Abstract
Purpose
Pain and fatigue are common symptoms in oncology patients. In a sample of oncology outpatients receiving chemotherapy (n = 1342), the study purposes were to identify subgroups of patients with distinct worst pain and morning fatigue profiles and evaluate for differences among the subgroups in demographic and clinical characteristics, as well as the severity of common symptoms and quality of life (QOL) outcomes.
Methods
Oncology outpatients receiving chemotherapy (n = 1342) completed self-report questionnaires to assess pain and morning fatigue, a total of six times over two cycles of chemotherapy. Joint latent profile analysis was used to identify subgroups of patients with distinct pain and morning fatigue profiles. Differences among the classes were evaluated using parametric and non-parametric tests.
Results
Five distinct profiles were identified (no pain and low morning fatigue (27.6%), moderate pain and low morning fatigue (28.2%), moderate pain and morning fatigue (28.0%), moderate pain and increasing and decreasing morning fatigue (6.9%), severe pain and very high morning fatigue (9.3%)). Patients with the three worst profiles had clinically meaningful levels of depression and sleep disturbance and decrements in QOL.
Conclusions
Over 44% of the sample had moderate to high levels of both pain and morning fatigue. Unrelieved pain may contribute to disturbed sleep which results in higher levels of morning fatigue. Clinicians need to assess for pain and fatigue, as well as sleep disturbance during chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain and fatigue are two of the most common symptoms reported by oncology patients [19]. Results of a landmark conference, which aimed to examine the co-occurrence of pain, fatigue, and depression, and associated interventions [59], identified that most studies focused on an evaluation of single symptoms. Since then, several studies have evaluated relationships between pain and fatigue in heterogenous samples of oncology patients and survivors [6, 8, 13, 20, 31, 37, 53]. However, only two of these studies evaluated patients receiving chemotherapy [20, 31].
In a study of elderly patients receiving chemotherapy [20], higher levels of pain were associated with increases in fatigue severity. In another study, which used latent profile analysis (LPA) to identify subgroups of patients with distinct pain and fatigue profiles [31], LPAs were done separately for two different cycles of chemotherapy (i.e., 4 days after (Time 1) and 43 to 60 days after the first treatment (Time 2)). At Time 1, three subgroups were identified (i.e., low pain and low fatigue; low pain and high fatigue; high pain and high fatigue). At Time 2, two subgroups were identified (i.e., low pain and low fatigue; high pain and high fatigue). Across both assessments, patients in the high pain and high fatigue subgroups reported higher levels of depression and poorer function.
Recent evidence from our group [17, 23, 33,34,35, 46, 47, 71,72,73,74,75,76] and others [40, 43] suggests diurnal variations in the occurrence and severity of fatigue. While daily fluctuations in fatigue severity are common in the general population [18], general patterns of lower levels of fatigue in the morning and higher levels of fatigue in the evening occur in patients with HIV infection [40, 43], renal failure [1], multiple sclerosis [11], and cancer [17, 23, 33,34,35, 46, 47, 71,72,73,74,75,76]. While most studies focus on evaluations of changes in average fatigue in patients with chronic conditions, like cancer, given this growing literature on diurnal variations in fatigue severity, an evaluation of various aspects of morning fatigue versus evening fatigue is warranted.
While the use of LPA provides insights into distinct pain and fatigue profiles, additional research is warranted on modifiable demographic and clinical characteristics associated with the worst profiles. In addition, as noted above, the growing body of evidence suggests that morning and evening fatigue are distinct symptoms [16, 33, 43, 44], and person-centered analytic approaches should be used to evaluate for distinct profiles associated with pain and morning fatigue, as well as the relationships between these profiles and other common symptoms and QOL outcomes.
Current study builds on our previous LPAs of pain [65] and morning fatigue [74] as single symptoms. Based on ratings of worst pain [65], four distinct classes were identified (i.e., none, mild, moderate, severe). Compared to none class, severe pain class had fewer years of education, a lower annual household income, and were less likely to be married or employed. For morning fatigue [74], four distinct groups were identified (i.e., very low, low, high, very high). Compared to the two lowest classes, the very high morning fatigue class was younger, not married, lived alone, and had a higher comorbidity burden. Given the clinical recognition that pain and fatigue are common symptoms in oncology patients; that diurnal variations in fatigue severity occur; and that the paucity of longitudinal studies on the co-occurrence of these two symptoms, the study’s purposes were to identify subgroups of patients with distinct worst pain and morning fatigue profiles and evaluate for differences among the subgroups in demographic, clinical, and symptom characteristics and QOL. We hypothesized that patients with higher levels of both symptoms would report a higher symptom burden and poorer QOL.
Methods
Patients and settings
This longitudinal study evaluated the symptom experience of oncology outpatients receiving chemotherapy. Eligible patients (n = 1343) were ≥ 18 years of age; had a diagnosis of breast, gastrointestinal, gynecological, or lung cancer; had received chemotherapy within the preceding four weeks; were scheduled to receive at least two additional cycles of chemotherapy; were able to read and write English; and gave written informed consent. Patients were recruited from two Comprehensive Cancer Centers, one Veteran’s Affairs hospital, and four community-based oncology programs.
Instruments
Demographic and clinical characteristics
Patients completed a demographic questionnaire, Karnofsky Performance Status (KPS) scale [30], Alcohol Use Disorders Identification Test (AUDIT) [63], and Self-Administered Comorbidity Questionnaire (SCQ) [4]. Chemotherapy regimen toxicity was evaluated using the MAX2 index [21]. Medical records were reviewed for disease and treatment characteristics.
Pain and fatigue measures
Worst pain severity was assessed using the Brief Pain Inventory (BPI) [12]. Patients were asked to indicate whether they were generally bothered by pain (yes/no). Those who were bothered by pain rated its worst pain severity in the past 24 h using a 0 (no pain) to 10 (worst pain imaginable) numeric rating scale (NRS).
The 18-item Lee Fatigue Scale (LFS) was designed to assess physical fatigue and energy [39]. Each item was rated on a 0 to 10 NRS. Total fatigue and energy scores were calculated as the mean of the 13 fatigue items and the 5 energy items, respectively. Higher scores indicate greater fatigue severity and higher levels of energy. Using separate LFS questionnaires, patients were asked to rate each item based on how they felt within 30 min of awakening (i.e., morning fatigue, morning energy) and prior to going to bed (i.e., evening fatigue, evening energy). The LFS has established cut-off scores for clinically meaningful levels of fatigue (i.e., ≥ 3.2 for morning fatigue, ≥ 5.6 for evening fatigue) and energy (i.e., ≤ 6.2 for morning energy, ≤ 3.5 for evening energy) [22]. Cronbach’s alphas were 0.96 for morning and 0.93 for evening fatigue and 0.95 for morning and 0.93 for evening energy. Patients’ ratings of morning fatigue were used in this analysis.
Symptom measures
An evaluation of other common symptoms was done using valid and reliable instruments. The symptoms and their respective measures were state and trait anxiety (Spielberger State-Trait Anxiety Inventories (STAI-S and STAI-T) [66]), depression (Center for Epidemiologic Studies-Depression Scale (CES-D) [62]), sleep disturbance (General Sleep Disturbance Scale (GSDS) [38]), and cognitive function (Attentional Function Index (AFI) [10]).
QOL measures
QOL was evaluated using general (i.e., Medical Outcomes Study-Short Form-12 (SF-12)) and disease-specific (i.e., Quality of Life Scale-Patient Version (QOL-PV)) measures. The SF-12 consists of 12 questions about physical and mental health, as well as overall health status. The instrument is scored into two components (i.e., physical component summary (PCS) and mental component summary (MCS) scores). Higher PCS and MCS scores indicate a better QOL [70].
The 41-item QOL-PV assesses four dimensions of QOL (i.e., physical, psychological, social, and spiritual well-being) in cancer patients, as well as a total QOL score. Each item was rated on a 0 to 10 NRS with higher scores indicating a better QOL [58].
Study procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and the Institutional Review Board at each site. Eligible patients were approached by a research staff member in the infusion unit during their first or second cycle of chemotherapy to discuss participation. Written informed consent was obtained from all patients. Patients completed questionnaires in their homes, a total of six times over two cycles of chemotherapy (i.e., prior to chemotherapy administration, approximately 1 week after chemotherapy administration, approximately 2 weeks after chemotherapy administration).
Data analysis
LPA was used to identify subgroups of patients with distinct pain and morning fatigue profiles. Before performing the LPA, patients who reported the occurrence of pain for ≤ 1 of the six assessments were identified and labeled as the “None” class (n = 371, 28.4%). Then, the LPA was performed on the remaining 971 patients. This LPA was done with the combined set of variables over time (i.e., using the worst pain and morning LFS scores obtained during the six assessments in a single LPA). This approach provides a profile description of these two symptoms with two profiles over time. The LPA was done using Mplus version 8.4 [57].
As described previously [65, 74], in order to incorporate expected correlations among the repeated measures of the same variable and cross-correlations of the series of the two variables (i.e., worst pain and morning LFS scores), we included covariance parameters among measures at the same occasion and those that were one or two occasions apart. Covariances of each variable with the other at the same assessments were included in the model, and autoregressive covariances were estimated with a lag of two with the same measures and with a lag of one for each variable’s series with the other variable [29]. Model fit was evaluated to identify the solution that best characterized the observed latent class structure with the Bayesian Information Criterion, Vuong-Lo-Mendell-Rubin likelihood ratio test, entropy, and latent class percentages that were large enough to be reliable [56]. Missing data were accommodated for with the use of the Expectation–Maximization algorithm [55].
Data were analyzed using SPSS version 27 (IBM Corporation, Armonk, NY). Differences among the pain AND morning fatigue classes in demographic, clinical, and symptom characteristics and QOL outcomes were evaluated using parametric and nonparametric tests. Post hoc contrasts were done using a Bonferroni corrected p-value of < 0.005 (0.05/10 possible pairwise comparisons).
Results
LPA results
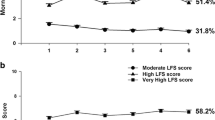
For the LPA that did not include patients who reported a pain score of ≤ 1 for the six assessments, a detailed description of the rationale for the selection of the 4-class solution is provided in Table 1. Figure 1A–E display the trajectories for worst pain and morning fatigue for the five classes of patients. The classes were named based on clinically meaningful cutoff scores for each symptom.
Trajectories of pain in relation to morning fatigue differed among most of the latent classes. For the No Pain and Low AM Fatigue class (i.e., None, 28.2%), while AM fatigue scores were below the clinically meaningful cutoff, they increase slightly in the weeks following the administration of chemotherapy (i.e., assessments 2 and 5). For Moderate Pain and Low AM Fatigue (i.e., Moderate Pain, 28.2%), Moderate Pain and Moderate AM Fatigue (i.e., Both Moderate, 28.0%), and Severe Pain and Very High AM Fatigue (i.e., Severe, 9.3%) classes, both scores remained relatively constant over the two cycles of chemotherapy. For the Moderate Pain and Increasing–Decreasing AM Fatigue class (i.e., Fluctuating, 6.9%), both pain and fatigue scores fluctuated over time.
Demographic and clinical characteristics
Differences in demographic and clinical characteristics are listed in Table 2. In brief, compared to None class, Moderate and Severe classes had fewer years of education, were less likely to be employed, and had received a higher number of cancer treatments. Compared to None and Moderate Pain classes, other three subgroups were more likely to be female and to have lower KPS scores. Compared to other four classes, Severe class reported a lower annual household income, had a higher number of comorbid conditions, had a higher SCQ score, was less likely to exercise on a regular basis, and was more likely to self-report diagnosis of depression.
Symptom characteristics
Differences in symptom characteristics are listed in Table 3. In brief, compared to the other four classes, Severe class had higher levels of depressive symptoms, trait anxiety, state anxiety, evening fatigue, and sleep disturbance and lower levels of morning energy, evening energy, and attentional function. Compared to None and Moderate Pain classes, both Moderate and Fluctuating classes had higher levels of depressive symptoms trait anxiety, state anxiety, evening fatigue, and sleep disturbance and lower levels of attentional function.
QOL outcomes
Differences in QOL outcomes are listed in Table 4. In brief, for the SF-12, compared to None and Moderate Pain classes, the other three classes had lower scores for the physical functioning, role physical, social functioning, role emotional, and mental health scales. For the QOL-PV, compared to None and Moderate Pain classes, the other three classes had lower scores for the physical, psychological, and social well-being and total QOL scales.
Discussion
This study is the first to use LPA to identify five distinct pain and morning fatigue profiles in oncology patients. Consistent with our a priori hypothesis, compared to the 27.6% of patients without pain and low morning fatigue, patients in the three highest classes (i.e., 44.2%) had a significantly higher symptom burden and poorer QOL outcomes (Table 5). While our previous LPAs of pain [65] and morning fatigue [74] as single symptoms identified four distinct profiles, the joint analysis of the two symptoms identified five distinct profiles. The Fluctuating profile identified in our current study was not readily apparent in either of our previous analyses. In terms of unique characteristics, compared to None class, the Fluctuating class was significantly younger and less likely to have gastrointestinal cancer and had received the most toxic chemotherapy regimens. While none of these characteristics are modifiable, these patients may warrant more aggressive symptom management to reduce their high levels of pain and fluctuating levels of morning fatigue.
Compared to a meta-analysis that reported a prevalence rate of 55% [68], 72.4% of our patients reported moderate to severe pain. Between 27.3 and 61.2% of our patients reported both non-cancer and cancer pain and pain interference scores in the moderate range. These findings suggest that unrelieved pain remains a significant clinical problem.
In the studies that determined fatigue prevalence rates of 80 to 100% in oncology patients, average fatigue severity was evaluated [2, 3, 49]. Therefore, our 44.2% prevalence rate for moderate to very high levels of morning fatigue, in our sample, warrants confirmation in future studies. One can hypothesize that these high levels of morning fatigue may be related to sleep disturbances caused by unrelieved pain. This hypothesis is supported by the fact that patients in our three highest profiles reported very high total GDSD scores.
One of this study’s goals was to identify modifiable and non-modifiable characteristics associated with worst pain and morning fatigue profiles. The remainder of the “Discussion” highlights some of the risk factors associated with membership in the higher pain and morning fatigue classes (Table 5).
Demographic characteristics
While no demographic characteristics differentiated None class from Moderate Pain class, patients in the other three classes were more likely to be female. This finding is consistent with previous studies that found that women with cancer report higher rates and severity of both pain [6, 28] and fatigue [28, 49]. While our gender differences may be related to the higher percentage of female patients, several chronic pain problems are more common in women (e.g., osteoarthritis [60]). In addition, while no differences were found among our profiles (i.e., approximately 20% of our sample reported child care responsibilities), women may experience higher levels of morning fatigue associated with child care and elder care responsibilities. This hypothesis warrants confirmation in future studies.
The remaining demographic characteristics (i.e., fewer years of education, less likely to be married or partnered, less likely to be employed, lower annual income) differentiated None from both Moderate and Severe classes. For both pain and fatigue, previous studies found that these symptoms restrict patients’ abilities to work and remain employed which can contribute to significant disability and lower QOL (75). Moreover, even after the completion of chemotherapy, financial distress may persist because patients can only return to work on a part-time basis or need to remain on disability [8]. In terms of marital status, previous research found that the lack of social support may contribute to higher rates of fatigue [52], heightened pain experiences [27], and lower overall well-being (76).
Clinical characteristics
The higher number of comorbidities and higher comorbidity burden were associated with a worse symptom profile. Of note, compared to None class, other four classes reported higher occurrence rates for depression, back pain, and/or osteoarthritis. Given that each of these conditions is independently associated with pain and fatigue, it is not surprising that the patients in our highest pain and morning fatigue classes reported these common comorbid conditions.
For example, oncology patients are two to three times more likely to be diagnosed with depression compared to general population [36]. As noted in the DSM-IV criteria for a major depressive episode [67], fatigue or the loss of energy are two of the required criteria. In addition, associations between unrelieved pain and depression are well documented [9]. Of note, patients in our three highest classes reported CES-D scores near or above clinically meaningful cutoff score.
An equally plausible explanation for the high levels of both pain and morning fatigue is the higher percentages of patients who reported back pain and osteoarthritis. In our three highest classes, the occurrence rates for back pain ranged from 26.9 to 45.2%. These rates are within the range of prevalence rates of 4% [24] to 69% [15] reported in the US population. In addition, prevalence rates for osteoarthritis in our three highest classes ranged from 10.8 to 16.9% which is lower than the 23.7% reported by individuals the USA [5]. However, both of these painful conditions are associated with sleep disturbances [64, 69] that may lead to higher levels of morning fatigue. While causes of non-cancer pain are not routinely assessed, our findings suggest that these common comorbidities need to be evaluated and managed by oncology clinicians or primary care providers.
Symptom characteristics
Consistent with our a priori hypothesis, compared to None class, the three worst classes reported higher scores for depression, state and trait anxiety, evening fatigue, sleep disturbance, and cognitive dysfunction. In addition, for these three classes, all of the symptom scores exceeded the clinically meaningful cutoffs. Of note, co-occurrence of sleep disturbance, depression, pain, and fatigue is one of the most common symptom clusters in oncology patients, and these symptoms appear to exacerbate one another [8, 48, 51, 61]. While none of these symptom cluster studies accounted for diurnal variability in fatigue severity, in its guideline on cancer-related fatigue [7], National Comprehensive Cancer Network noted that the co-occurrence of pain, depression, anxiety, and sleep disturbance contributes to increases in fatigue severity. An equally plausible hypothesis to explain the occurrence of these symptoms is that unrelieved pain can lead to increases in depressive symptoms, anxiety, sleep disturbance, and fatigue and decreases in attentional function [9, 25, 32]. Of note, the pain scores in the three worst classes were in the severe range and were associated with moderate levels of interference with function.
Diurnal variations in both fatigue and energy in the three worst classes warrant consideration. While the terms fatigue and energy are often used interchangeably, patients’ perceptions of their energy levels are not routinely assessed. As noted by Lerdal [41, 42], energy is defined as an “individual’s potential to perform physical and mental activity.” Of note, in a Rasch analysis of the LFS, fatigue and energy were found to be distinct symptoms [45]. Across our five classes, increases in evening fatigue paralleled increases in morning fatigue. However, for morning energy, all five classes had scores that were below the clinically meaningful cutoff for this symptom. In terms of decrements in evening energy, while all five classes had relatively low scores, only the two worst classes had scores below the clinically meaningful cutoff. Given that no studies have examined the relationships between fatigue and energy in oncology patients, these analyses are currently in progress.
Similar to morning energy, all of the sleep disturbance scores for the five classes were at or above the clinically meaningful cutoff. It is reasonable to suggest that a patient who reports a poor night’s sleep would report decrements in morning energy. Of note, the sleep disturbance scores for the three worst classes are comparable to scores reported by shift workers [38] and parents of newborn infants [26]. Examination of the relationships between sleep disturbance and decrements in morning energy warrants additional research.
In terms of cognitive dysfunction, the three worst classes had AFI scores in the low and moderate ranges. These findings are congruent with a systematic review that suggested that while physical fatigue and cognitive fatigue are distinct symptoms, reciprocal relationships exist between them [14]. In fact, in our previous joint LPA of evening fatigue and cognitive fatigue [54], 80% of the patients had moderate to high levels of both evening fatigue and cognitive fatigue (i.e., cognitive dysfunction).
QOL outcomes
Consistent with our a priori hypothesis, except for the spiritual well-being scale of the QOL-PV, patients in the three worst pain and morning fatigue classes had significant decrements in all of the other domains of QOL that were assessed using both the general and disease-specific measures. For the PCS scores, all of the classes had scores that were lower than the normative score of 50 for the general US population [50]. Of particular interest in terms of this joint LPA is the extremely low vitality scores reported by the three worst classes. For the MCS, the three worst classes had scores below the normative score of 50. In terms of the cancer-specific QOL, while the None and Moderate Pain classes had relatively high scores for all of its domains, as well as overall QOL, the other three classes reported marked decrements in all of its scores.
Limitations
While our sample was large, it was relatively homogeneous in terms of gender, race/ethnicity, and income. Future studies need to include more diverse samples to be able to evaluate the impact of other social determinants of health on co-occurrence of pain and morning fatigue. Future studies need to assess patients from prior to through to the completion of chemotherapy and evaluate the effects of pharmacologic interventions (e.g., analgesics, sleep medications) on changes in pain and morning fatigue severity during chemotherapy. The use of analytic techniques like parallel process growth modeling may allow for a determination of which symptom is “driving” the severity of the other symptom. Future studies need to evaluate the pharmacologic and nonpharmacologic interventions that patients are using to manage pain and other symptoms.
Conclusions
This study identified five groups of oncology patients with distinct pain and morning fatigue profiles who exhibited distinct risk factors, high levels of co-occurring symptoms, and decrements in QOL. Based on our findings, while oncology clinicians do assess for pain and fatigue, these routine assessments should expand to include morning fatigue and sleep disturbance. The demographic and clinical characteristics associated with the worst profiles can be used to identify high-risk patients. While effective interventions for fatigue are limited, given the high prevalence and intensity of pain reported by our patients, clinicians need to focus on the implementation of a pain management plan and an evaluation of its efficacy for both pain and morning fatigue.
Data availability
Available with reasonable request.
Code availability
Not applicable.
References
Abdel-Kader K, Jhamb M, Mandich LA, Yabes J, Keene RM, Beach S, Buysse DJ, Unruh ML (2014) Ecological momentary assessment of fatigue, sleepiness, and exhaustion in ESKD. BMC Nephrol 15:29
Al Maqbali M (2021) Cancer-related fatigue: an overview. Br J Nurs 30:S36–S43
Al Maqbali M, Al Sinani M, Al Naamani Z, Al Badi K, Tanash MI (2021) Prevalence of fatigue in patients with cancer: a systematic review and meta-analysis. J Pain Symptom Manag 61(167–189):e114
Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG (2001) AUDIT: The Alcohol Use Disorders Identification Test: guidelines for use in primary care. World Health Organization, Geneva, Switzerland
Barbour KE, Helmick CG, Boring M, Zhang X, Lu H (2014) Holt JB (2016) Prevalence of doctor-diagnosed arthritis at state and county levels - United States. MMWR Morb Mortal Wkly Rep 65:489–494
Beck SL, Dudley WN, Barsevick A (2005) Pain, sleep disturbance, and fatigue in patients with cancer: using a mediation model to test a symptom cluster. Oncol Nurs Forum 32:542
Berger AM, Mooney K, Banerjee A, Breitbart WS, Carpenter CM, Chang Y, Cleeland C, Davis E, Dest V, DuBenske LL, Esclante CP, Fernandes Robles C, Garcia S, Jankowski C, Jatoi A, Kinczewski LE, Loggers ET, Mandrell B, McInnes S, Meyer F, Murphy BA, Palesh O, Patel H, Riba MB, Roshal A, Rugo HS, Salvador C, Wagner-Johnston N, Walter MB, Webb JA (2020) NCCN Guidelines Version 1 2020 - cancer-related fatigue. In: NCCN Guidelines Version 1 2020 - Cancer-Related Fatigue. National Comprehensive Cancer Network
Bjerkeset E, Rohrl K, Schou-Bredal I (2020) Symptom cluster of pain, fatigue, and psychological distress in breast cancer survivors: prevalence and characteristics. Breast Cancer Res Treat 180:63–71
Broemer L, Hinz A, Koch U, Mehnert-Theuerkauf A (2021) Prevalence and severity of pain in cancer patients in Germany. Front Pain Res (Lausanne) 2:703165
Cimprich B, So H, Ronis DL, Trask C (2005) Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology 14:70–78
Claros-Salinas D, Bratzke D, Greitemann G, Nickisch N, Ochs L, Schroter H (2010) Fatigue-related diurnal variations of cognitive performance in multiple sclerosis and stroke patients. J Neurol Sci 295:75–81
Daut RL, Cleeland CS, Flanery RC (1983) Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain 17:197–210
Davis MP, Rybicki LA, Samala RV, Patel C, Parala-Metz A, Lagman R (2021) Pain or fatigue: which correlates more with suffering in hospitalized cancer patients? Support Care Cancer 29:4535–4542
de Raaf PJ, de Klerk C, van der Rijt CC (2013) Elucidating the behavior of physical fatigue and mental fatigue in cancer patients: a review of the literature. Psychooncology 22:1919–1929
Deyo RA, Mirza SK, Martin BI (2006) Back pain prevalence and visit rates: estimates from US national surveys. Spine 31:2724–2727
Dhruva A, Aouizerat BE, Cooper B, Paul SM, Dodd M, West C, Wara W, Lee K, Dunn LB, Langford DJ, Merriman JD, Baggott C, Cataldo J, Ritchie C, Kober K, Leutwyler H, Miaskowski C (2013) Differences in morning and evening fatigue in oncology patients and their family caregivers. Eur J Oncol Nurs 17:841–848
Dhruva A, Aouizerat BE, Cooper B, Paul SM, Dodd M, West C, Wara W, Lee K, Dunn LB, Langford DJ, Merriman JD, Baggott C, Cataldo J, Ritchie C, Kober KM, Leutwyler H, Miaskowski C (2015) Cytokine gene associations with self-report ratings of morning and evening fatigue in oncology patients and their family caregivers. Biol Res Nurs 17:175–184
Dimsdale JE, Ancoli-Israel S, Elsmore TF, Gruen W (2003) Taking fatigue seriously: I Variations in fatigue sampled repeatedly in healthy controls. J Med Eng Technol 27:218–222
Dodd MJ, Miaskowski C, Paul SM (2001) Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum 28:465–470
Erturk M, Yildirim Y, Kilic SP, Ozer S, Aykar FS (2015) Pain and fatigue in elderly cancer patients: Turkey example Holist. Nurs Pract 29:167–173
Extermann M, Bonetti M, Sledge GW, O’Dwyer PJ, Bonomi P, Benson AB 3rd (2004) MAX2–a convenient index to estimate the average per patient risk for chemotherapy toxicity; validation in ECOG trials. Eur J Cancer 40:1193–1198
Fletcher BS, Paul SM, Dodd MJ, Schumacher K, West C, Cooper B, Lee K, Aouizerat B, Swift P, Wara W, Miaskowski CA (2008) Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol 26:599–605
Flowers E, Miaskowski C, Conley Y, Hammer MJ, Levine J, Mastick J, Paul S, Wright F, Kober K (2018) Differential expression of genes and differentially perturbed pathways associated with very high evening fatigue in oncology patients receiving chemotherapy. Support Care Cancer 26:739–750
Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, Castel LD, Kalsbeek WD, Carey TS (2009) The rising prevalence of chronic low back pain. Arch Intern Med 169:251–258
Friedman DR, Patil V, Li C, Rassmussen KM, Burningham Z, Hamilton-Hill S, Kelley MJ, Halwani AS (2022) Integration of patient-reported outcome measures in the electronic health record: The Veterans Affairs Experience JCO Clin. Cancer Informatics 6:e2100086
Gay CL, Lee KA, Lee SY (2004) Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs 5:311–318
Holtzman S, Newth S, Delongis A (2004) The role of social support in coping with daily pain among patients with rheumatoid arthritis. J Health Psychol 9:677–695
Jhamb M, Abdel-Kader K, Yabes J, Wang Y, Weisbord SD, Unruh M, Steel JL (2019) Comparison of fatigue, pain, and depression in patients with advanced kidney disease and cancer-symptom burden and clusters. J Pain Symptom Manage 57:566–575
Jung T, Wickrama KAS (2008) An introduction to latent class growth analysis and growth mixture modeling Soc Personal Psychol. Compass 2:302–317
Karnofsky D, Abelmann WH, Craver LV, Burchenal JH (1948) The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer 1:634–656
Kim HJ, Malone PS, Barsevick AM (2014) Subgroups of cancer patients with unique pain and fatigue experiences during chemotherapy. J Pain Symptom Manage 48:558–568
Ko HJ, Seo SJ, Youn CH, Kim HM, Chung SE (2013) The association between pain and depression, anxiety, and cognitive function among advanced cancer patients in the hospice ward. Korean J Fam Med 34:347–356
Kober KM, Cooper BA, Paul SM, Dunn LB, Levine JD, Wright F, Hammer MJ, Mastick J, Venook A, Aouizerat BE, Miaskowski C (2016) Subgroups of chemotherapy patients with distinct morning and evening fatigue trajectories. Support Care Cancer 24:1473–1485
Kober KM, Dunn L, Mastick J, Cooper B, Langford D, Melisko M, Venook A, Chen LM, Wright F, Hammer M, Schmidt BL, Levine J, Miaskowski C, Aouizerat BE (2016) Gene expression profiling of evening fatigue in women undergoing chemotherapy for breast cancer. Biol Res Nurs 18:370–385
Kober KM, Roy R, Dhruva A, Conley YP, Chan RJ, Cooper B, Olshen A, Miaskowski C (2021) Prediction of evening fatigue severity in outpatients receiving chemotherapy: less may be more Fatigue. Biomed Health Behav 9:14–32
Krebber AM, Buffart LM, Kleijn G, Riepma IC, de Bree R, Leemans CR, Becker A, Brug J, van Straten A, Cuijpers P, Verdonck-de Leeuw IM (2014) Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology 23:121–130
Kwekkeboom K, Zhang Y, Campbell T, Coe CL, Costanzo E, Serlin RC, Ward S (2018) Randomized controlled trial of a brief cognitive-behavioral strategies intervention for the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. Psychooncology 27:2761–2769
Lee KA (1992) Self-reported sleep disturbances in employed women Sleep 15:493–498
Lee KA, Hicks G, Nino-Murcia G (1991) Validity and reliability of a scale to assess fatigue. Psychiatry Res 36:291–298
Lee KA, Portillo CJ, Miramontes H (1999) The fatigue experience for women with human immunodeficiency virus. J Obstet Gynecol Neonatal Nurs 28:193–200
Lerdal A (1998) A concept analysis of energy Its meaning in the lives of three individuals with chronic illness. Scand J Caring Sci 12:3–10
Lerdal A (2002) A theoretical extension of the concept of energy through an empirical study. Scand J Caring Sci 16:197–206
Lerdal A, Gay CL, Aouizerat BE, Portillo CJ, Lee KA (2011) Patterns of morning and evening fatigue among adults with HIV/AIDS. J Clin Nurs 20:2204–2216
Lerdal A, Kottorp A, Gay C, Aouizerat BE, Lee KA, Miaskowski C (2016) A Rasch analysis of assessments of morning and evening fatigue in oncology patients using the Lee Fatigue Scale. J Pain Symptom Manage 51:1002–1012
Lerdal A, Kottorp A, Gay CL, Lee KA (2013) Lee fatigue and energy scales: exploring aspects of validity in a sample of women with HIV using an application of a Rasch model. Psychiatry Res 205:241–246
Lin Y, Bailey DE, Docherty SL, Porter LS, Cooper B, Paul S, Kober K, Hammer MJ, Wright F, Conley Y, Levine J, Miaskowski C (2021) Distinct morning and evening fatigue profiles in gastrointestinal cancer during chemotherapy. BMJ Support Palliat Care
Lin Y, Bailey DE, Xiao C, Hammer M, Paul SM, Cooper BA, Conley YP, Levine JD, Kober KM, Miaskowski C (2022) Distinct co-occurring morning and evening fatigue profiles in patients with gastrointestinal cancers receiving chemotherapy. Cancer Nurs
Loh KP, Zittel J, Kadambi S, Pandya C, Xu H, Flannery M, Magnuson A, Bautista J, McHugh C, Mustian K, Dale W, Duberstein P, Mohile SG (2018) Elucidating the associations between sleep disturbance and depression, fatigue, and pain in older adults with cancer. J Geriatr Oncol 9:464–468
Ma Y, He B, Jiang M, Yang Y, Wang C, Huang C, Han L (2020) Prevalence and risk factors of cancer-related fatigue: a systematic review and meta-analysis. Int J Nurs Stud 111:103707
Maglinte GA, Hays RD, Kaplan RM (2012) US general population norms for telephone administration of the SF-36v2. J Clin Epidemiol 65:497–502
Miaskowski C, Cooper BA, Paul SM, Dodd M, Lee K, Aouizerat BE, West C, Cho M, Bank A (2006) Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum 33:E79-89
Miaskowski C, Paul SM, Snowberg K, Abbott M, Borno HT, Chang SM, Chen LM, Cohen B, Cooper BA, Hammer MJ, Kenfield SA, Kober KM, Laffan A, Levine JD, Pozzar R, Rhoads K, Tsai KK, Van Blarigan EL, Van Loon K (2021) Loneliness and symptom burden in oncology patients during the COVID-19 pandemic. Cancer 127:3246–3253
Miladinia M, Baraz S, Ramezani M, Malehi AS (2018) The relationship between pain, fatigue, sleep disorders and quality of life in adult patients with acute leukaemia: during the first year after diagnosis. Eur J Cancer Care (Engl) 27(1)
Morse L, Kober KM, Viele C, Cooper BA, Paul SM, Conley YP, Hammer M, Levine JD, Miaskowski C (2021) Subgroups of patients undergoing chemotherapy with distinct cognitive fatigue and evening physical fatigue profiles. Support Care Cancer 29:7985–7998
Muthen B, Shedden K (1999) Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics 55:463–469
Muthén L, Muthén B (2009) Mplus statistical analysis with latent variables User’s guide 7
Muthen LK, Muthen BO (1998-2020) Mplus User's Guide (8th ed.). Muthen & Muthen, Los Angeles, CA
Padilla GV, Ferrell B, Grant MM, Rhiner M (1990) Defining the content domain of quality of life for cancer patients with pain. Cancer Nurs 13:108–115
Patrick DL, Ferketich SL, Frame PS, Harris JJ, Hendricks CB, Levin B, Link MP, Lustig C, McLaughlin J, Reid LD, Turrisi AT 3rd, Unutzer J, Vernon SW, National Institutes of Health State-of-the-Science P (2004) National Institutes of Health State-of-the-Science Conference Statement: symptom management in cancer: pain, depression, and fatigue, July 15–17. J Natl Cancer Inst Monogr 2002:9–16
Pereira D, Peleteiro B, Araujo J, Branco J, Santos RA, Ramos E (2011) The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthr Cartil 19:1270–1285
Pud D, Ben Ami S, Cooper BA, Aouizerat BE, Cohen D, Radiano R, Naveh P, Nikkhou-Abeles R, Hagbi V, Kachta O, Yaffe A, Miaskowski C (2008) The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. J Pain Symptom Manage 35:162–170
Radloff LS (1977) The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401
Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN (2003) The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 49:156–163
Schepman P, Thakkar S, Robinson R, Malhotra D, Emir B, Beck C (2021) Moderate to severe osteoarthritis pain and its impact on patients in the United States: a national survey. J Pain Res 14:2313–2326
Shin J, Harris C, Oppegaard K, Kober KM, Paul SM, Cooper BA, Hammer M, Conley Y, Levine JD, Miaskowski C (2022) Worst pain severity profiles of oncology patients are associated with significant stress and multiple co-occurring symptoms. J Pain 23:74–88
Spielberger CG, Gorsuch RL, Suchene R, Vagg PR, Jacobs GA (1983) Manual for the State-Anxiety (Form Y): self evaluation questionnaire. Consulting Psychologists Press, Palo Alto, CA
Tolentino JC, Schmidt SL (2018) DSM-5 Criteria and depression severity: implications for clinical practice Front Psychiatry 9:450
van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ (2016) Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage 51(1070–1090):e1079
Van Looveren E, Bilterys T, Munneke W, Cagnie B, Ickmans K, Mairesse O, Malfliet A, De Baets L, Nijs J, Goubert D, Danneels L, Moens M, Meeus M (2021) The association between sleep and chronic spinal pain: a systematic review from the last decade J Clin Med 10(17):3836
Ware J Jr, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233
Wright F, Cooper BA, Conley YP, Hammer MJ, Chen LM, Paul SM, Levine JD, Miaskowski C, Kober KM (2017) Distinct evening fatigue profiles in oncology outpatients receiving chemotherapy Fatigue. Biomed Health Behav 5:131–144
Wright F, D’Eramo Melkus G, Hammer M, Schmidt BL, Knobf MT, Paul SM, Cartwright F, Mastick J, Cooper BA, Chen LM, Melisko M, Levine JD, Kober K, Aouizerat BE, Miaskowski C (2015) Predictors and trajectories of morning fatigue are distinct from evening fatigue. J Pain Symptom Manage 50:176–189
Wright F, D’Eramo Melkus G, Hammer M, Schmidt BL, Knobf MT, Paul SM, Cartwright F, Mastick J, Cooper BA, Chen LM, Melisko M, Levine JD, Kober K, Aouizerat BE, Miaskowski C (2015) Trajectories of evening fatigue in oncology outpatients receiving chemotherapy. J Pain Symptom Manage 50:163–175
Wright F, Dunn LB, Paul SM, Conley YP, Levine JD, Hammer MJ, Cooper BA, Miaskowski C, Kober KM (2019) Morning fatigue severity profiles in oncology outpatients receiving chemotherapy. Cancer Nurs 42:355–364
Wright F, Hammer M, Paul SM, Aouizerat BE, Kober KM, Conley YP, Cooper BA, Dunn LB, Levine JD, Melkus GD, Miaskowski C (2017) Inflammatory pathway genes associated with inter-individual variability in the trajectories of morning and evening fatigue in patients receiving chemotherapy. Cytokine 91:187–210
Wright F, Kober KM, Cooper BA, Paul SM, Conley YP, Hammer M, Levine JD, Miaskowski C (2020) Higher levels of stress and different coping strategies are associated with greater morning and evening fatigue severity in oncology patients receiving chemotherapy. Support Care Cancer 28:4697–4706
Funding
This work was supported by a grant from the National Cancer Institute (NCI, CA134900). Dr. Miaskowski is an American Cancer Society Clinical Research Professor.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Dr. Miaskowski, Dr. Cooper, and Dr. Paul. The first draft of the manuscript was written by Ms. Bouvron and Dr. Miaskowski. All of the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study procedures were approved by the Committee on Human Research at the University of California, San Francisco and the Institutional Review Board at each of the study sites. This study was performed in accordance with the ethical standards as laid down in the 1964 Helsinki Declaration.
Consent to participate
Informed consent was obtained from all individual participants included in this study.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouvron, B., Mackin, L., Kober, K.M. et al. Impact of worst pain severity and morning fatigue profiles on oncology outpatients’ symptom burden and quality of life. Support Care Cancer 30, 9929–9944 (2022). https://doi.org/10.1007/s00520-022-07431-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07431-6