Abstract
Purpose
The impact of supportive medications on patient-reported outcomes (PROs) has not been systematically evaluated. We describe the supportive medications used by treatment-naïve lung cancer patients and assess their association with PROs from MD Anderson Symptom Inventory (MDASI).
Methods
Treatment-naïve lung cancer patients who completed PROs from MDASI at the initial visit to MD Anderson Cancer Center were included. Medications from the initial visit were abstracted from the electronic medical records system and categorized into therapeutic classes based on U.S. Pharmacopeia v7.0. A chi-square or Mann-Whitney U test was conducted as appropriate.
Results
Among 459 patients, ~ 50% took any analgesics and 25% were on opioids. One-third of patients with moderate-severe pain were not on any analgesics. Patients taking opioids had significantly worse median pain scores (6 vs. 0) compared with those not taking any analgesics (p < 0.0001). Higher proportion of patients with moderate-severe pain took opioids compared with those with mild pain (52% vs. 16%, p < 0.0001). Patients on opioids also reported significantly worse scores for five other cancer-specific core symptoms and all six symptoms rating interference with daily life. Only 15% of patients with higher composite score for depression-related symptoms were on antidepressants. However, patients taking antidepressants did not significantly differ in any individual MDASI symptom scores compared with those not on antidepressants (p = 0.4858).
Conclusions
Our results suggest a need for better screening for pain and depression and optimization of pain management in treatment-naïve lung cancer patients since their poor functional status may result in suboptimal cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patient-reported outcomes (PROs) are increasingly being incorporated in both interventional trials and in clinical practice especially in cancer patients [1, 2]. While the NCI Common Terminology Criteria for Adverse Events (CTCAE) aids in the objective assessment and grading of the severity of adverse effects of therapy, they do not allow the clinicians to determine the impact of treatment on the patients’ perception of symptoms and their quality of life (QOL) [3,4,5,6,7]. Therefore, PROs are important in determining baseline symptom burden of patients as well as assessing the impact of various treatments over time. With aggressive cancers such as lung cancer, where most patients are diagnosed with advanced stage disease, the baseline symptom burden of these patients is high [8, 9]. The psychological impact of the diagnosis and prognosis itself adds significant burden to the QOL of patients requiring additional treatment [10,11,12]. This results in polypharmacy, which may further impact the symptom burden of the patients, especially in older patients [13, 14]. Therefore, careful evaluation of medications is an important factor while evaluating symptom burden regardless of them being clinician-evaluated or patient-reported.
Very few studies have examined the symptom status and supportive care of lung cancer patients before they start definitive cancer treatment. In treatment-naïve patients, understanding of baseline symptom burden and comorbidities is of utmost importance to select appropriate modes of cancer therapies as well as individualized drug therapy regimen for patients to maintain dose intensity without compromising medication adherence [15, 16]. However, determining the cause of the symptoms and their appropriate management is equally important for the patient-centered care model [17, 18]. PROs especially in treatment-naïve patients are thought to be due to the cancer itself. Hence, the impact of medications and comorbidities as the causes of symptoms has not been assessed in greater detail. Conversely, how effective the supportive care medications are in relieving the symptom burden has also not been evaluated.
The MD Anderson Symptom Inventory (MDASI) is a comprehensive questionnaire designed to evaluate the severity of symptoms reported by the cancer patients themselves [19]. It is completed by all patients at their first visit to MD Anderson Cancer Center (MDACC). The score (ranging from 0 to 10) reported by patients rather than clinicians eliminates an external bias by the investigator and reflects the patient’s perspectives. Since patients with lung cancer undergo extensive symptom burden [20], MDASI is an essential tool in quantifying this burden. In this study, we aimed to describe the supportive care medications used by treatment-naïve lung cancer patients and assess the association between the use of analgesics or antidepressants and the severity of common symptoms as reported by patients.
Methods
Patients
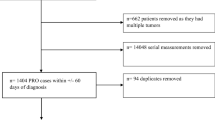
The study was a retrospective analysis of medication and symptom data in treatment-naïve patients with lung cancer seen at MDACC between February 2008 and February 2015. We defined treatment-naïve patients as those who had received no prior cancer therapy including surgery, radiation, or systemic drug therapy at the time of MDASI completion. The study was approved by the Institutional Review Board at MDACC and University of Houston.
The inclusion criteria included patients with diagnosis of either non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC) and completion of MDASI 30 days pre-diagnosis and 45 days of post-diagnosis. The exclusion criteria were patients who received any treatment for their lung cancer including surgery, radiation, or systemic therapy and those with missing data.
Data collection
The data was extracted between June 2016 and September 2017. Baseline patient and tumor characteristics (age, gender, race, days from diagnosis, number of medications, clinical stage of cancer, histopathology, and tumor location) at the first visit were abstracted using the EPIC electronic medical records system at MDACC. The list of medications was generated by the clinical oncology team at the first visit. Histopathological description of the tumor was categorized based on the 2015 WHO Classification of Lung Tumors [21].
Patient-reported symptoms were based from MDASI, which is a validated multi-symptom assessment measure developed for use in the patients with lung as well as other types of cancer [19, 22, 23]. The intensity of 12 common cancer-related symptoms (pain, drowsiness, fatigue, nausea/vomiting, disturbed sleep, shortness of breath, lack of appetite, dry mouth, distress, sadness, numbness/tingling, and difficulty remembering) was rated on 0–10 numeric scales ranging from “not present” to “as bad as you can imagine.” Six items related to how much symptoms have interfered with enjoyment of life, which were relations with others, walking, general activity, work, and mood were rated on 0–10 numeric scales ranging from “did not interfere” to “interfered completely.” In addition to MDASI, patients also responded to four measures of overall well-being/quality of life items (QOL, physical well-being, emotional well-being, and social support) that were rated on a 0–10 scale, with lower scores indicating poorer function. Seven items were used to represent depression based on their relevance to PHQ9 Depression questionnaire [24]. These were emotional well-being, enjoyment of life, mood, disturbed sleep, fatigue, lack of appetite, and sadness. The direction of emotional well-being was reversed to account for its lower scores indicating poorer function. These seven items were composited to create a depression scale of 0 to 70 ranging from “did not interfere” to “interfered completely”.
A complete medication list from the first visit was obtained from the EPIC electronic medical record system at the MDACC. Each medication was categorized into various classes based on the classification from the USP Therapeutic Categories Model Guidelines (USP Medicare Model Guidelines v7.0. Available from http://www.usp.org/sites/default/files/usp/document/our-work/healthcare-quality-safety/uspmmg_v7_0_cat-class.pdf. Accessed September 12, 2017). Individual components were categorized into separate classes for drug products containing two or more active ingredients. In this study, we evaluated various analgesic regimens (opioid, non-opioid, and combination therapy) and antidepressants for their impact on MDASI symptoms. For this analysis, bupropion was categorized as a smoking cessation agent rather than antidepressant because of lung cancer patients.
Data analysis
Descriptive statistics of patients’ demographics (gender and race) and tumor characteristics (stages, histopathology and tumor location) were presented for overall and each lung cancer subtypes (NSCLC or SCLC). Median and interquartile range (IQR) were reported for continuous variable, whereas frequency and percentage were reported for categorical variables. Chi-square tests were used to compare the demographics (age and sex), cancer stages (advanced vs. early), and proportion of patients taking various analgesics or antidepressants categorized based on the symptom scores. A Mann-Whitney U test was conducted to compare the median/IQR of MDASI symptoms (ordinal variable). All analyses were two-sided and were carried out using SAS® 9.3, Cary, NC, USA.
Results
Patient and tumor characteristics
The patient and tumor characteristics are summarized in Table 1. One thousand five hundred and three patients with lung cancer were seen during the study period and 941 had no prior treatment. A total of 459 patients diagnosed with either NSCLC (N = 429 (93.5%)) and SCLC (N = 30 (6.5%)) met the inclusion criteria and were evaluated in this analysis (Table 1).
Medications
A complete list of therapeutic categories of medications is provided in Supplementary Table 1. The following medications were taken by at least 15% of lung cancer patients: Dyslipidemics (38.1%), vitamins (37.3%), renin-angiotensin-aldosterone system inhibitors (34.3%), electrolyte/mineral/metal modifiers and replacements (29.6%), opioid analgesics (27.2%), beta-adrenergic blocking agents (25.5%), acetaminophen (23.5%), sympathomimetic bronchodilators (23.3%), non-steroidal anti-inflammatory drugs (21.6%), diuretics (20.9%), proton pump inhibitors (20.0%), low-dose aspirin (20.0%), thyroid agents (18.1%), benzodiazepines (17.6%), antidepressants (15.7%), other gastrointestinal agents (15.5%), antihistamines (15.5%), and inhaled corticosteroids (15.0%).
Use of analgesics and its association with pain and related symptom scores
Among 459 lung cancer patients, about half were on some type of analgesic therapy, with about one-fourth of them taking opioid-containing regimens (opioids only or combination of opioids and non-opioids) (Table 2). One-third of patients with moderate-severe pain (5 or above on a 0–10 scale) were not on any analgesic therapies. Patients younger than 65 years of age and those with advanced-stage lung cancer had more moderate-severe pain. There was no significant difference in mild versus moderate-severe pain based on gender. Type of analgesic therapy did not have any association with age, gender, or stage of cancer (Supplementary Table 2).
Significantly worse pain scores were reported by patients taking only opioids (median score: 5) or combination of opioids and non-opioids (median score: 6) compared with those not taking any analgesics (median score; 0) (p < 0.0001, Mann-Whitney U test) (Table 3). Median pain scores were not significantly different in patients taking only non-opioid analgesics (median score; 1) compared with those not on any analgesic therapy (Table 3). Furthermore, moderate-severe pain (vs. mild pain) was reported by a higher proportion of patients taking only opioids (13% vs. 4%, p < 0.0001) or combination of opioids and non-opioids (40% vs. 11%, p < 0.0001) (Table 2). Patients on opioid-containing pain regimens also reported worse scores for drowsiness, fatigue, nausea/vomiting, disturbed sleep, shortness of breath, lack of appetite, dry mouth, distress, enjoyment of life, relations with others, walking, general activity, work, quality of life, and physical well-being (p < 0.001 for Mann-Whitney U median test) (Table 3). In addition, four MDASI symptoms (sadness, numbness/tingling, mood, and emotional well-being) had worse scores only in patients taking combination of opioids and non-opioids and not in those on opioids only regimens likely due to larger sample size in the combination group (Table 3).
Use of antidepressants and its association with depression-related symptom scores
About one-fifth of treatment-naïve lung cancer patients were on antidepressants (Table 4) with most taking serotonin or norepinephrine reuptake inhibitors. More than two-thirds of patients with a composite depression score of ≥ 35 were not on antidepressants (Table 4). A composite depression score of ≥ 35 was reported more by younger patients and those with advanced-stage disease. There was no significant difference in composite scores of ≥ 35 versus < 35 based on gender. Females were more likely to be on antidepressants compared with male gender (Supplementary Table 3). Age and stage of cancer did not have any association with the use of antidepressants. The median scores for any MDASI symptoms including the individual depression-related symptoms (enjoyment of life, mood, disturbed sleep, lack of appetite, emotional well-being, sadness, and relations with others) were not significantly different between patients taking antidepressants compared with those not on the therapy (Table 5).
Discussion
In this study, we describe the supportive medications used by treatment-naïve lung cancer patients at baseline and assess the association between their use and PROs. We report that one-third of patients with moderate-severe pain scores (5 or above) were not on any analgesic therapy, and over two-thirds of patients with composite depression scores (35 or above) were not on any antidepressants. Furthermore, patients on opioid-containing analgesic therapy for pain had significantly worse scores for pain and 15 out of 22 PROs compared with those on no pain medications.
Our study highlights pain as a major symptom burden of treatment-naïve lung cancer patients that is poorly managed in community practice before the patients are referred to MDACC. First, one-third of patients with moderate-severe pain scores were not taking any analgesic therapy. In addition, one-fifth of patients with moderate-to-severe pain were taking only non-opioid regimens despite opioids being considered as a first-line approach in this setting [25]. Since pain impacts daily living as well as QOL of patients, undertreated pain is associated with higher severity of multiple other cancer-related symptoms. The relationship between pain and mood as well as activities of daily living has been well-recognized [26,27,28]. The NCCN guidelines on Adult Cancer Pain state that the goals of pain management are to optimize pain relief, activities of daily living, and mood while minimizing adverse effects and avoiding addiction [25]. This is emphasized by our study finding that the group of patients with worse pain scores also had worse scores for most other symptoms related to daily living, mood, and QOL. The inadequate pain control in treatment-naïve lung cancer patients may be a result of deficient knowledge of pain management among community providers and stigma associated with pain among patients as well as providers [29,30,31,32,33]. Opioid overdoses and death rates associated with opioid abuse have increased substantially in the past few years. The fear of drug abuse in patients may contribute to prescribers’ reservations for using opioids in some cases [34, 35]. There is also a lack of consensus among prescribers on how to use opioids for pain in non-cancer patients, emphasizing the need to educate community providers about these issues [36].
The pain scores along with the scores for drowsiness and nausea/vomiting were higher in patients taking opioid-containing regimen, suggesting inadequate pain control as well as undesired adverse effects. An optimal analgesic treatment in cancer patients with chronic pain should typically include regularly scheduled analgesics for persistent pain control along with supplemental doses of analgesics to manage breakthrough pain [25]. The analgesic regimen needs to be designed based on this principle with proper understanding of pharmacology of opioids as well as dose conversions for many different opioid agents with varying doses and dosage forms available in the market to achieve patient-specific goals for comfort and function for optimal pain management [37, 38].
Our findings are based on retrospective analysis of the data and need to be confirmed in prospective studies. In addition, the cross-sectional analysis conducted in this study limits the evaluation of changes to the supportive care regimen made by the clinical oncology team. Other limitations include assessment of medications for only nociceptive and not neuropathic pain (such as tricyclic antidepressants and gabapentin) due to multiple indications of these agents, and the reliability of electronic medical records as a source of current medication. A future prospective longitudinal study that includes patient interviews to determine the reason for using each medications and recording of the current medications cross-checked by pharmacy records may allow more comprehensive assessment of supportive care medications. In our study, we were also not able to assess the appropriateness of the analgesic therapy without additional clinical data to determine the site and etiology of pain. While pain is likely due to cancer itself, coincidental non-cancer pain may also add to the symptom burden. Consideration of non-cancer comorbidities, such as arthritis, neuropathy, fibromyalgia, trauma/injury, or migraine, may also help in assessing appropriateness of therapy as well as impact of the medications on symptoms. In this study, we did not evaluate patients’ comorbidities that can influence PROs. However, future prospective studies should evaluate the impact of comprehensive clinical assessment and interventions on pain and other symptoms to maximize the utility of PROs in clinical management of cancer patients.
While the major emphasis of this study was on pain management, we also found that only 15% of patients with higher composite score for depression-related symptoms were on antidepressants. However, patients taking antidepressants did not significantly differ in any individual MDASI symptoms compared with patients not on antidepressants. These results may suggest that antidepressants, when properly used, are effective in managing the depression-related symptoms. However, these findings need to be confirmed in the prospective studies. Our findings that more female compared with male patients were on antidepressants are also consistent with other reports [39]. A comprehensive evaluation of depressive symptoms using PHQ-2 and PHQ-9 is a common practice to screen for depression [40]. However, the “sadness” symptom from MDASI has been suggested as a useful initial screen for assessing depression [24]. Future studies to incorporate a pre-screening approach for cancer patients may improve the efficiency and acceptability of such a simple tool for clinicians and patients.
In conclusion, this is the first study to systematically evaluate and report the associations between supportive care medication and PROs in cancer patients prior to definitive cancer treatment. Our study highlights the importance of assessing the need for concomitant supportive care medication at the beginning of cancer therapy and suggests that symptom assessment and evaluation of the etiologies for higher symptom burden should be included in the initial plan for treatment selection. Our study also emphasizes the need for longitudinal and multi-institutional studies to evaluate the effectiveness and impact of supportive care medications on PROs over the course of cancer care. Screening for pain and depression and optimization of pain management in treatment-naïve lung cancer patients need attention of community providers as well as oncology clinicians. Since patients’ poor functional status may result in suboptimal cancer therapy, our results highlight a need for early symptom assessment and therapeutic interventions in these patients.
References
Basch E (2016) Missing patients’ symptoms in cancer care delivery--the importance of patient-reported outcomes. JAMA Oncol 2:433–434
Kluetz PG, Slagle A, Papadopoulos EJ, Johnson LL, Donoghue M, Kwitkowski VE, Chen WH, Sridhara R, Farrell AT, Keegan P, Kim G, Pazdur R (2016) Focusing on core patient-reported outcomes in cancer clinical trials: symptomatic adverse events, physical function, and disease-related symptoms. Clin Cancer Res 22:1553–1558
Atkinson TM, Ryan SJ, Bennett AV, Stover AM, Saracino RM, Rogak LJ, Jewell ST, Matsoukas K, Li Y, Basch E (2016) The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer 24:3669–3676
Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris MG, Scher HI, Schrag D (2006) Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol 7:903–909
Basch E, Jia X, Heller G, Barz A, Sit L, Fruscione M, Appawu M, Iasonos A, Atkinson T, Goldfarb S, Culkin A, Kris MG, Schrag D (2009) Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst 101:1624–1632
Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, Mendoza TR, Hay J, Atkinson TM, Abernethy AP, Bruner DW, Cleeland CS, Sloan JA, Chilukuri R, Baumgartner P, Denicoff A, St. Germain D, O'Mara AM, Chen A, Kelaghan J, Bennett AV, Sit L, Rogak L, Barz A, Paul DB, Schrag D (2014) Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 106:dju244
Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM (2004) How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the quality-of-life questionnaire C30. J Clin Oncol 22:3485–3490
Iyer S, Roughley A, Rider A, Taylor-Stokes G (2014) The symptom burden of non-small cell lung cancer in the USA: a real-world cross-sectional study. Support Care Cancer 22:181–187
Lou VW, Chen EJ, Jian H et al (2017) Respiratory symptoms, sleep, and quality of life in patients with advanced lung cancer. J Pain Symptom Manag 53:250–256 e251
Lemonnier I, Baumann C, Jolly D, Arveux P, Woronoff-Lemsi MC, Velten M, Guillemin F (2011) Solitary pulmonary nodules: consequences for patient quality of life. Qual Life Res 20:101–109
Lheureux M, Raherison C, Vernejoux JM, Nguyen L, Nocent C, Tunon de Lara M, Taytard A (2004) Quality of life in lung cancer: does disclosure of the diagnosis have an impact? Lung Cancer 43:175–182
Montazeri A, Milroy R, Hole D, McEwen J, Gillis CR (2001) Quality of life in lung cancer patients: as an important prognostic factor. Lung Cancer 31:233–240
Blanco R, Maestu I, de la Torre MG, Cassinello A, Nuñez I (2015) A review of the management of elderly patients with non-small-cell lung cancer. Ann Oncol 26:451–463
Repetto L, Balducci L (2002) A case for geriatric oncology. Lancet Oncol 3:289–297
Cleeland CS, Allen JD, Roberts SA, Brell JM, Giralt SA, Khakoo AY, Kirch RA, Kwitkowski VE, Liao Z, Skillings J (2012) Reducing the toxicity of cancer therapy: recognizing needs, taking action. Nat Rev Clin Oncol 9:471–478
Yates JW (2001) Comorbidity considerations in geriatric oncology research. CA Cancer J Clin 51:329–336
Basch E (2013) Toward patient-centered drug development in oncology. N Engl J Med 369:397–400
Tirodkar MA, Acciavatti N, Roth LM, Stovall E, Nasso SF, Sprandio J, Tofani S, Lowry M, Friedberg MW, Smith-McLallen A, Chanin J, Scholle SH (2015) Lessons from early implementation of a patient-centered care model in oncology. J Oncol Pract 11:456–461
Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, Engstrom MC (2000) Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 89:1634–1646
Giuliani ME, Milne RA, Puts M et al (2016) The prevalence and nature of supportive care needs in lung cancer patients. Curr Oncol 23:258–265
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I (2015) The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 10:1243–1260
Cleeland CS, Zhao F, Chang VT, Sloan JA, O’Mara AM, Gilman PB, Weiss M, Mendoza TR, Lee JW, Fisch MJ (2013) The symptom burden of cancer: evidence for a core set of cancer-related and treatment-related symptoms from the eastern cooperative oncology group symptom outcomes and practice patterns study. Cancer 119:4333–4340
Mendoza TR, Wang XS, Lu C, Palos GR, Liao Z, Mobley GM, Kapoor S, Cleeland CS (2011) Measuring the symptom burden of lung cancer: the validity and utility of the lung cancer module of the M. D. Anderson Symptom Inventory. Oncologist 16:217–227
Jones D, Vichaya EG, Cleeland CS, Cohen L, Thekdi SM, Wang XS, Fisch MJ (2014) Screening for depressed mood in patients with cancer using the MD Anderson Symptom Inventory: investigation of a practical approach for the oncologist. J Oncol Pract 10:e95–e102
National Comprehensive Cancer Network. Adult cancer pain (version 1.2018). https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf. Accessed April 11
Cleeland CS, Nakamura Y, Mendoza TR, Edwards KR, Douglas J, Serlin RC (1996) Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain 67:267–273
Geisser ME, Cano A, Foran H (2006) Psychometric properties of the mood and anxiety symptom questionnaire in patients with chronic pain. Clin J Pain 22:1–9
Shacham S, Dar R, Cleeland CS (1984) The relationship of mood state to the severity of clinical pain. Pain 18:187–197
Collier R (2018) “Complainers, malingerers and drug-seekers” - the stigma of living with chronic pain. CMAJ 190:E204–E205
Jamison RN, Sheehan KA, Scanlan E et al (2014) Beliefs and attitudes about opioid prescribing and chronic pain management: survey of primary care providers. J Opioid Manag 10:375–382
Kanouse AB, Compton P (2015) The epidemic of prescription opioid abuse, the subsequent rising prevalence of heroin use, and the federal response. J Pain Palliat Care Pharmacother 29:102–114
Spitz A, Moore AA, Papaleontiou M, Granieri E, Turner BJ, Reid MC (2011) Primary care providers’ perspective on prescribing opioids to older adults with chronic non-cancer pain: a qualitative study. BMC Geriatr 11:35
Waugh OC, Byrne DG, Nicholas MK (2014) Internalized stigma in people living with chronic pain. J Pain 15:550 e551–550 e510
Centers for Disease C, Prevention (2011) Vital signs: overdoses of prescription opioid pain relievers---United States, 1999--2008. MMWR Morb Mortal Wkly Rep 60:1487–1492
Reuben DB, Alvanzo AA, Ashikaga T et al (2015) National Institutes of Health pathways to prevention workshop: the role of opioids in the treatment of chronic pain. Ann Intern Med 162:295–300
Paulozzi LJ, Mack KA, Hockenberry JM et al (2014) Vital signs: variation among states in prescribing of opioid pain relievers and benzodiazepines - United States. 2012 MMWR Morb Mortal Wkly Rep 63:563–568
Besson JM The complexity of physiopharmacologic aspects of pain(1997) La complexité des aspects physiopharmacologiques de la douleur. Drugs 53(Suppl 2):1–9
Peppin JF, Cheatle MD, Kirsh KL, McCarberg BH (2015) The complexity model: a novel approach to improve chronic pain care. Pain Med 16:653–666
Janberidze E, Hjermstad MJ, Brunelli C, Loge JH, Lie HC, Kaasa S, Knudsen AK, on behalf of EURO IMPACT (2014) The use of antidepressants in patients with advanced cancer--results from an international multicentre study. Psychooncology 23:1096–1102
Mitchell AJ, Yadegarfar M, Gill J, Stubbs B (2016) Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic meta-analysis of 40 studies. BJPsych Open 2:127–138
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 32 kb)
Rights and permissions
About this article
Cite this article
Hoang, J.M., Upadhyay, N., Dike, D.N. et al. Patient-reported outcomes in light of supportive medications in treatment-naïve lung cancer patients. Support Care Cancer 28, 1809–1816 (2020). https://doi.org/10.1007/s00520-019-05004-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-05004-8