Abstract
Aims
Evaluate the abundance of the selected targets, alpha-1-antitrypsin (A1AT) and macrophage migration inhibitory factor (MIF), and correlate these findings with the risk of developing severe oral mucositis (OM).
Materials and methods
Head and neck squamous cell carcinoma (HNSCC) patients submitted to radiotherapy (RT) or chemoradiotherapy (CRT) were assessed. OM grade and pain were evaluated daily during treatment. Two protein targets, A1AT and MIF, were evaluated, using selected reaction monitoring-mass spectrometry (SRM-MS), in whole saliva, collected prior to oncologic treatment. The results obtained from the targeted proteomic analysis were correlated with OM clinical outcomes.
Results
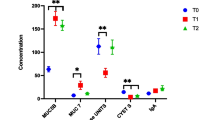
A total of 27 patients were included, of whom 21 (77.8%) had locally advanced disease (clinical stage III or IV). Most patients (70.4%) received CRT. OM grades 2 (40.8%) and 3 (33.3%) were the most prevalent during RT with a mean highest reported OM-related pain of 3.22 through the visual analogue scale (VAS). The abundance of A1AT and MIF correlated significantly with severe (grades 3 or 4, p < 0.02) compared with moderate-low (grades 1 or 2, p < 0.04) OM grade.
Conclusions
There is a correlation between the abundance of salivary A1AT and MIF and oncologic treatment–induced OM. The correlation of MIF expression with severe OM appears to be compatible with its physiological pro-inflammatory role. These results open up great possibilities for the use of salivary MIF and A1AT levels as prognostic markers for effective therapeutic interventions, such as photobiomodulation therapy, patient-controlled analgesia, or personalized medicaments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck squamous cell carcinoma (HNSCC) treatment is multimodal, including surgery, radiotherapy (RT), and chemotherapy (CT) [1,2,3]. Despite the notable benefit of RT, this treatment modality can be associated with several acute and chronic side effects that affect non-target tissues present within the radiation field [4]. In this context, oral mucositis (OM) is a highly prevalent acute toxicity that affects up to 80 to 100% of patients undergoing RT for oral and oropharyngeal cancer. According to the literature, chemoradiotherapy (CRT) protocols can increase the frequency and clinical severity of OM [4, 5]. Besides the effect of combining CT with RT, other factors that have been correlated as risk factors for RT-induced OM are poor oral hygiene, young age, female gender, poor renal function, poor nutritional status, total cumulative radiation dose, smoking, and decreased salivary secretion [6].
OM has a complex pathophysiology characterized by persistent oral mucosal ulcers associated with severe pain and reduced oral functions, such as swallowing, speech, taste, and chewing. These consequent morbidities can, in severe cases, lead to the interruption of cancer treatment and, consequently, cause tumor progression and negatively impact on survival rates [1,2,3, 7]. These comorbidities may also increase the cost of healthcare due to increased hospitalization, expensive drugs such as opioids or growth factors (e.g., keratinocyte growth factor (KGF)), and the use of probes for nasogastric feeding [8,9,10,11].
Improved molecular characterization of oncologic treatment–induced OM will not only aid in an improved understanding of the disease process but also enable development of robust prognostic biomarkers to improve effective interventions. A study by Jehmlich et al. [12] outlined the salivary proteomic profile of HNSCC cancer patients and noted the expression of 48 proteins related to an increased risk for the development of OM. Considering the above-mentioned information and the need for better understanding of the proteins associated with OM development, the aim of the present study was to characterize two proteins, alpha-1-antitrypsin (A1AT) and macrophage migration inhibitory factor (MIF), and correlate them with the risk of developing severe OM post-oncologic treatment.
Materials and methods
Study design
This was a single-center cohort study designed to evaluate the salivary proteomic profile of HNSCC patients submitted to RT or CRT protocols at the Instituto do Câncer do Estado de São Paulo (ICESP, Brazil) from January 2011 to February 2018. This study was approved by the Ethics Committee of the School of Medicine, University of São Paulo, Sao Paulo, Brazil (Protocol # 2.647.153). Written informed consent was obtained from all participants and the study was conducted in accordance with the Declaration of Helsinki.
Inclusion criteria
This study included oral and oropharynx cancer patients who underwent RT or CRT protocols (with or without previous surgery) using a 6MV linear accelerator and 3-dimensional conformal or intensity-modulated radiation therapy technique (IMRT) (Synergy Platform, Elekta AB, Stockholm, Sweden). The target radiation volumes encompassed the primary site and areas of regional lymph nodes at risk and received cumulative doses that ranged from 60 to 70 Gy. We applied the recommendations for treatment planning and constraints for organs at risk, as previously reported [13,14,15].
All included patients completed the institutional dental conditioning protocol prior to beginning RT. Besides routine oral care, all patients were submitted to the standard-of-care daily photobiomodulation (PBM) protocol for prevention of OM as per our institutional (Dental Oncology Service, ICESP, Brazil) protocol [11]. Finally, all patients needed to have complete demographic and clinicopathological data available on electronical medical charts, including gender, age, tumor location, clinical cancer stage (according to the American Joint Committee on Cancer Staging System, 7th edition [16]), cancer treatment modalities, and information regarding weekly dental follow-up during RT and OM outcomes.
Exclusion criteria
Patients who missed one or more RT or PBM sessions were considered to have received incomplete treatment and were excluded from the study.
Oral mucositis assessment
A trained dental surgeon conducted OM grading using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, Version 4.0, 2010 [17]) on the last day of each week of treatment (day 5, 10, 15, 20, 25, 30, and 35) as a part of standard of care. The highest OM grade developed throughout RT was used for comparison with the salivary proteomic profiles. Patient self-reported OM pain was recorded using the visual analogue scale (VAS) with scores ranging from 0 to 10, where 0 represents no pain and 10 is the highest level of pain. Assessments were recorded at the end of each week of RT and mean VAS values throughout RT were noted.
Saliva collection and preparation
Saliva samples were obtained voluntarily and with signed consent from HNSCC patients. Whole saliva collection was performed immediately prior to the beginning of RT. Individuals first rinsed their mouths with 5 mL of drinking water and then harvested the saliva into a sterile recipient. Saliva samples were aliquoted in 2-mL tubes and immediately frozen at − 80 °C for long-term storage.
Whole saliva protein preparation
The preparation of saliva samples was performed as previously described [18, 19]. Briefly, saliva was first centrifuged for 5 min at 1500g at 4 °C to remove intact cells and debris. A volume of 100 μL of whole saliva was then used in the protein extraction procedure by homogenizing with 100 μL of urea buffer (100 mM Tris-HCl pH 7.5, 8 M urea, and 2 M thiourea) containing the complete Mini Protease Inhibitor Cocktail (Roche, Auckland, New Zealand), 5 mM EDTA, 1 mM PMSF, and 1 mM DTT. Samples were sonicated for 10 min and centrifuged at 10,000g for 5 min. Total protein was quantified using a Bradford assay kit (Bio-Rad, São Paulo, Brazil).
Mass spectrometry and data analysis
The proteotypic peptides and their respective transitions of the proteins alpha-1-antitrypsin (A1AT) and macrophage migration inhibitory factor (MIF) were selected based on the criteria described [20, 21]: MIF_HUMAN (ASVPDGFLSELTQQLAQATGK, m/z 721.04, +3 and its respective transitions [y6] m/z 575.31+; [y4] m/z 376.21+; [y2] m/z 204.13+); A1AT_HUMAN (LQHLENELTHDIITK, m/z 451.74, +4; and its respective transitions [y2] m/z 248.16+; [y8] m/z 470.77++; [y7] m/z 414.23++ and EINDYVEK, m/z 505.24,+2 and its respective transitions [y6] m/z 767.35+; [y5] m/z653.31+; [y4] m/z 538.28+).
Three proteotypic peptides were purchased as crude heavy-isotope-labeled peptide standards (Thermo Fisher Scientific). The stable isotope-labeled peptides (SIL) were synthesized with heavy isotopes on lysine (+8 Da), localized at the C-terminal of the peptide (Thermo Fisher Scientific). Three transitions were monitored for the light and heavy counterparts of each peptide. Eight or nine peptides with their respective transitions of the internal retention time standard (Pierce™ Peptide Retention Time Calibration Mixture, Thermo Fisher Scientific) were monitored as a control for retention time shifts in liquid chromatography.
In order to avoid bias in the analyses, samples were randomized using the software R (v3.4.0) and separated in blocks with each sample analyzed in triplicate for SRM analysis. All samples were assessed within each block in different orders to further reduce systematic bias.
Samples were analyzed on a Xevo TQ-XS triple quadrupole mass spectrometer (Waters, Milford, MA, USA) equipped with an electrospray ion source (Ion Key, Waters, Milford, MA, USA) with MassLynx software (version 4.2), as described by Carnielli et al. [19]. The data analysis was manually performed in Skyline.
Statistical analysis
Demographic data, clinicopathological features, and clinical outcome results were grouped into a spreadsheet for descriptive statistical analyses based on mean, median, standard deviation, and proportion values. For the SRM analysis, the comparison of the levels of the monitored peptides between the patients who developed grades 1 or 2 OM (M1) and those who developed grades 3 or 4 OM (M2) was performed using a Wilcoxon Mann-Whitney test (not log-transformed data) with the significance level set at α = 0.05.
Results
Patient characteristics
A total of 27 patients were included. The major clinicopathological features, including age, gender, tumor site, clinical staging, treatments, and RT modality, are summarized in Table 1. The mean age was 57 years (range 20–81 years) and 24 (88.9%) were males. The most frequent primary tumor site was the lateral border of the tongue (12 cases, 44.5%) followed by oropharynx (10 cases, 37%), and the floor of the mouth (2 cases, 7.4%). Most patients (n = 21, 77.8%) were diagnosed with an advanced stage of disease (III/IV), and none of the 27 (100%) patients had their surgery at the time of saliva collection (presented with visible oral cancer lesions). All included patients received complete and uninterrupted RT and 19 (70.4%) received concomitant CRT protocols. Regarding the RT technique, 20 (74.1%) of patients were treated by 3-dimensional conformal RT; 7 (25.9%) with IMRT, with an overall mean dose of 67.7 Gy (ranging from 66 to 68.3 Gy).
Oral mucositis assessment
The majority of the patients (n = 24, 88.9%) developed OM during the treatment period, even when performing PBM protocol. During the first 2 weeks of treatment (days 5 to 10), OM grades varied from 0 to 2. Grade 3 lesions started to develop from the third week of treatment (day 15), while grade 4 lesions developed from the 5th week (day 25) of treatment (Fig. 1). Three (11.1%) patients did not develop OM throughout the treatment. From the patients that developed OM, a total of 14 (51.9%) patients developed minimal OM (grade 1 (3/11.1%) or 2 (11/40.8%)) and 10 (37%) patients developed severe OM (grade 3 (9/33.3%) or 4 (1/3.7%)) as the highest grade throughout the treatment. The mean highest reported VAS throughout the treatment was 3.22 (range 0 to 9) and the highest level of OM-related pain was observed on the last week of radiotherapy with a mean VAS of 1.6. Data regarding OM grades and mean VAS per week of radiotherapy are summarized in Table 2.
Representative clinical images of subjects in the present study who developed oral mucositis lesions. a Subject presenting with grade 1 OM demonstrating erythema on the right buccal mucosa. b Subject presenting with grade 2 OM demonstrating small, punctuate ulcers covered with a pseudomembrane. c Subject presenting with grade 3 OM demonstrating confluent ulcers with pseudomembrane affecting the lateral border, dorsum, and ventral surface of the tongue. d Subject presenting with grade 4 OM demonstrating deep ulcers and bleeding, note the crust formations on the upper and lower lip
Salivary protein assessment
Targeted proteomic analysis was performed for the samples of the 27 patients. Figure 2 demonstrates, individually, the light/heavy intensity ratio (not log transformed) of two proteins, MIF and A1AT, between M1 and M2 saliva samples. We demonstrated that alpha-1-antitrypsin and macrophage migration inhibitory factor showed a statistically significant correlation between patients who developed severe grades 3 or 4 OM (p < 0.02 and 0.04, respectively) as compared with patients who developed grades 1 or 2 OM. The increase in A1AT and MIF abundance was 2.97- and 21.4-fold, respectively, in grades 3 or 4 subjects compared with patients who developed low grades 1 or 2 or no OM.
Discussion
This study evaluated the proteomic profile of whole saliva in HNSCC patients treated with RT or CRT, associated or not with previous surgery for tumor resection, and correlated this with OM severity. We found two proteins A1AT and MIF that could be possible prognostic biomarkers for the development of severe oncologic treatment–induced OM. The relevance of this finding is driven by the great interest in the development of personalized treatments based on the molecular profile of diseases that could lead to increased personalized treatment efficacy [22].
The patient-related findings in the present study are consistent with those reported in the literature, with the highest prevalence of HNSCC noted among middle-aged men diagnosed with advanced squamous cell carcinomas at the lateral border of the tongue and oropharynx, with most of these lesions considered clinically unresectable [23, 24]. In terms of the frequency and severity of OM, the present study also observed conventionally reported incidences, with over 80% of patients presenting some degree of lesions for patients submitted to RT or CRT protocols and treated with prophylactic PBM therapy. Over 40% of the patients in this study developed grade 2 OM as the highest grade throughout the treatment which is similar to reports in the literature of approximately 50% of grade 2 OM incidence by the end of RT with PBM treatments [25, 26]. The use of PBM therapy prophylactically is now considered standard of care and has likely provided additional benefit in this study to mitigate OM severity [11, 27].
The A1AT protein, also known as serpin 1, is a major liver-derived circulating protein that functions as a natural inhibitor of various serine proteases. It serves as a key component of the acute-phase response with roles in modulating the local and systemic inflammatory responses [28]. Jehmlich et al. [12] were the first to correlate the A1AT protein with the presence of OM, based on proteome analysis of whole saliva samples [12]. We also observed a significant modulation of this marker in the present study and this appeared to correlate with increased susceptibility to develop severe OM post-oncologic treatment. However, the precise role of A1AT in the OM pathogenesis remains unclear and further studies are warranted [12].
The MIF protein is a T cell–derived factor and its primary role is as a pro-inflammatory protein [29, 30]. MIF acts by activating pathways such as mitogen-activated protein (MAP) kinase and phosphoinositide-3-kinase and enhancing the expression of pro-inflammatory genes [31]. Additionally, MIF can both act on macrophages and be secreted by them in response to stimulation by cytokines such as tumor necrosis factor-alpha (TNF-α) [29, 32]. Interestingly, MIF is also responsible for the production and release of TNF-α, a pro-inflammatory cytokine that, in turn, further upregulates production of MIF in an autocrine manner and thereby promoting a pro-inflammatory response [29]. MIF also regulates the innate immune response and has been shown to be important in various autoimmune diseases. It has been noted to be involved in cell proliferation, cell survival, migration, and metastasis in several types of cancers, particularly oral squamous cell carcinomas [30, 31, 33].
The role of MIF as a pro-inflammatory marker appears to be compatible with the results of the present study, in that we found a higher abundance of MIF in samples from subject who developed severe grades 3 or 4 OM. This is the first clinical demonstration, to our knowledge, of this correlation in human subjects that has been previously observed in animal studies [34, 35]. Other studies have reported reduced cytokine levels of TNF-α and MIF following effective OM treatment, likely reflecting a reduction in the underlying inflammatory pathophysiology [34, 35].
As previously mentioned, OM presents a complex pathophysiology which involves several signaling pathways [12, 34]. One of the known signaling pathways implicated in OM is the nuclear factor kappa beta (NFκB) signal transduction pathway [36]. This pathway, when activated, enhances the expression of the pro-inflammatory cytokine TNF-α which, in turn, drives tissue damage leading to the development of OM lesions. In addition, both TNF-α and NFκB signaling have been shown to modulate MIF expression, potentially corroborating the results in this study for an important role for MIF in OM pathogenesis [34, 35].
Strengths and limitations
The major strengths of the current study were the rigorous clinical inclusion and exclusion criteria that enabled a relatively homogeneous cohort of subjects. Nonetheless, limitations of the present work include a single institution study design, availability of only pre-RT/CRT (and not intermediate and post-treatment) saliva samples, and variations in tumor burden during saliva sampling. These limitations justify the need for a larger and independent cohort and long-term prospective studies that are being pursued currently.
Conclusions
This study indicates the correlation of two protein levels with the development of severe OM and noted increased MIF and A1AT levels correlated with more severe grades of OM following oncologic treatment. Besides providing potential insights into their role in OM pathogenesis, these potential salivary biomarkers could serve as valuable prognostic aids to enhance and precisely calibrate effective OM interventions such as PBM treatments, patient-controlled analgesia, or personalized medicaments.
References
Bensadoun RJ, Franquin JC, Ciais G, Darcourt V, Schubert MM, Viot M, Dejou J, Tardieu C, Benezery K, Nguyen TD, Laudoyer Y, Dassonville O, Poissonnet G, Vallicioni J, Thyss A, Hamdi M, Chauvel P, Demard F (1999) Low-energy He/Ne laser in the prevention of radiation-induced mucositis. A multicenter phase III randomized study in patients with head and neck cancer. Support Care Cancer 7:244–252
Gouvêa de Lima A, Villar RC, de Castro G Jr et al (2012) Oral mucositis prevention by low-level laser therapy in head-and-neck cancer patients undergoing concurrent chemoradiotherapy: a phase III randomized study. Int J Radiat Oncol Biol Phys 82:270–275. https://doi.org/10.1016/j.ijrobp.2010.10.012
Oberoi S, Zamperlini-Netto G, Beyene J, Treister NS, Sung L (2014) Effect of prophylactic low level laser therapy on oral mucositis: a systematic review and meta-analysis. PLoS One 9:e107418. https://doi.org/10.1371/journal.pone.0107418
Sonis TS, Hashemi S, Epstein JB, Nair RG, Raber-Durlacher JE (2016) Could the biological robustness of low level laser therapy (Photobiomodulation) impact its use in the management of mucositis in head and neck cancer patients. Oral Oncol 54:7–14. https://doi.org/10.1016/j.oraloncology.2016.01.005
Treister N, Sonis S (2007) Mucositis: biology and management. Curr Opin Otolaryngol Head Neck Surg 15:123–129. https://doi.org/10.1097/MOO.0b013e3280523ad6
Maria OM, Eliopoulos N, Muanza T (2017) Radiation-induced oral mucositis. Front Oncol 7:89. https://doi.org/10.3389/fonc.2017.00089
Murphy CT, Galloway TJ, Handorf EA, Egleston BL, Wang LS, Mehra R, Flieder DB, Ridge JA (2016) Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. J Clin Oncol 34(2):169–178. https://doi.org/10.1200/JCO.2015.61.5906
Ferreira KASL, Kimura M, Teixeira MJ (2006) The WHO analgesic ladder for cancer pain control, twenty years of use. How much pain relief does one get from using it? Support Care Cancer 14:1086–1093. https://doi.org/10.1007/s00520-006-0086-x
Gautam AP, Fernandes DJ, Vidyasager MS, Maiya AG, Vadhiraja BJ (2013) Low level laser therapy for concurrent chemoradiotherapy induced oral mucositis in head and neck cancer patients. A triple blinded randomized controlled trial. Radiother Oncol 21(5):1421–1428. https://doi.org/10.1016/j.radonc.2012.06.011
Antunes HS, Schluckebier LF, Herchenhorn D, Small IA, Araújo CMM, Viégas CMP, Rampini MP, Ferreira EMS, Dias FL, Teich V, Teich N, Ferreira CG (2016) Cost-effectiveness of low-level laser therapy (LLLT) in head and neck cancer patients receiving concurrent chemoradiation. Oral Oncol 52:85–90. https://doi.org/10.1016/j.oraloncology.2015.10.022
Brandão TB, Morais-Faria K, Ribeiro ACP, Rivera C, Salvajoli JV, Lopes MA, Epstein JB, Arany PR, de Castro G Jr, Migliorati CA, Santos-Silva AR (2018) Locally advanced oral squamous cell carcinoma patients treated with photobiomodulation for prevention of oral mucositis: retrospective outcomes and safety analyses. Support Care Cancer 26:2417–2423. https://doi.org/10.1007/s00520-018-4046-z
Jehmlich N, Stegmaier P, Golatowski C, Salazar MG, Rischke C, Henke M, Völker U (2015) Differences in the whole saliva baseline proteome profile associated with development of oral mucositis in head and neck cancer patients undergoing radiotherapy. J Proteome 125:98–103. https://doi.org/10.1016/j.jprot.2015.04.030
Grégoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, Lee A, le QT, Maingon P, Nutting C, O’Sullivan B, Porceddu SV, Lengele B (2014) Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol 110(1):172–181. https://doi.org/10.1016/j.radonc.2013.10.010
Mendez LC, Moraes FY, Poon I, Marta GN (2016) The management of head and neck tumors with high technology radiation therapy. Expert Rev Anticancer Ther 16(1):99–110. https://doi.org/10.1586/14737140.2016.1121111
Grégoire V, Evans M, Le QT et al (2018) Delineation of the primary tumour clinical target volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother Oncol 126(1):3–24. https://doi.org/10.1016/j.radonc.2017.10.016
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474. https://doi.org/10.1245/s10434-010-0985-4
National Cancer Institute (2010) Common terminology criteria for adverse events (CTCAE) version 4.0 published: May 28, 2009 (v4.03: June14, 2010). Downloaded from http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickRef erence_5x7.pdf on Mar 30, 2011. Last accessed in 19 of May of 2020
Winck FV, Ribeiro AC, Domingues RR et al (2015) Insights into immune responses in oral cancer through proteomic analysis of saliva and salivary extracellular vesicles. Sci Rep 5:16305. https://doi.org/10.1038/srep16305
Carnielli CM, Macedo CCS, De Rossi T et al (2018) Combining discovery and targeted proteomics reveals a prognostic signature in oral cancer. Nat Commun 9(1):3598. https://doi.org/10.1038/s41467-018-05696-2
Lange V, Picotti P, Domon B, Aebersold R (2008) Selected reaction monitoring for quantitative proteomics: a tutorial. Mol Syst Biol 4:222. https://doi.org/10.1038/msb.2008.61
Gallien S, Duriez E, Domon B (2011) Selected reaction monitoring applied to proteomics. J. Mass Spectrom 46:298–312. https://doi.org/10.1002/jms.1895
Cohen EEW, LaMonte SJ, Erb NL, Beckman KL et al (2016) American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin 66(3):203–239. https://doi.org/10.3322/caac.21343
Kowalski LP, Carvalho AL, Priante AVM, Magrin J (2005) Predictive factors for distant metastasis from oral and oropharyngeal squamous cell carcinoma. Oral Oncol 41(5):534–541. https://doi.org/10.1016/j.oraloncology.2005.01.012
Scully C, Felix DH (2006) Oral medicine - update for the dental practitioner oral cancer. Br Dent J 200(1):13–17. https://doi.org/10.1038/sj.bdj.4813117
Bensadoun RJ, Le Page F, Darcourt V et al (2006) Radiation-induced mucositis of the aerodigestive tract: prevention and treatment. MASCC/ISOO mucositis group's recommendations. Bull Cancer 93:201–211
Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya AG, Vadhiraja BM (2012) Low level laser therapy for concurrent chemoradiotherapy induced oral mucositis in head and neck cancer patients - a triple blinded randomized controlled trial. Radiother Oncol 104:349–354. https://doi.org/10.1016/j.radonc.2012.06.011
Zadik Y, Arany PR, Fregnani ER et al (2019) Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 27(10):3969–3983. https://doi.org/10.1007/s00520-019-04890-2
Ehlers MR (2014) Immune-modulating effects of alpha-1 antitrypsin. Biol Chem 395(10):1187–1193. https://doi.org/10.1515/hsz-2014-0161
Bach JP, Rinn B, Meyer B, Dodel R, Bacher M (2008) Role of MIF in inflammation and tumorigenesis. Oncology 75(3–4):127–133. https://doi.org/10.1159/000155223
Wang SS, Cen X, Liang XH, Tang YL (2017) Macrophage migration inhibitory factor: a potential driver and biomarker for head and neck squamous cell carcinoma. Oncotarget 8(6):10650–10661. https://doi.org/10.18632/oncotarget.12890
Kang Y, Zhang Y, Sun Y (2018) Macrophage migration inhibitory factor is a novel prognostic marker for human oral squamous cell carcinoma. Pathol Res Pract 214(8):1192–1198. https://doi.org/10.1016/j.prp.2018.06.020
Calandra T, Bernhagen J, Mitchell RA, Bucala R (1994) The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med 179(6):1895–1902
Chang KP, Lin SJ, Liu SC, Yi JS, Chien KY, Chi LM, Kao HK, Liang Y, Lin YT, Chang YS, Yu JS (2015) Low-molecular-mass secretome profiling identifies HMGA2 and MIF as prognostic biomarkers for oral cavity squamous cell carcinoma. Sci Rep 5:11689. https://doi.org/10.1038/srep11689
Ribeiro SB, de Araújo AA, Araújo Júnior RF et al (2017) Protective effect of dexamethasone on 5-FU-induced oral mucositis in hamsters. PLoS One 12(10):e0186511. https://doi.org/10.1371/journal.pone.0186511
Araújo AA, Araújo LS, Medeiros CACX et al (2018) Protective effect of angiotensin II receptor blocker against oxidative stress and inflammation in an oral mucositis experimental model. J Oral Pathol Med 47(10):972–984. https://doi.org/10.1111/jop.12775
Sonis ST (2007) Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol 5(9 Suppl 4):3–11
Acknowledgments
We would like to thank Waters Corporation for providing us with access to a Xevo TQ-XS triple quadrupole mass spectrometer.
Funding
The authors would like to gratefully acknowledge the financial support of the São Paulo Research Foundation (FAPESP 2018/04657-8, 2018/02233-6, 2016/22862-2, 2013/18402-8, 2012/06138-1, 2015/12431-1, and 2016/07846-0) as well as the National Council for Scientific and Technological Development (CNPq), Brazil. The authors also state that this study was financed in part by the Coordination for the Improvement of Higher Education Personnel - Brazil (CAPES) - Finance Code 001.
Author information
Authors and Affiliations
Contributions
Natália Rangel Palmier, Ana Carolina Prado-Ribeiro, Alan Roger Santos-Silva, and Thaís Bianca Brandão performed the conception, design of the study, and draft of the manuscript. Natália Rangel Palmier, Karina Morais-Faria, Tatiane de Rossi, and Guilherme Pimentel Telles performed the analysis and interpretation of data. Adriana Franco Paes Leme, César Augusto Migliorati, Gustavo Nader Marta, Luiz Paulo Kowalski, and Praveen R. Arany revised the manuscript for important intellectual content. Final approval of the version to be published was performed by Ana Carolina Prado-Ribeiro, Alan Roger Santos-Silva, and Thaís Bianca Brandão.
Corresponding author
Ethics declarations
This study was approved by the Ethics Committee of the School of Medicine, University of São Paulo, Sao Paulo, Brazil (Protocol# 2.647.153).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palmier, N.R., Leme, A.F.P., De Rossi, T. et al. Salivary alpha-1-antitrypsin and macrophage migration inhibitory factor may be potential prognostic biomarkers for oncologic treatment–induced severe oral mucositis. Support Care Cancer 29, 2939–2946 (2021). https://doi.org/10.1007/s00520-020-05805-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05805-2