias. Third, the patients we chose only received one kind of targeted drug, which might compromise the reproducibility of the results. Additionally, only two HRQOL-related factors were systematically investigated, and the relationship between others variables and HRQOL and the interrelationship among these variables should be explored in the future.
Conclusion
The HRQOL level among NSCLC patients with skin ADRs undergoing targeted therapy is suboptimal and needs to be improved. Our findings indicated a significant association between age, education level, combination of drugs, comorbidities, stages of disease, coping styles, self-management, and HRQOL in NSCLC patients undergoing targeted therapy.
Data availability
The data generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author who was an organizer of the study.
Code availability
Not applicable.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) CA Cancer J Clin 71(3):209–249
Rongshou Z, Siwei Z, Hongmei Z, Changfa X (2022) J National Cancer Cent 12(3):525–537
Galvez-Nino M, Ruiz R, Pinto JA, Roque K, Mas L (2020) Lung Cancer 198(1):195–200
Thomas A, Chen Y, Yu T, Jakopovic M (2015) Front Oncol 5(33):113–119
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Int J Cancer 136(5):E359–E386
Liu X, Luo X, Jiang C, Zhao H (2019) Clin Genet 95(6):569–574
Wilkes GM (2018) Asia Pac J Oncol Nurs 5(2):137–155
Singal G, Miller PG, Agarwala V, Li G, Kaushik G, Backenroth D, Gossai A, Frampton GM, Torres AZ, Lehnert EM (2019) JAMA J Am Med Assoc 321(14):1391–1402
Staats H, Cassidy C, Kelso J, Mack S, Nemunaitis J (2020) Biomedicine 5(13):27–49
Wu Y, Zhou C, Lu S, Qin S (2019) Lung Cancer 130(20):18–24
Ettinger DS, Wood DE, Akerley W, Bazhenova LA (2016) J Natl Compr Canc Netw Jnccn 14(3):255–264
Wang MC, Wang CL, Chen TL, Chang WC, Lu JJ, Chang PY, Chiou CC (2017) Clin Chem Lab Med 55(12):1979–1986
Kale HP, Mays AP, Nadpara PA, Slattum PW (2019) Urol Oncol 356(37):19–28
Peng Y, Li Q, Zhang J, Shen W, Zhang X, Sun C, Cui H (2018) Biosci Trends 33(32):3817–3825
JLee JL, Jeong Y (2019) Cancer Nurs 42(6):475–483
Barrios DM, Phillips GS, Freites-Martinez A, Hsu M, Lacouture ME (2019) J Eur Acad Dermatol 34(6):27–39
Chan JC, Lee YH, Liu CY, Shih HH, Tang WR (2019) J Nurs Res 27(6):51–59
Yagasaki K, Komatsu H, Soejima K, Naoki K, Hamamoto Y (2018) Asia Pac J Oncol Nurs 5(2):172–177
Sano K, Nakadate K, Hanada K (2020) BMC Cancer 20(1):279–287
Komatsu H, Yagasaki K, Yamaguchi T, Mori A, Tamura K (2020) Eur J Oncol Nurs 23(8):1017–1028
Wagner LI, Lacouture ME (2007) Oncology 21(5):34–36
Wagner LI, Berg SR, Gandhi M, Hlubocky FJ (2013) Support Care Cancer 21(4):1033–1041
Liao Y-C, Liao W-Y, Sun J-L, Ko J-C, Yu C-J (2018) Support Care Cancer 26(2):989–996
Yuan M, Huang LL, Chen JH, Wu J, Xu Q (2019) Signal Transduct Target Ther 55(5):103–111
Tantoy IY, Cataldo JK, Aouizerat BE, Dhruva A (2016) Cancer Nurs 39(6):437–445
Lacouture ME, Anadkat M, Jatoi A, Garawin T, Bohac C, Mitchell E (2018) Clin Colorectal Canc 17(2):85–96
Huang FF, Yang Q, Zhang J, Han XY, Zhang JP, Chirico A (2018) PLoS ONE 13(9):332–339
Chirico A, Lucidi F, Merluzzi T, Alivernini F, Giordano A (2017) Oncotarget 8(22):36800–36811
Chen H, Senan S, Nossent EJ, Boldt RG, Warner A, Palma DA, Louie AV (2017) Int J Radiat Oncol 47(6):327–336
Charan J, Biswas T (2013) Indian J Psychol Med 35(2):121–126
Qing L, Yanmei P, Jingyi Z, Shen W (2020) J China-Japan Friendship Hospital 34(5):263–267
Lingling C (2017) S Yuqian. Chin J Nurs 52(9):1082–1087
Sun V, Reb A, Debay M, Fakih M, Ferrell B (2021) J Cancer Educ 36(4):421–428
Xiaohong S, Qianjin J (2000) Chin J Behav Med Brain Sci 9(1):18–20
Wu XD, Qin HY, Zhang JE, Zheng MC, Xin MZ, Liu L, Wu XJ, Jiang CN, Zhang MF (2015) Eur J Oncol Nurs 21(5):725–732
He Y, Jian H, Yan M, Zhu J, Chen J (2019) BMJ Open 9(5):233–239
Hauke J, Kossowski T (2011) Quaest Geogr 30(2):87–93
Barnes TA, Amir E, Templeton AJ, Gomez-Garcia S, Navarro B, Seruga B, Ocana A (2017) Cancer Treat Rev 56(6):1–7
Yamamoto K, Yano I (2018) Med Oncol 35(2):16–26
Du R, Wang X, Ma L, Larcher LM, Wang T (2021) BMC Cancer 21(1):231–239
Occhipinti M, Brambilla M, Galli G, Manglaviti S, Prelaj A, Ferrara R, Toma AD, Beninato T, Zattarin E, Proto C (2021) J Thorac Oncol 16(4):1257–1265
Chen CB, Wu MY, Yee NC, Lu CW, Wu J, Pei-Han K, Yang CK, Peng MT, Huang CY, Chang WC (2018) Cancer Manag Res 10:1259–1273
Deutsch A, Leboeuf NR, Lacouture ME, Mclellan BN (2020) Proc Am Soc Clin Oncol 40(40):485–500
He Y, Jian H, Yan M, Zhu J, Chen J (2019) BMJ Open 9(5):236–242
Cheng T, Respiration DO (2016) China Health Industry 13(16):532–536
De W, Derijcke S, Galdermans D, Daenen M, Surmont V, Droogh ED, Lefebure A, Saenen E, Vandenbroucke E, Morel AM (2020) Clin Lung Cancer 2(22):146–152
Kafatos G, Dube S, Burdon P, Demonty G, Flinois A, Leclerc M, Lowe K, Feudjo-Tepie M, Segaert S (2020) Clin Colorectal Canc 19(2):100–108
Sun V, Reb A, Debay M, Fakih M, Ferrell B (2021) J Cancer Educ 25(4):788–796
Chen Q, Di S, Ying Y, Yue Z, Xiaoying Z (2019) Qual Life Res Int J Qual Life Asp Treat Care Rehabil 67(5):1245–1253
Liao Y-C, Liao W-Y, Sun J-L, Ko J-C, Yu C-J (2018) Support Care Cancer 12(23):236–243
Huang W, Li J, Qiu F, Wu X, Zhu S (2020) J Clin Pharm Ther 45(12):29–39
Acknowledgements
We thank the First Affiliated Hospital of Zhengzhou University and Henan Tumor Hospital and the First Affiliated Hospital of Henan University of Science and Technology for their assistance and support and the Follow-up Registration System and data provided by the Follow-up Center of the First Affiliated Hospital of Zhengzhou University. We also thank the oncologists and nurses of the three hospitals. We appreciate all participants for their generous participation.
Funding
This study received financial support from the China Postdoctoral Science Foundation in 2018 (2018M630839) and the National Natural Science Foundation of China (no. 81773175)
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. WT and CCY were responsible for the overall design and quality control of the study as well as the communication with the hospital and departments reviewed. DRF, WX, and WT made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; WX, LML, and MLX were involved in drafting the manuscript or revising it critically for important intellectual content; ZHY contributed to the training and management of nurses and students who collected data. DRF, ZHY, WX, LML, CCY, and WT gave final approval of the version to be published. MLX contributed to data analysis and solving statistical problems. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content; DRF, CCY, and WT agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of our university and administrative permissions were obtained from directors of oncology departments. All methods were carried out in accordance with relevant guidelines and regulations. Information about the study was provided to the participants, and we obtained written informed consent from all participants prior to their inclusion in the study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, R., Wang, X., Zhou, H. et al. The health-related quality of life of lung cancer patients with EGFR-TKI-related skin adverse drug reactions and its relationship with coping style and self-management. Support Care Cancer 30, 9889–9899 (2022). https://doi.org/10.1007/s00520-022-07451-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07451-2