Abstract
Introduction
Despite the recommendations for cancer survivors to engage in physical activity (PA), little is known about the effects of both PA and sedentary time (ST) on key health symptoms. This study prospectively examined the lifestyle behaviors of moderate-to-vigorous PA (MVPA) and ST as predictors of depressive symptoms, pain, and fatigue in breast cancer survivors using longitudinal data from early post-treatment to 4-year survivorship.
Methods
Breast cancer survivors (n = 199, mean(SD) age = 55.0(11.0) years) self-reported depressive symptoms, pain, and fatigue, and wore an accelerometer to measure MVPA and ST every 3 months during the first year (times 1 to 5) and 2 and 4 years (times 6 and 8) post-cancer treatment. Linear mixed models were adjusted for personal (e.g., age, BMI, education) and cancer (e.g., stage, time since treatment) variables.
Results
MVPA and ST were independent predictors of depressive symptoms, but not fatigue, and only ST was associated with pain over 4 years post-treatment. Higher levels of MVPA were associated with lower scores of depressive symptoms (\(\beta\) (95%CI): − 0.062 (− 0.092, − 0.031) p < .001), whereas higher levels of ST were associated with higher scores of depressive symptoms (\(\beta\) (95%CI): 0.023 (0.017, 0.028) p < .001). Higher levels of ST were associated with increased pain level over time (\(\beta\) (95%CI): 0.017 (0.007, 0.027) p = .001).
Conclusions
Rehabilitation interventions should aim to both increase MVPA and reduce ST to promote health and well-being among breast cancer survivors, in particular during the early post-treatment period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer remains the highest incident cancer diagnosis in Canadian females [1]. Valuable progress in breast cancer survival has been achieved (5-year survival rate of 88% in Canada) as a result of advances in prevention, screening, early detection, and combined modality treatment [1]. As survival rates increase, a rise in symptom burden secondary to breast cancer survivorship is common [2]. Side effects can develop as early as immediately after cancer diagnosis, usually becoming more severe during cancer treatment and can continue for months and even years [3]. Fatigue, pain, and depressive symptoms are among the most prevalent long-term breast cancer effects that undermine physical and psychosocial functioning [2].
Breast cancer survivors report severe chronic pain [4], debilitating fatigue [5], and depressive symptoms [6, 7] that independently and together interfere with physical and psychological functioning, quality of life, distress, and many comorbidities long after treatments have ended [5,6,7,8,9,10]. As such, it is important to identify strategies to reduce the experience of these symptoms.
Physical activity (PA) is a safe, feasible, and effective strategy that could be targeted to prevent and reduce late-effect symptoms of pain [11], fatigue [12, 13], and depression [14] among breast cancer survivors. The American College of Sports Medicine published the first recommendations in 2010 suggesting that cancer patients and survivors should (i) avoid physical inactivity and (ii) aim for 150 min of moderate-to-vigorous PA (MVPA) per week [15]. Recently, updated evidence-based guidelines suggest that 90 min per week of MVPA may be valuable to health outcomes [16]. Specifically, MVPA has been associated with a significant reduction in cancer-related fatigue in breast cancer survivors [13]. Higher levels of MVPA [17] and even light intensity PA [18] are associated with fewer depressive symptoms and breast cancer survivors who were meeting MVPA guidelines were less likely to report above-average pain [19].
In addition to PA, sedentary time (ST) (i.e., resting activity < 1.5 METS in a sitting or reclined position) [20] is an independent risk factor of cancer mortality and morbidity [21]. Reducing ST time in breast cancer survivors may be a more feasible behavioral target to reduce symptoms of pain, fatigue, and depression, when compared to attempts at increasing MVPA [22]. ST has been associated with poorer quality of life [23], decreased wellbeing, and increased fatigue duration [24] in breast cancer patients and survivors. Little is known about the relationships and interactions among MVPA and ST on pain, fatigue, and depression symptoms over time among breast cancer survivors.
The purpose of this study was to prospectively examine the association between lifestyle behaviors of MVPA and ST and depressive symptoms, fatigue, and pain in early post-treatment breast cancer survivors using long-term follow-up over 4 years. The target sample of early post-treatment women following breast cancer diagnosis helps us understand the associations among MVPA, ST, and the common effects of depression, fatigue, and pain during a cancer phase that is rarely explored. Addressing limitations of self-reported behaviors, MVPA and ST were device-measured. It was hypothesized that an increase in ST, in addition to a decrease in MVPA level, would be associated with more symptoms of depression, fatigue, and pain.
Methods
Participants and procedures
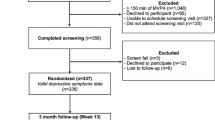
Women who had completed primary treatment (i.e., surgery, radiotherapy, chemotherapy) for stages I to III breast cancer were invited to participate in a longitudinal study investigating the natural developmental changes in lifestyle behaviors. Survivors were recruited through advertisements and oncologist referrals from various local medical clinics and hospitals in Montreal (Canada). Interested survivors were asked to contact the research team by phone to obtain additional details on the study. Screening for eligibility was conducted using the following inclusion criteria: (i) at least 18 years of age, (ii) recently (i.e., 0–20 weeks) post-primary treatment (i.e., surgery, chemotherapy, radiation therapy) for stages I to III breast cancer, (iii) treated for a first cancer diagnosis, (iv) report no health concerns that may inhibit participation in PA, and (v) provide written informed consent in either English or French. Involvement in this study included completing self-report questionnaires every 3 months for 5 times over the first 12 months of the study (times 1 to 5) and once a year thereafter respectively 24, 36, 48, and 60 months after study inception (times 6 to 9). Participants wore an accelerometer to measure PA and ST for 7 consecutive days at data collection times 1 to 5, 6, and 8. The study methods are reported in more detail elsewhere [25]. This study was approved by appropriate hospital and university research ethics committees. All participants provided written informed consent.
Measures
Lifestyle behaviors (MVPA and ST) were measured using Actigraph GT3X accelerometers (Actigraph, Pensacola, FL) every 3 months during the first year (times 1 to 5) and 2 and 4 years (times 6 and 8) post-cancer treatment. During each data collection cycle used in the present study, breast cancer survivors were asked to wear the accelerometer on their hip during waking hours over 7 days, except when participating in water activities (e.g., bathing/showering, pool activities). Movement data were recorded every minute and the number of daily minutes of light (100 to 1951 counts/min), moderate (1952–5724 counts/min), and vigorous (> 5725 counts/min) PA was calculated using established cut points [26]. MVPA was a sum score of weekly minutes in MVPA. ST was analyzed as < 100 counts/minute, adjusted for non-wear time operationalized as at least 60 min of consecutive zeros [27]. Data were included in the analyses if there were no extreme counts (> 20,000) and if data were available for at least 500 min on 4 or more days. The lower limit of wear time compared to established criteria of 600 min was consistent with participants’ daily diary records for time awake [25]. For the current study, MVPA and ST variables reflect the average percent of the day spent in MVPA and ST (e.g., time in MVPA and ST accounting for time the accelerometer was worn) using the following formula: Total = [Day 1(Total time in MVPA /Total time accelerometer worn) + Day 2(Total time in MVPA /Total time accelerometer worn) + ….Day 7 (Total time in MVPA /Total time accelerometer worn)]/7.
Depressive symptoms were assessed using the 10-item Center for Epidemiologic Studies Depression Scale (CES-D) [28]. Participants were asked to rate how often they felt each of the 10 items state (e.g., I felt depressed; I felt fearful) on a 4-point Likert scale (rarely or none of the time (0), some or little of the time (1), occasionally or a moderate amount of time (2); and most or all of the time (3)). The global depressive symptoms score was calculated by taking the average of these 10 items at each of the seven data collections. Similar to previous reports in HIV-positive people [28], our findings support the internal consistency of the score across data collections; Cronbach’s alpha coefficients range α = 0.82–0.86.
Fatigue was measured using the Brief Fatigue Inventory (BFI) [29] which provides an assessment of the fatigue severity, amount of interference with function caused by fatigue, and the presence of factors that worsen fatigue, such as pain and medications. Three items ask participants to rate the severity of their fatigue (e.g., weariness, tiredness) by circling the one number that best describes their fatigue RIGHT NOW (item 1), USUALLY in the past 24 h (item 2), and their WORST fatigue in the past 24 h (item 3), ranging from no fatigue (0) to as bad as you can imagine (10). Six items assess the amount that fatigue has interfered with different aspects of the participant’s life during the past 24 h: general activities, mood, walking ability, normal work, relations with others, enjoyment of life. Each item is scored on a 0–10 continuum, ranging from does not interfere (0) to completely interferes (10). A global score was calculated by taking the average of the 9 items. Cronbach’s alpha coefficients for internal consistency range from α = 0.94 to 0.95 across data collections. Fatigue was assessed using the BFI starting at time 2 (3 months after study inception) and then at all time points thereafter.
Pain was assessed asking participants to report whether (yes/no) they experienced any of 6 acute pain symptoms during the day for 2 nonconsecutive days in each week of data collection: stomach pain, back pain, pain in arms, legs or joints (knees, hips, etc.), pain or problems during sexual intercourse, headaches, and chest pain [30]. A score (0–6) is obtained by summing the number of acute pain symptoms experienced during this period, with higher score representing higher level of pain and averaging across the 2 days per data collection. Many of these symptoms have been shown to be prevalent among breast cancer survivors and were drawn from the Primary Care Evaluation of Mental Disorders screening questionnaire (PRIME MD) [31].
Covariates
Participants were asked to self-report their age, highest education level attained, income, marital status, breast cancer stage, and time since treatment was received. A trained lab technician measured and recorded baseline height and weight, which were used to calculate body mass index (BMI; i.e., weight in kilograms divided by height in meters squared).
Analysis
Preliminary analyses comprised descriptive statistics to assess distributions, identify outliers, and compute proportions, means, and standard deviations. Linear mixed effects modeling (LMM) was used to account for repeated measures. Separate models were estimated for each health outcome (i.e., depressive symptoms, fatigue, pain). The final estimated models included fixed effects for MVPA and ST, a time variable, and were adjusted for covariates. The models also included random intercepts and slopes, to allow participants to differ at baseline and also over time (i.e., women can follow different trajectories over time), as well as an “exchangeable” one-parameter correlation matrix. Models were compared using Akaike information criterion (AIC) goodness of fit index [32]. The software R 3.4.2 was used as well as the package nlme, Version 3.1–137. A significance level of 5% was used. In sensitivity analyses, we considered models in which covariances varied over time with no meaningful difference observed.
Results
Participants include 199 women breast cancer survivors (mean (SD) age = 55.0 (11.0) years, 85% White/Caucasian) recruited at baseline with stage I (n = 83), II (n = 78), or III (n = 38) breast cancer (n = 2; did not indicate). On average, participants had a household income of $102,041 (SD = 185,217), a BMI of 26.06 (SD = 6.15) kg/m2, and were 3.5 months (SD = 2.3) post-surgery, chemotherapy, and/or radiation. Most participants reported having a university degree or higher education (n = 101; 50.7%) and being married (n = 128; 64.3%) (see Table 1 for sample descriptive statistics). A total of 199 participants provide data at study inception (time 1); in times 2, 3, 4, 5, 6, and 8, n (% of the initial sample) 186 (93.5%), 181 (91.0%), 180 (90.4%), 180 (90.4%), 100 (50.3%), and 85 (42.7%) participants provide data. For the main analysis, only observations with complete data for all variable of interest (questionnaire and accelerometer data) were included in the analysis: a total of 835 observations were included in the analyses for depression symptoms, 826 observations were included in the analyses for pain, and 671 observations were included in the analyses exploring fatigue as the outcome. There was no difference between participants at time 8 (60 months) (n = 85) and those who were lost to follow-up (n = 114) over a 4-year and seven data collection points timeframe for MVPA (mean(SD) = 1.79(1.32) vs. 2.01(1.49), p = 0.286), depression symptoms (mean(SD) = 1.75(0.49) vs. 1.73(0.55), p = 0.779), and pain (mean(SD) = 1.26(1.10) vs. 1.25(1.08), p = 0.988) at time 1 (0 month) and fatigue (mean(SD) = 3.45(2.18) vs. 3.13(2.36), p = 0.342) at time 2 (3 months) (fatigue was not assessed at time 1; this is a 3-month difference between time 1 and time 2). Participants who remain involve in the study at time 8 (60 months) report lower ST at time 1 (0 month) compared to those lost to follow-up (mean(SD) = 79.13(5.74) vs. 77.06(5.49), p = 0.011).
Descriptive statistics of percent of day spent in MVPA and ST, depressive symptoms, fatigue, and pain scores across all data collections are reported in Table 2. Across the seven data collections, mean accelerometer wear times were 812.6 (SD = 123.9) to 841.1 (SD = 94.1) min/day, and the median number of days worn per week was seven. Average percent of day spent in MVPA remained relatively stable over the first-year post-treatment (ranged from 1.81% (SD = 1.37) to 1.98% (1.52)) and increased at 24 months post-treatment (3.04%, SD = 2.63) and then remained stable at 48 months post-treatment (2.98%, SD = 2.72). Average percent of day spent in ST ranged from 77.75% (SD = 5.84) to 78.55% (SD = 5.92) during the first-year post-treatment, then decreased to 64.01% (SD = 9.45) at 24 months and 65.92% (SD = 11.23) at 48 months post treatment. Average scores for depressive symptoms, fatigue, and pain all decreased over time during the 4 years post-treatment.
In linear mixed effects models, MVPA and ST were independent predictors of depressive symptoms, but not fatigue, and only ST was associated with pain over 4 years post-treatment, after adjustment for potential confounders. Higher levels of MVPA were associated with lower scores of depressive symptoms (\(\beta\) (95%CI): − 0.062 (− 0.092, − 0.031) p < 0.001), whereas higher levels of ST were associated with higher scores of depressive symptoms (\(\beta\) (95%CI): 0.023 (0.017, 0.028) p < 0.001). Higher levels of ST were associated with increased pain level over time (\(\beta\) (95%CI): 0.017 (0.007, 0.027) p = 0.001). No statistically significant association was found for MVPA in the pain model (\(\beta\) (95%CI): − 0.053 (− 0.113, 0.007) p > 0.05). The interaction between MVPA and ST was statistically non-significant. Detailed results are presented in Table 3.
Discussion
This study shows that both MVPA and ST were independently associated with fewer depressive symptoms over time. ST, but not MVPA, was associated with higher pain levels. Neither MVPA nor ST were associated with fatigue over time. This study innovates by including both device-measured MVPA and ST in the same model and by combining the use of multiple time points during the first year following treatment (5 time-points every 3 months) with a 24-month and a 48-month follow-up.
The link between MVPA and depressive symptoms is well established in the literature, both during and after treatment [17, 18]. Similarly, we found in the current study that higher levels of MVPA were associated with lower depressive symptoms. Moreover, our results show that the association is maintained even when including time points up to 48 months after treatment completion. The results of the current study expand on previous cross-sectional findings reported by Trinh et al. [22] suggesting that higher level of ST is associated with more depressive symptoms over time. However, Phillips et al. [24] report no cross-sectional association between ST and depression in breast cancer survivors. Based on the current study results, observing that depressive symptoms diminish over time allows us to capture the variation in the severity of symptoms while also exploring the association with ST and MVPA.
Contrary to previous studies [12, 13], our results did not show an association between MVPA or ST and fatigue. This difference could be explained by including both MVPA and ST in the analysis model. Current literature contains mixed results regarding the association between ST and fatigue. Phillips et al. [24] found that ST was positively associated with fatigue duration, but not with fatigue interference or severity whereas George et al. (2013) found no association between ST and fatigue 3.5 years post-diagnosis in breast cancer survivors [24, 33]. These inconsistencies may be due to fatigue measurement [12]. Moreover, fatigue measurement in longitudinal studies can be altered by the patient experience factors such as the beliefs and attitudes that patients have about PA as well as their evolving perception of fatigue over time [34].
In the current study, MVPA was not statistically significantly associated with pain; however, the magnitude of the estimate and the upper confidence interval was close to zero suggesting that there might be an association that we were not able to detect due to the relatively small sample size. Nonetheless, increased ST was associated with increased pain. Similarly, previous study found that ST was positively associated with pain; however, the association only appears to be present when MVPA levels are low [22]. Although several studies have shown that PA interventions reduce pain in people with cancer [14, 16], the longitudinal effects of MVPA on pain are less well established. Forsythe et al. (2013) found a negative association between self-reported MVPA and pain only in women who continued to be active 5 to 10 years after diagnosis [19]. In contrast, Alfano et al. (2007) found that higher levels of PA 39 months after diagnosis were associated with increased breast pain [35]. Pain levels were potentially not severe enough in our sample for an effect to be detected, suggesting a ceiling effect; it is more common to see fairly high pain symptoms in women with breast cancer [19].
Strengths and limitations
Strengths of the current study include multiple data collection immediately after cancer treatment and a 4-year follow-up for a total of seven data collections included in the current analysis. Also, MVPA and ST were device-measured which reduce information bias and misclassification [36]. Wearing an accelerometer could encourage participants to be more active and less sedentary which could be reflected in higher MVPA and lower ST levels. However, information bias due to social desirability is likely to be constant across participants and data collections and consequently is unlikely to distort the association between PA, ST, and each health outcomes assessed in the study. Limitations also include the use of self-report questionnaires for depressive symptoms, pain, and fatigue which are subject to misclassification. A multidimensional measure of pain may be more accurate to consider intensity and interference in a person’s life separately. At study inception (time 1), fatigue was only assessed as one item within a list of symptoms rather than a multidimensional scale examining the impact of fatigue on daily functioning. The sequential measurement of fatigue was a study design decision to explore predictors of health outcomes in a sequential way. Consequently, fatigue was assessed using the BFI for the first time at time 2 and the main analysis for fatigue started at time 2. High loss at follow-up may result in selection bias. It is possible that participants remaining in the study who provided data at T6 and T8 (respectively 24 and 48 months after study inception) may be more active and less sedentary compared to women who did not continue in the study. For example, Shi et al. (2020) investigated PA and ST trajectories in female breast cancer survivors and observed decreasing or stable low self-reported MVPA trajectories during the first 24 months following cancer [37]. They also found four distinct ST trajectories (high maintainer (18%), high decreaser (27%), low increaser (24%), and low maintainer (31%)), suggesting that a quarter of the participants show decreasing ST over time while the other three quarters show increasing or stable ST [37]. The device-measured increasing PA and decreasing ST level observed at 24 and 48 months in the current study need to be replicated.
Although the association between MVPA, ST, and depressive symptoms was found over time, the observational design of the current study and the modeling strategy do not allow inferences of causality. Moreover, the current analyses are exploring the general association between MVPA, ST, and each of depressive symptoms, fatigue, and pain over a 4-year follow-up. However, it is possible that these associations differ throughout the survivorship (early vs. long-term survivorship). This study is underpowered to test this proposition, and future work is needed. Finally, because the study is limited to women with breast cancer, it is not possible to generalize the results of this study to other cancer populations.
Conclusion
This is the first study to demonstrate that lower ST, in addition to higher MVPA, is associated to lower depressive symptoms and that ST is associated with lower fatigue over a 4-year follow-up. The study shows the importance of these associations over time, not only during early post-treatment when there is a heightened emphasis on care. These findings support international guidelines suggesting targeting physical inactivity and reduce ST among cancer patients to improve physical and psychological outcomes. Our findings support that PA promotion as part of routine care for breast cancer patients and survivors could improve both physical and mental health functioning. Rehabilitation interventions should aim to both increase PA and reduce ST to promote health among breast cancer survivors, both during the early post-treatment period and on the long-term survivorship. Recommendations for cancer patients and survivors should target small changes in lifestyle habits, which are known to be associated not only with the uptake of PA, but also with its maintenance over time [38]. Developing specific interventions for people with cancer that focus on reducing ST could be less of a challenge for people with cancer than increasing MVPA and therefore make it possible to initiate a change in the lifestyle behaviors of this population.
Data availability
The data that supports the findings of this study are available on request from the corresponding author.
Code availability
The R code that supports the findings of this study are available on request from the corresponding author.
References
Canadian Cancer Statistics Advisory Committee (2019) Canadian Cancer Statistics 2019. Canadian Cancer Society, Toronto, ON
Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M (2010) It’s not over when it’s over: long-term symptoms in cancer survivors—a systematic review. The International Journal of Psychiatry in Medicine 40(2):163–181
Stanton AL, Rowland JH, Ganz PA (2015) Life after diagnosis and treatment of cancer in adulthood: contributions from psychosocial oncology research. Am Psychol 70(2):159
Glare PA, Davies PS, Finlay E, Gulati A, Lemanne D, Moryl N et al (2014) Pain in cancer survivors. J Clin Oncol 32(16):1739
Abrahams H, Gielissen M, Verhagen C, Knoop H (2018) The relationship of fatigue in breast cancer survivors with quality of life and factors to address in psychological interventions: a systematic review. Clin Psychol Rev 63:1–11
Frazzetto P, Vacante M, Malaguarnera M, Vinci E, Catalano F, Cataudella E et al (2012) Depression in older breast cancer survivors. BMC Surg 12(1):S14
Reyes-Gibby CC, Anderson KO, Morrow PK, Shete S, Hassan S (2012) Depressive symptoms and health-related quality of life in breast cancer survivors. Journal of women’s health 21(3):311–318
Green CR, Hart-Johnson T, Loeffler DR (2011) Cancer-related chronic pain: examining quality of life in diverse cancer survivors. Cancer 117(9):1994–2003
Jensen MP, Chang H-Y, Lai Y-H, Syrjala KL, Fann JR, Gralow JR (2010) Pain in long-term breast cancer survivors: frequency, severity, and impact. Pain Med 11(7):1099–1106
Berger AM, Gerber LH, Mayer DK (2012) Cancer-related fatigue: implications for breast cancer survivors. Cancer 118(S8):2261–2269
Albrecht TA, Taylor AG (2012) Physical activity in patients with advanced-stage cancer: a systematic review of the literature. Clin J Oncol Nurs 16(3):293
Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR et al (2017) Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol 3(7):961–968
Kessels E, Husson O, van der Feltz-Cornelis CM (2018) The effect of exercise on cancer-related fatigue in cancer survivors: a systematic review and meta-analysis. Neuropsychiatr Dis Treat 14:479
Craft LL, VanIterson EH, Helenowski IB, Rademaker AW, Courneya KS (2012) Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiology and Prevention Biomarkers 21(1):3–19
Schmitz KH, Courneya KS, Matthews C, et al (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42(7):1409–1426. https://doi.org/10.1249/MSS.0b013e3181e0c112
Campbell KL, Winters-stone KM, Wiskemann J, May AM, Schwartz AI, Courneya KS et al (2019) Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 51(11):2375–2390
Brunet J, O’Loughlin JL, Gunnell KE, Sabiston CM (2018) Physical activity and depressive symptoms after breast cancer: cross-sectional and longitudinal relationships. Health Psychol 37(1):14
Sylvester BD, Ahmed R, Amireault S, Sabiston CM (2017) Changes in light-, moderate-, and vigorous-intensity physical activity and changes in depressive symptoms in breast cancer survivors: a prospective observational study. Support Care Cancer 25(11):3305–3312
Forsythe LP, Alfano CM, George SM, McTiernan A, Baumgartner KB, Bernstein L et al (2013) Pain in long-term breast cancer survivors: the role of body mass index, physical activity, and sedentary behavior. Breast Cancer Res Treat 137(2):617–630
Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE et al (2017) Sedentary behavior research network (SBRN)–terminology consensus project process and outcome. Int J Behav Nutr Phys Act 14(1):75
Lynch BM (2010) Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiology and Prevention Biomarkers 19(11):2691–2709
Trinh L, Amireault S, Lacombe J, Sabiston CM (2015) Physical and psychological health among breast cancer survivors: interactions with sedentary behavior and physical activity. Psychooncology 24(10):1279–1285
Hartman SJ, Marinac CR, Bellettiere J, Godbole S, Natarajan L, Patterson RE et al (2017) Objectively measured sedentary behavior and quality of life among survivors of early stage breast cancer. Support Care Cancer 25(8):2495–2503
Phillips SM, Awick EA, Conroy DE, Pellegrini CA, Mailey EL, McAuley E (2015) Objectively measured physical activity and sedentary behavior and quality of life indicators in survivors of breast cancer. Cancer 121(22):4044–4052
Sabiston CM, Wrosch C, Fong AJ, Brunet J, Gaudreau P, O’Loughlin J et al (2018) Life after breast cancer: moving on, sitting down or standing still? A prospective study of Canadian breast cancer survivors. BMJ Open 8(7):e021770
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14(5):377–381
Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M (2008) Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40(1):181
Zhang W, O’Brien N, Forrest JI, Salters KA, Patterson TL, Montaner JS et al (2012) Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS ONE 7(7):e40793
Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK et al (1999) The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 85(5):1186–1196
Hadd V, Sabiston CM, McDonough MH, Crocker PR (2010) Sources of stress for breast cancer survivors involved in dragon boating: examining associations with treatment characteristics and self-esteem. Journal of Women’s Health 19(7):1345–1353
Spitzer RL, Williams JB, Kroenke K, Linzer M, Verloin deGruy F, Hahn SR, et al (1994) Utility of a new procedure for diagnosing mental disorders in primary care: the PRIME-MD 1000 study. Jama 272(22):1749–56. https://doi.org/10.1001/jama.1994.03520220043029
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH et al (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24(3):127–135
George SM, Alfano CM, Smith AW, Irwin ML, McTiernan A, Bernstein L et al (2013) Sedentary behavior, health-related quality of life, and fatigue among breast cancer survivors. J Phys Act Health 10(3):350–358
Kampshoff C, van Dongen J, Van Mechelen W, Schep G, Vreugdenhil A, Twisk J et al (2018) Long-term effectiveness and cost-effectiveness of high versus low-to-moderate intensity resistance and endurance exercise interventions among cancer survivors. J Cancer Surviv 12(3):417–429
Alfano CM, Smith AW, Irwin ML, Bowen DJ, Sorensen B, Reeve BB et al (2007) Physical activity, long-term symptoms, and physical health-related quality of life among breast cancer survivors: a prospective analysis. J Cancer Surviv 1(2):116
Broderick J, Ryan J, O’Donnell D, Hussey J (2014) A guide to assessing physical activity using accelerometry in cancer patients. Support Care Cancer 22(4):1121–1130
Shi Z, Rundle A, Genkinger JM, Cheung YK, Ergas IJ, Roh JM et al (2020) Distinct trajectories of moderate to vigorous physical activity and sedentary behavior following a breast cancer diagnosis: the Pathways Study. J Cancer Surviv 14(3):393–403
Rhodes RE (2017) The evolving understanding of physical activity behavior: a multi-process action control approach. Advances in motivation science. 4: Elsevier. p 171–205. https://doi.org/10.1016/bs.adms.2016.11.001
Funding
This research was supported by a Canadian Institutes of Health Research grant (#186128).
Author information
Authors and Affiliations
Contributions
ID conceptualized the objectives, conducted all analyses, drafted, and revised the manuscript. CS designed the main study and critically reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was obtained from the University of Toronto (REB# 28180).
Consent to participate
All participants provided written informed consent.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doré, I., Plante, A., Peck, S.S. et al. Physical activity and sedentary time: associations with fatigue, pain, and depressive symptoms over 4 years post-treatment among breast cancer survivors. Support Care Cancer 30, 785–792 (2022). https://doi.org/10.1007/s00520-021-06469-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06469-2