Abstract
Purpose
The purpose was to model cognitive fatigue and evening physical fatigue together to determine subgroups of patients with distinct cognitive fatigue AND evening physical fatigue profiles. Once these profiles were identified, differences among the subgroups in demographic and clinical characteristics, co-occurring symptoms, and quality of life outcomes were evaluated.
Methods
Oncology patients (n = 1332) completed self-report measures of cognitive fatigue and evening physical fatigue, six times over two cycles of chemotherapy. Latent profile analysis, which combined the two symptom scores, was done to identify subgroups of patients with distinct cognitive fatigue AND evening physical fatigue profiles.
Results
Three distinct profiles (i.e., Low [20.5%], Moderate [39.6%], and High [39.6%]) were identified. Compared to the Low class, patients in the High class were younger, female, and more likely to live alone and had a higher comorbidity burden and a lower functional status. In addition, these patients had a higher symptom burden and a poorer quality of life.
Conclusion
Based on clinically meaningful cutoff scores, 80% of the patients in this study had moderate to high levels of both cognitive fatigue and evening physical fatigue. In addition, these patients experienced high levels of other common symptoms (e.g., anxiety, depression, sleep disturbance, and pain). These co-occurring symptoms and other modifiable characteristics associated with membership in the Moderate and High classes may be potential targets for individualized symptom management interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatigue occurs in approximately 80% of oncology patients [6]. This symptom decreases patients’ adherence with treatments [28] and impairs their quality of life (QOL) [21]. Cancer-related fatigue is defined as “a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” [6]. This definition emphasizes the multidimensional nature of fatigue.
As noted in one review [15], consensus does not exist on the number of dimensions of fatigue that warrant evaluation. For example, the Fatigue Questionnaire evaluates two dimensions (i.e., physical and mental). In contrast, both the Multidimensional Fatigue Inventory (MFI; i.e., general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity) [55] and the Multidimensional Fatigue Symptom Inventory (i.e., global, somatic, affective, cognitive, and behavioral) [57] evaluate five dimensions. The authors of this review commented that one expert panel endorsed the existence of at least physical and cognitive or mental fatigue in oncology patients [48].
Given the large amount of inter-individual variability in fatigue occurrence [17] and severity [64,65,66,67,68], one needs to consider how to characterize the multiple dimensions of fatigue in oncology patients. As noted by deRaaf and colleagues [15], three possible conceptualizations of fatigue exist (i.e., unidimensional, multidimensional, and multiple symptoms). Based on the results of their systematic review that focused on an evaluation of the “behavior of physical fatigue and mental fatigue in cancer patients” [15], they concluded that physical fatigue and mental fatigue may be separate phenomena.
Two of the studies cited in the review [15] evaluated for changes over time in physical [12] and cognitive [13] fatigue in breast cancer patients receiving adjuvant chemotherapy. Fatigue was assessed at the first, third, and fifth cycles, and at 4 and 12 weeks after the last cycle of chemotherapy using the MF [55]. While physical fatigue increased over the course of chemotherapy and declined following the completion of treatment, cognitive fatigue remained relatively stable over time. While changes over time were evaluated, in the same sample of patients, each dimension of the fatigue experience was analyzed separately.
In a cross-sectional study of long-term survivors of colorectal cancer [58], latent class analysis was used to identify subgroups of survivors with distinct fatigue profiles using the five dimensions of the MFI. Three distinct profiles were identified (i.e., no fatigue and distress [56%], low fatigue and moderate distress [22%], and high fatigue and moderate distress [22%]). Compared to the no fatigue and distress class, the high fatigue and moderate distress class were more likely to be female and overweight, had co-occurring diabetes, and had received radiation therapy. In addition, survivors in the two higher classes were more likely to have comorbid heart disease and higher levels of anxiety and sleep disturbance. Across the three classes, cognitive fatigue scores were low. While this study provides insights into fatigue subtypes, it was cross-sectional and focused on only cancer survivors.
Recent work from our group evaluated for inter-individual differences in and risk factors for physical fatigue, using the Lee Fatigue Scale (LFS) [37], in a sample of patients with heterogeneous types of cancer undergoing chemotherapy [64,65,66,67,68]. Using latent profile analysis (LPA), four subgroups of patients with distinct morning (i.e., Very Low, Low, High, and Very High) [67] and four subgroups with distinct evening (i.e., Low, Moderate, High, and Very High) [64] fatigue profiles were identified. Given that the severity and trajectories of fatigue differed between the morning and evening fatigue, latent classes and different demographic and clinical characteristics were associated with membership in the higher morning and evening fatigue classes, we concluded that diurnal variations in physical fatigue occurred over two cycles of chemotherapy and that morning and evening fatigue were distinct but related symptoms. In terms of cognitive fatigue [4], in the same sample, we used LPA to evaluate for distinct cognitive fatigue profiles using the Attentional Function Index (AFI) [10]. Three subgroups of patients with distinct cognitive fatigue profiles were identified (i.e., Low, Moderate, and High). Patients with moderate and high levels of cognitive fatigue were younger, more likely to be female, and were less likely to be employed.
Given the paucity of research on the relationships among multiple dimensions of the fatigue experience and on the identification of subgroups of patients with distinct cognitive fatigue AND evening physical fatigue profiles, in this study, we modeled cognitive fatigue and evening physical fatigue together to determine subgroups of patients with distinct cognitive fatigue AND evening physical fatigue profiles. Once these profiles were identified, we evaluated for differences among the subgroups in demographic and clinical characteristics, co-occurring symptoms, and QOL outcomes.
Methods
Patients and settings
This longitudinal study is described in detail elsewhere [40, 41]. Eligible patients were ≥ 18 years of age; had a diagnosis of breast, gastrointestinal, gynecological, or lung cancer; had received chemotherapy within the preceding four weeks; were scheduled to receive at least two additional cycles of chemotherapy; were able to read, write, and understand English; and gave written informed consent. Patients were recruited from two Comprehensive Cancer Centers, one Veteran’s Affairs hospital, and four community-based oncology programs. A total of 2234 patients were approached and 1343 consented to participate (60.1% response rate). The major reason for refusal was being overwhelmed with their cancer treatment.
Instruments
Demographic and clinical characteristics
Patients completed a demographic questionnaire, Karnofsky Performance Status (KPS) scale [31], Alcohol Use Disorders Identification Test (AUDIT) [5], and Self-Administered Comorbidity Questionnaire (SCQ). The SCQ evaluates the occurrence, treatments for, and impact of 13 common medical conditions [51]. A MAX-2 score was calculated for each patient’s chemotherapy regimen. This score is a valid and reliable indicator of the toxicity of various chemotherapy regimens [19].
Cognitive fatigue and evening physical fatigue measures
Attentional Function Index assesses an individual’s perceived effectiveness in performing daily activities that are supported by attention and working memory [10]. Total scores can be grouped into categories of attentional function (i.e., < 5.0 low function, 5.0 to 7.5 moderate function, and > 7.5 high function) [9].
Lee Fatigue Scale was designed to assess physical fatigue and energy [37]. Total fatigue and energy scores were calculated as the mean of the 13 fatigue items and the 5 energy items, respectively. Higher scores indicate greater fatigue severity and higher levels of energy. Using separate questionnaires, patients rated each item based on how they felt within 30 min of awakening (i.e., morning fatigue and morning energy) and prior to going to bed (i.e., evening fatigue and evening energy). The LFS has established cut-off scores for clinically meaningful levels of fatigue (i.e., ≥ 3.2 for morning fatigue and ≥ 5.6 for evening fatigue) [20] and energy (i.e., ≤ 6.2 for morning energy and ≤ 3.5 for evening energy) [20].
Symptom measures
To assess the severity of common symptoms associated with cancer and its treatment, patients completed Center for Epidemiological Studies-Depression scale (CES-D) [49], Spielberger State-Trait Anxiety Inventories (STAI-S, STAI-T) [56], General Sleep Disturbance Scale (GSDS) [36], and Brief Pain Inventory [11].
QOL measures
QOL was evaluated using generic (i.e., Medical Outcomes Study-Short Form-12 [SF-12] [60]) and disease-specific (i.e., QOL-Patient Version [QOL-PV] [45]) measures. The individual items on the SF-12 were evaluated and the instrument was scored into two components (i.e., physical component summary [PCS] and mental component summary [MCS] scores). QOL-PV measures four dimensions of QOL (i.e., physical, psychological, social, and spiritual well-being), as well as a total QOL score. For both measures, higher scores indicate a better QOL.
Study procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco, and by the Institutional Review Board at each of the study sites. Written informed consent was obtained from all patients. Patients completed questionnaires in their homes, a total of six times over two cycles of chemotherapy. Medical records were reviewed for disease and treatment information.
Data analysis
LPA was used to identify subgroups of patients with distinct cognitive fatigue AND evening physical fatigue profiles. Using Mplus version 8.4 [43], this LPA was done with the combined set of variables over time (i.e., using the AFI AND evening LFS scores obtained during the six assessments in a single LPA). This approach provides a profile description of these two symptoms with parallel profiles over time.
In order to incorporate expected correlations among the repeated measures of the same variable and cross-correlations of the series of the two variables (i.e., evening LFS and AFI scores), we included covariance parameters among measures at the same occasion and those that were one or two occasions apart. Covariances of each variable with the other at the same assessments were included in the model and autoregressive covariances were estimated with a lag of two with the same measures and with a lag of one for each variable’s series with the other variable. We limited the covariance structure to a lag of two to accommodate the expected reduction in the correlations that would be introduced by two chemotherapy cycles within each set of three measurement occasions and to reduce model complexity [30].
Data were analyzed using SPSS version 27 (IBM Corporation, Armonk, NY). Differences among the cognitive fatigue AND evening physical fatigue classes in demographic, clinical, and symptom characteristics, and QOL were evaluated using parametric and nonparametric tests. Bonferroni corrected p-value of < 0.017 was considered statistically significant for the pairwise contrasts.
Results
Latent class solution
Three-class solution was selected because the Bayesian Information Criterion (BIC) for that solution was lower than the BIC for the 2-class solution. In addition, the Vuong-Lo-Mendell-Rubin likelihood ratio test was significant for the three-class solution, indicating that three classes fit the data better than two classes (Table 1). This approach allowed for the identification of three groups of patients with distinct evening physical fatigue AND cognitive fatigue profiles.
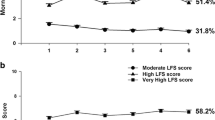
Cognitive fatigue AND evening physical fatigue classes were labeled as Low cognitive fatigue and Low evening physical fatigue (i.e., Low), Moderate cognitive fatigue and Moderate evening physical fatigue (i.e., Moderate), and High cognitive fatigue and high evening physical fatigue (i.e., High) based on clinically meaningful cut-off scores for cognitive fatigue and for evening physical fatigue. As shown in Fig. 1, the trajectories for the two symptoms that were modeled together were relatively similar across the three latent classes. For the Low (20.5%), Moderate (39.6%), and High (39.9%) classes, cognitive fatigue and evening physical fatigue scores increased slightly at the second and fifth assessments (i.e., assessments following the administration of chemotherapy).
Changes in cognitive fatigue (CF, left y-axis; lower scores indicate higher levels of cognitive fatigue) and evening physical fatigue (PF, right y-axis; higher scores indicate higher levels of physical fatigue) scores over two cycles of chemotherapy for subgroups of oncology patients with High Cognitive Fatigue and High Evening Physical Fatigue (panel A), Moderate Cognitive Fatigue and Moderate Evening Physical Fatigue (panel B) and Low Cognitive Fatigue and Low Evening Physical Fatigue (panel C)
Demographic and clinical characteristics
Significant differences were found among the latent classes for many of the demographic and clinical characteristics (Table 2). Compared to the Low class, the other two classes were significantly younger, more likely to be female, more likely to be White, less likely to be Black, less likely to exercise on a regular basis, more likely to be diagnosed with breast cancer, less likely to be diagnosed with gastrointestinal cancer, more likely to self-report a diagnosis of depression, and more likely to have received previous cancer treatments.
Compared to the other two classes, the High class was less likely to be married/partnered, less likely to be employed, and more likely to self-report a diagnosis of back pain and had a higher number of comorbidities. Compared to the Low class, the High class was more likely to live alone, more likely to have child care responsibilities, and more likely to report a past or current history of smoking; had received a higher number of previous cancer treatments; and had a higher MAX-2 score.
Compared to the other two classes, the Moderate class had more years of education and a higher annual household income. Among the three classes, KPS scores followed the expected pattern (Low > Moderate > High).
Symptom severity
For trait anxiety, state anxiety, depressive symptoms, morning fatigue, evening fatigue, and sleep disturbance, the scores followed the expected pattern (i.e., Low < Moderate < High). In terms of evening energy and cognitive fatigue, the scores followed the expected pattern (i.e., Low > Moderate > High; Table 3). Compared to the Low and Moderate classes, the High class had lower evening energy scores. In terms of types of pain, the proportion of patients who reported no pain was in the expected direction (i.e., Low > Moderate > High). Compared to the other two classes, a higher percentage of patients in the High class reported the occurrence of both cancer and non-cancer pain and higher worst pain intensity scores. Pain interference scores followed a similar pattern to other symptoms (i.e., Low < Moderate < High).
Differences in QOL
For the physical functioning, role functioning, bodily pain, vitality, social functioning, mental health, and for the PCS and MCS, SF-12 scores followed the expected pattern (i.e., Low > Moderate > High). For general health and role emotional subscales, compared to the other two classes, patients in the High class reported lower scores (Table 4). For the physical well-being, psychological well-being, social well-being subscales, and total QOL-PV scale, scores followed the expected pattern (i.e., Low > Moderate > High). For spiritual well-being, compared to the Low class, the High class reported lower scores.
Discussion
This study is the first to use LPA to identify subgroups of oncology patients with distinct cognitive fatigue AND evening physical fatigue profiles over two cycles of chemotherapy. While our previous LPAs found three distinct profiles for cognitive fatigue [4] and four distinct profiles for evening physical fatigue [64], when these two dimensions of fatigue were modeled together, three distinct profiles were identified. Consistent with previous reports [6, 7, 24], based on the clinically meaningful cutoff scores for these two symptoms, 80% of our patients were categorized in either the Moderate or High classes.
Comparison of the trajectories of the cognitive fatigue and evening physical fatigue scores among the latent classes suggests that when the two symptoms are modeled together, both scores fluctuate in a similar pattern regardless of class assignment. Consistent with previous reports of the individual symptoms from our group [4, 64] and others [12,13,14], both types of fatigue increase following the administration of chemotherapy and then decline prior to the next infusion.
In their systematic review [15], de Raaf and colleagues suggested that if cognitive fatigue and physical fatigue were different symptoms within the multiple symptom concept of fatigue (versus fatigue as a multidimensional concept that is experienced in different ways), these two symptoms would differ in intensity in different types of cancer; differ in intensity across courses of treatment; have different characteristics associated with each symptom; and have different responses to interventions. Unfortunately, like the findings from the systematic review, no definitive conclusions can be made regarding this question. While we found three groups of patients with distinct cognitive fatigue and evening physical fatigue profiles, the severity of the pairs of symptoms and their changes over time remained relatively similar across the three groups. However, as shown in Table 5, some of the characteristics associated with the Moderate and High groups were different. One criterion that was not listed in this review was whether the mechanisms that underlie the single symptoms are similar or different. Information on the common and distinct aspects of cognitive and physical fatigue, including common and distinct underlying mechanisms, are essential in order to answer the multiple symptom versus multidimensional symptom question.
One of our study purposes was to identify demographic, clinical, and symptom characteristics that were associated with a higher symptom burden (Table 5). Compared to the Low class, some of characteristics associated with membership in the Moderate and High classes were common while others were distinct. For example, compared to the Low class, the other two classes shared the following characteristics: younger age, more likely to be female, more likely to be White, and less likely to be Black. While findings regarding age differences in cognitive and physical fatigue are inconsistent [3, 25], for both symptoms, potential explanations for the association with younger age include that older patients may be given lower doses of chemotherapy [61]; age-related changes in inflammatory responses [7]; and/or a “response shift” occurs in the symptom perceptions of older patients [22]. Another potential explanation is the emerging evidence that suggests that younger age is associated higher rates of social isolation [44]. In addition, recent evidence suggests that compensatory neural changes may occur in older adults that offset cognitive fatigue [16].
Findings regarding gender differences in cognitive fatigue and physical fatigue are inconclusive [3]. These inconsistencies may be related to the gender distribution of patients in previous studies. Future studies need to evaluate for gender differences in these two symptoms, in cancers with an equal gender distribution (e.g., lung). Given the paucity of research on the association between ethnicity and either symptom, direct comparisons with our findings cannot be made.
A larger number of unique characteristics were associated with membership in the High class (Table 5). Consistent with previous studies of physical fatigue [64, 66], patients who were not married or partnered, were living alone, had child care responsibilities, and did not exercise on a regular basis were in the High class. It is readily apparent why patients with the additional responsibilities of child care would be classified in the High class. A plausible explanation for the associations between marital status and living arrangements and membership in the High class is the recent findings that perceptions of lack of social support and loneliness are associated with higher levels of cancer-related fatigue [47, 53]. Finally, the findings regarding exercise are consistent with meta-analyses that demonstrated the beneficial effects of exercise in reducing cancer-related fatigue [8, 32]. Future studies should incorporate measures of loneliness, social isolation, and social support to evaluate these associations.
In terms of clinical characteristics, compared to the Low class, the other two classes had a higher comorbidity burden and a lower functional status, and were more likely to have breast cancer, were less likely to have gastrointestinal cancer, were more likely to have received prior cancer treatments, and were more likely to self-report depression. Previous studies of oncology patients found that both symptoms are associated with a higher comorbidity burden [64] and poorer functional status [64]. As noted in one review [69], the prevalence of cancer-related fatigue increases as the number of comorbid conditions increases. A potential explanation for this finding is that the fatigue associated with various chronic conditions may share similar underlying mechanisms [39]. In addition, the occurrence of multiple chronic conditions may potentiate symptom severity in a synergistic manner [27].
In terms of differences in the occurrence of cognitive and physical fatigue among patients with different types of cancer, comparisons are difficult because of differences in the measures used and the timing of the measures. In one study that controlled for age and sex in their analysis [54], the highest prevalence rates for fatigue were found in patients with gall bladder cancer. Findings from a study, which used a multidimensional fatigue inventory to assess physical, cognitive, and emotional fatigue in patients with fifteen different types of cancer [52], suggest that all three types of fatigue were lowest in patients with breast cancer.
In terms of the unique clinical characteristics associated with membership in the High class, these patients reported a higher number of comorbidities and a higher number of previous cancer treatments, were receiving a more toxic chemotherapy regimen, and were more likely to have back pain. All of these characteristics may potentiate cognitive fatigue and physical fatigue in a synergistic manner [27].
This suggestion of synergistic interactions among co-occurring symptoms is supported by the differences in symptom severity scores among the three classes. For the majority of the symptoms, the severity scores increased in a stepwise fashion. Equally important, all of the symptom severity scores for the High class were above the clinically meaningful cutpoints. While some evidence suggests that pain, fatigue, sleep disturbance, cognitive dysfunction, and depression occur as a psychoneurological symptom cluster and have shared biological mechanisms [33], additional research is warranted to determine the common and unique mechanisms that contribute to a higher symptom burden.
Less is known about the relationship between anxiety and fatigue. Our findings are consistent with previous reports that found that higher rates of trait anxiety were associated with higher levels of fatigue in patients with breast cancer undergoing chemotherapy [62] and in cancer survivors [29, 47, 59]. One potential explanation is that higher levels of anxiety cause dysregulation of the hypothalamic–pituitary–adrenal axis, which may increase cytokine production and associated increases in both symptoms [7].
For the majority of the QOL outcomes, the scores decreased in a stepwise fashion. As noted in two reviews [1, 50], this association is well established. It stands to reason that patients who are not able to engage fully in their daily activities due to both cognitive and physical fatigue would experience decrements in QOL. These decreases were found in both the general and disease-specific measures of QOL. In fact, the PCS and MCS scores for the High class were below the normative scores for the US population.
Several limitations warrant consideration. While a total of six assessments were done over two cycles of chemotherapy, patients were not assessed prior to the initiation of chemotherapy. Second, our assessment of cognitive function was limited to a self-report measure that primarily evaluates attention and executive function. Third, the findings related to ethnicity need to be interpreted with caution given the relatively small sample sizes for the different ethnic groups. However, this large representative sample of oncology patients undergoing chemotherapy, the evaluation of both cognitive fatigue and evening physical fatigue across two cycles of chemotherapy, and the use of LPA to identify risk factors associated with cognitive fatigue AND evening physical fatigue are major strengths of this study.
The phenotypic characteristics associated with membership in the High class can be used to identify high-risk patients. The identification of nonmodifiable (e.g., age and gender) and modifiable (e.g., childcare responsibilities, depressive symptoms, sleep disturbance, and lack of regular exercise) risk factors allows clinicians to tailor interventions for specific patients. For example, a growing body of evidence suggests that exercise can decrease cognitive and physical fatigue [35, 42]. In addition, behavioral interventions to improve sleep may reduce both cognitive and physical fatigue. Equally important, programs that offer support to patients with childcare responsibilities and improve the perception of social connection may benefit patients with both types of fatigue.
Given that pre-treatment fatigue was found to predict post-treatment fatigue [38, 46], future studies should include measures of pre-treatment fatigue. In addition, given the diurnal variations in fatigue severity, future studies need to determine if the same profiles and risk factors are identified when morning physical fatigue and cognitive fatigue are modeled together in the same LPA. To determine whether cognitive fatigue and evening physical fatigue are multiple symptom or a multidimensional concept, future research should investigate whether the mechanisms that underlie the single symptoms are similar or different. In addition, studies are needed that use objective measures of cognitive and physical fatigue to determine if latent class membership differs depending on the assessment method used and the domains of cognitive and physical fatigue that are evaluated. Finally, given the compelling evidence that childhood adversity [23, 34, 63], coping styles [18, 26], and perceptions of social support [2] influence the severity of fatigue, future studies should include measures of psychosocial and behavioral risk factors for both cognitive and physical fatigue.
Data availability
Data will be provided to the publisher after they obtain a material transfer agreement from the University of California, San Francisco.
References
Abrahams HJG, Gielissen MFM, Verhagen C, Knoop H (2018) The relationship of fatigue in breast cancer survivors with quality of life and factors to address in psychological interventions: a systematic review. Clin Psychol Rev 63:1–11
Adams RN, Mosher CE, Winger JG, Abonour R, Kroenke K (2018) Cancer-related loneliness mediates the relationships between social constraints and symptoms among cancer patients. J Behav Med 41:243–252
Al Maqbali M, Al Sinani M, Al Naamani Z, Al Badi K, Tanash MI (2021) Prevalence of fatigue in patients with cancer: a systematic review and meta-analysis. J Pain Symptom Manage 61:167–189
Atallah M, Cooper B, Munoz RF, Paul SM, Anguera J, Levine JD, Hammer M, Wright F, Chen LM, Melisko M, Conley YP, Miaskowski C, Dunn LB (2020) Psychological symptoms and stress are associated with decrements in attentional function in cancer patients undergoing chemotherapy. Cancer Nurs 43:402–410
Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG (2001) AUDIT: The alcohol use disorders identification test: guidelines for use in primary care. World Health Organization, Geneva
Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, Cleeland C, Dotan E, Eisenberger MA, Escalante CP, Jacobsen PB, Jankowski C, LeBlanc T, Ligibel JA, Loggers ET, Mandrell B, Murphy BA, Palesh O, Pirl WF, Plaxe SC, Riba MB, Rugo HS, Salvador C, Wagner LI, Wagner-Johnston ND, Zachariah FJ, Bergman MA, Smith C, National comprehensive cancer n (2015) Cancer-Related Fatigue, Version 2.2015. J Natl Compr Cancer Netw 13:1012–1039
Bower JE (2019) The role of neuro-immune interactions in cancer-related fatigue: biobehavioral risk factors and mechanisms. Cancer 125:353–364
Chen YJ, Li XX, Ma HK, Zhang X, Wang BW, Guo TT, Xiao Y, Bing ZT, Ge L, Yang KH, Han XM (2020) Exercise training for improving patient-reported outcomes in patients with advanced-stage cancer: a systematic review and meta-analysis. J Pain Symptom Manage 59:734-749.e710
Cimprich B, So H, Ronis DL, Trask C (2005) Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology 14:70–78
Cimprich B, Visovatti M, Ronis DL (2011) The Attentional Function Index-a self-report cognitive measure. Psychooncology 20:194–202
Daut RL, Cleeland CS, Flanery RC (1983) Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain 17:197–210
de Jong N, Candel MJ, Schouten HC, Abu-Saad HH, Courtens AM (2004) Prevalence and course of fatigue in breast cancer patients receiving adjuvant chemotherapy. Ann Oncol 15:896–905
de Jong N, Candel MJ, Schouten HC, Abu-Saad HH, Courtens AM (2005) Course of mental fatigue and motivation in breast cancer patients receiving adjuvant chemotherapy. Ann Oncol 16:372–382
de Jong N, Kester AD, Schouten HC, Abu-Saad HH, Courtens AM (2006) Course of fatigue between two cycles of adjuvant chemotherapy in breast cancer patients. Cancer Nurs 29:467–477
de Raaf PJ, de Klerk C, van der Rijt CC (2013) Elucidating the behavior of physical fatigue and mental fatigue in cancer patients: a review of the literature. Psychooncology 22:1919–1929
Dew IT, Buchler N, Dobbins IG, Cabeza R (2012) Where is ELSA? The early to late shift in aging. Cereb Cortex 22:2542–2553
Dimsdale JE, Ancoli-Israel S, Ayalon L, Elsmore TF, Gruen W (2007) Taking fatigue seriously, II: variability in fatigue levels in cancer patients. Psychosomatics 48:247–252
Dupont A, Bower JE, Stanton AL, Ganz PA (2014) Cancer-related intrusive thoughts predict behavioral symptoms following breast cancer treatment. Health Psychol 33:155–163
Extermann M, Bonetti M, Sledge GW, O’Dwyer PJ, Bonomi P, Benson AB 3rd (2004) MAX2—a convenient index to estimate the average per patient risk for chemotherapy toxicity; validation in ECOG trials. Eur J Cancer 40:1193–1198
Fletcher BS, Paul SM, Dodd MJ, Schumacher K, West C, Cooper B, Lee K, Aouizerat B, Swift P, Wara W, Miaskowski CA (2008) Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol 26:599–605
Gupta D, Lis CG, Grutsch JF (2007) The relationship between cancer-related fatigue and patient satisfaction with quality of life in cancer. J Pain Symptom Manage 34:40–47
Hamidou Z, Dabakuyo TS, Bonnetain F (2011) Impact of response shift on longitudinal quality-of-life assessment in cancer clinical trials. Expert Rev Pharmacoecon Outcomes Res 11:549–559
Han Tatiana J, Felger Jennifer C, Lee Anna, Mister Donna, Miller Andrew H, Torres Mylin A (2016) Association of childhood trauma with fatigue, depression, stress, and inflammation in breast cancer patients undergoing radiotherapy. Psychooncology 25:187–193
Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D (2008) Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the U.S. Support Care Cancer 16:791–801
Huehnchen P, van Kampen A, Boehmerle W, Endres M (2020) Cognitive impairment after cytotoxic chemotherapy. Neurooncol Pract 7:11–21
Hughes A, Suleman S, Rimes KA, Marsden J, Chalder T (2020) Cancer-related fatigue and functional impairment—towards an understanding of cognitive and behavioural factors. J Psychosom Res 134:110127
Institute of Medicine (2012) Living well with chronic illness: a call for public health sction. In: Living Well with Chronic Illness: A Call for Public Health Action. The National Academies Press, Washington, DC
Jacobs JM, Ream ME, Pensak N, Nisotel LE, Fishbein JN, MacDonald JJ, Buzaglo J, Lennes IT, Safren SA, Pirl WF, Temel JS, Greer JA (2019) Patient experiences with oral chemotherapy: adherence, symptoms, and quality of life. J Natl Compr Cancer Netw 17:221–228
Jung JY, Lee JM, Kim MS, Shim YM, Zo JI, Yun YH (2018) Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psychooncology 27:465–470
Jung T, Wickrama KAS (2008) An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass 2:302–317
Karnofsky D (1977) Performance scale. Plenum Press, New York
Kessels E, Husson O, van der Feltz-Cornelis CM (2018) The effect of exercise on cancer-related fatigue in cancer survivors: a systematic review and meta-analysis. Neuropsychiatr Dis Treat 14:479–494
Kim HJ, Barsevick AM, Fang CY, Miaskowski C (2012) Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer Nurs 35:E1–E20
Kuhlman KR, Chiang JJ, Horn S, Bower JE (2017) Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood adversity to disease. Neurosci Biobehav Rev 80:166–184
Lange M, Joly F, Vardy J, Ahles T, Dubois M, Tron L, Winocur G, De Ruiter MB, Castel H (2019) Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol 30:1925–1940
Lee KA (1992) Self-reported sleep disturbances in employed women. Sleep 15:493–498
Lee KA, Hicks G, Nino-Murcia G (1991) Validity and reliability of a scale to assess fatigue. Psychiatry Res 36:291–298
Liu L, Fiorentino L, Natarajan L, Parker BA, Mills PJ, Sadler GR, Dimsdale JE, Rissling M, He F, Ancoli-Israel S (2009) Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psychooncology 18:187–194
Matura LA, Malone S, Jaime-Lara R, Riegel B (2018) A systematic review of biological mechanisms of fatigue in chronic illness. Biol Res Nurs 20:410–421
Miaskowski C, Cooper BA, Aouizerat B, Melisko M, Chen LM, Dunn L, Hu X, Kober KM, Mastick J, Levine JD, Hammer M, Wright F, Harris J, Armes J, Furlong E, Fox P, Ream E, Maguire R, Kearney N (2017) The symptom phenotype of oncology outpatients remains relatively stable from prior to through 1 week following chemotherapy. Eur J Cancer Care 26(3):e12437. https://doi.org/10.1111/ecc.12437
Miaskowski C, Cooper BA, Melisko M, Chen LM, Mastick J, West C, Paul SM, Dunn LB, Schmidt BL, Hammer M, Cartwright F, Wright F, Langford DJ, Lee K, Aouizerat BE (2014) Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer 120:2371–2378
Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, Mohr D, Palesh OG, Peppone LJ, Piper BF, Scarpato J, Smith T, Sprod LK, Miller SM (2017) Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol 3:961–968
Muthen LK, Muthen BO (1998–2020) Mplus user’s guide, 8th edn. Muthen & Muthen, Los Angeles
Nguyen TT, Lee EE, Daly RE, Wu TC, Tang Y, Tu X, Van Patten R, Jeste DV, Palmer BW (2020) Predictors of loneliness by age decade: study of psychological and environmental factors in 2,843 community-dwelling Americans aged 20–69 years. J Clin Psychiatry 81
Padilla GV, Ferrell B, Grant MM, Rhiner M (1990) Defining the content domain of quality of life for cancer patients with pain. Cancer Nurs 13:108–115
Pertl MM, Hevey D, Collier S, Lambe K, O’Dwyer AM (2014) Predictors of fatigue in cancer patients before and after chemotherapy. J Health Psychol 19:699–710
Puigpinos-Riera R, Serral G, Sala M, Bargallo X, Quintana MJ, Espinosa M, Manzanera R, Domenech M, Macia F, Grau J, Vidal E (2020) Cancer-related fatigue and its determinants in a cohort of women with breast cancer: the DAMA Cohort. Support Care Cancer 28:5213–5221
Radbruch L, Strasser F, Elsner F, Gonçalves JF, Løge J, Kaasa S, Nauck F, Stone P (2008) Fatigue in palliative care patients — an EAPC approach. Palliat Med 22:13–32
Radloff LS (1977) The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401
Rau KM, Shun SC, Chiou TJ, Lu CH, Ko WH, Lee MY, Huang WT, Yeh KH, Chang CS, Hsieh RK (2020) A nationwide survey of fatigue in cancer patients in Taiwan: an unmet need. Jpn J Clin Oncol 50:693–700
Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN (2003) The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 49:156–163
Schmidt ME, Hermann S, Arndt V, Steindorf K (2020) Prevalence and severity of long‐term physical, emotional, and cognitive fatigue across 15 different cancer entities. Cancer Med 9:8053–8061
Schmidt ME, Wiskemann J, Schneeweiss A, Potthoff K, Ulrich CM, Steindorf K (2018) Determinants of physical, affective, and cognitive fatigue during breast cancer therapy and 12 months follow-up. Int J Cancer 142:1148–1157
Singer S, Kuhnt S, Zwerenz R, Eckert K, Hofmeister D, Dietz A, Giesinger J, Hauss J, Papsdorf K, Briest S, Brown A (2011) Age- and sex-standardised prevalence rates of fatigue in a large hospital-based sample of cancer patients. Br J Cancer 105:445–451
Smets EM, Garssen B, Bonke B, De Haes JC (1995) The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 39:315–325
Spielberger CG, Gorsuch RL, Suchene R, Vagg PR, Jacobs GA (1983) Manual for the State-Anxiety (Form Y): Self Evaluation Questionnaire. Consulting Psychologists Press, Palo Alto
Stein KD, Martin SC, Hann DM, Jacobsen PB (1998) A multidimensional measure of fatigue for use with cancer patients. Cancer Pract 6:143–152
Thong MSY, Mols F, van de Poll-Franse LV, Sprangers MAG, van der Rijt CCD, Barsevick AM, Knoop H, Husson O (2018) Identifying the subtypes of cancer-related fatigue: results from the population-based PROFILES registry. J Cancer Surviv 12:38–46
Tsaras K, Papathanasiou IV, Mitsi D, Veneti A, Kelesi M, Zyga S, Fradelos EC (2018) Assessment of depression and anxiety in breast cancer patients: prevalence and associated factors. Asian Pac J Cancer Prev 19:1661–1669
Ware J, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233
Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, Falandry C, Artz A, Brain E, Colloca G, Flamaing J, Karnakis T, Kenis C, Audisio RA, Mohile S, Repetto L, Van Leeuwen B, Milisen K, Hurria A (2014) International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 32:2595–2603
Williams AM, Khan CP, Heckler CE, Barton DL, Ontko M, Geer J, Kleckner AS, Dakhil S, Mitchell J, Mustian KM, Peppone LJ, Kipnis V, Kamen CS, O’Mara AM, Janelsins MC (2021) Fatigue, anxiety, and quality of life in breast cancer patients compared to non-cancer controls: a nationwide longitudinal analysis. Breast Cancer Res Treat 187(1):275–285. https://doi.org/10.1007/s10549-020-06067-6
Witek Janusek L, Tell D, Albuquerque K, Mathews HL (2013) Childhood adversity increases vulnerability for behavioral symptoms and immune dysregulation in women with breast cancer. Brain Behav Immun 30 Suppl:S149-162
Wright F, Cooper BA, Conley YP, Hammer MJ, Chen LM, Paul SM, Levine JD, Miaskowski C, Kober KM (2017) Distinct evening fatigue profiles in oncology outpatients receiving chemotherapy Fatigue. Biomed Health Behav 5:131–144
Wright F, D’Eramo Melkus G, Hammer M, Schmidt BL, Knobf MT, Paul SM, Cartwright F, Mastick J, Cooper BA, Chen LM, Melisko M, Levine JD, Kober K, Aouizerat BE, Miaskowski C (2015) Predictors and trajectories of morning fatigue are distinct from evening fatigue. J Pain Symptom Manage 50:176–189
Wright F, D’Eramo Melkus G, Hammer M, Schmidt BL, Knobf MT, Paul SM, Cartwright F, Mastick J, Cooper BA, Chen LM, Melisko M, Levine JD, Kober K, Aouizerat BE, Miaskowski C (2015) Trajectories of evening fatigue in oncology outpatients receiving chemotherapy. J Pain Symptom Manage 50:163–175
Wright F, Dunn LB, Paul SM, Conley YP, Levine JD, Hammer MJ, Cooper BA, Miaskowski C, Kober KM (2019) Morning fatigue severity profiles in oncology outpatients receiving chemotherapy. Cancer Nurs 42:355–364
Wright F, Hammer M, Paul SM, Aouizerat BE, Kober KM, Conley YP, Cooper BA, Dunn LB, Levine JD, Dem G, Miaskowski C (2017) Inflammatory pathway genes associated with inter-individual variability in the trajectories of morning and evening fatigue in patients receiving chemotherapy. Cytokine 91:187–210
Wright F, Hammer MJ, D’Eramo Melkus G (2014) Associations between multiple chronic conditions and cancer-related fatigue: an integrative review. Oncol Nurs Forum 41:399–410
Funding
This study was funded by a grant from the National Cancer Institute (CA134900). Dr. Miaskowski is an American Cancer Society Clinical Research Professor.
Author information
Authors and Affiliations
Contributions
All of the authors participated in the revisions to this paper and the interpretation of the results and approved the final paper.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Committee on Human Research at the University of California.
Consent to participate
This study was exempted from written informed consent.
Consent for publication
All of the authors approved the final paper for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morse, L., Kober, K.M., Viele, C. et al. Subgroups of patients undergoing chemotherapy with distinct cognitive fatigue and evening physical fatigue profiles. Support Care Cancer 29, 7985–7998 (2021). https://doi.org/10.1007/s00520-021-06410-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06410-7