Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a prevalent and clinically relevant side effect of chemotherapy. The symptoms diminish patients’ quality of life and represent a decisive limiting factor for medical therapy. To date, effective treatment options are lacking. Specific exercise interventions have proven promising to target relevant symptoms. We conducted a prospective, four-armed, randomized, controlled trial, to evaluate the effects of sensorimotor training (SMT) and whole-body vibration training (WBV) on patients with CIPN. Participants (N = 40) were randomized to either one of two intervention groups (SMT N = 10 or WBV N = 10) or oncological control group (N = 10) and matched by gender and age with a healthy control (N = 10). The intervention groups exercised twice a week for 6 weeks. Primary endpoint was the reduction of CIPN-related symptoms (improve peripheral deep sensitivity, Achilles tendon reflex (ASR) and patellar tendon reflex (PSR), light-touch perception, sense of position, and lower leg strength). Secondary endpoints were nerve conduction velocity and amplitude, balance control, quality of life, and CIPN-related pain. Patients exercising improved sensory and associated motor symptoms. Significant intergroup differences were found for the tendon reflexes (ASR P = .017 and PSR P = .020), peripheral deep sensitivity (P = .010), and pain (P = .043). Furthermore, tendencies were found regarding the subjective improvement of symptoms (P = .075) and two subscales of the EORTC-QLQ-C30 questionnaire: pain (P = .054) and dyspnea (P = .054). The results for the SMT group were superior regarding the tendon reflexes, and a tendency regarding the subjective report of symptoms, while WBV was superior regarding pain. SMT and WBV behold a large potential to reduce CIPN-related symptoms and can be considered feasible and safe for patients with CIPN (compliance 97.5%, no adverse events).
Registration: DRKS00013027
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a highly prevalent and clinically relevant side effect of cancer treatment. According to a recent review, 68.1% of patients suffer from CIPN by the first month after completion of chemotherapy [1].
CIPN is induced by neurotoxic agents (e.g., platinum derivatives, vinca alkaloids, taxanes) [2]. Peripheral nerves are especially sensitive to these toxins. Damage caused to these fibers leads to various sensory and motor dysfunctions such as loss of sensation, apparent as numbness, tingling or burning, dysesthesia, reduced or absent Achilles tendon reflexes [3, 4], pain, and loss of balance control [5], leading to unstable gait, as well as an increased incidence of accidents and falls [6, 7]. Not only do patients have to deal with the debilitating side effects, but CIPN has become a decisive limiting factor for medical therapy, causing treatment delays, dose reductions, or even discontinuation of therapy, affecting the outcome and compromise survival [8]. To date, there is no agreement for the treatment of CIPN, as evidence for the effectiveness of currently circulating supportive measures such as vitamin E or high-dose vitamin B [9], electrolyte infusions (Ca/Mg), or electrotherapy in patients with neuropathies is lacking. Even neuroprotective treatments such as amifostine, nerve growth factors, or corticosteroids are not well evaluated or failed to demonstrate beneficial effects in clinical trials [2]. To the contrary, many have additional negative side effects [2]. In this line, the latest guidelines from the ASCO even suggest to refrain from CIPN-preventive medication [10]. Solely, pain can be targeted to some extent with duloxetine [10].
Promising results have recently been achieved with specific exercise interventions. In a first clinical trial by Streckmann et al. [11], an exercise intervention consisting of endurance, strength, and sensorimotor training (SMT) was conducted twice a week for 36 weeks, accompanying lymphoma patients from diagnosis to completion of treatment. The study revealed a significant reduction of neuropathic symptoms. Patients exercising were able to reduce CIPN-related symptoms (e.g., peripheral deep sensitivity) by 87%, while in the control group, no change (0%) was detected. After 36 weeks, 55% of the control group still had symptoms related to CIPN compared to 4% in the intervention group. Further, Streckmann et al. conducted a systematic review [12], evaluating all exercise intervention studies for neuropathic patients, independent of the derivation. It was found that for toxically induced neuropathies such as CIPN, balance exercises were most beneficial for motor as well as sensory symptoms. Studies with strength training alone or in combination with endurance showed little to no improvements. This strengthened the presumption that SMT plays a decisive role as studies in healthy adults have revealed that SMT is characterized by functional adaptations of the neuromuscular system [13], regeneration of neuromuscular structures [14], and the diminished prevalence of injuries [15], leading to improved proprioception [13], intramuscular coordination, and balance control; causing fewer falls [16]; and increasing mobility. It therefore has the potential to counteract some relevant side effects of PNP. In line with this hypothesis, Schwenk et al. [17] performed a sensor-based balance training with N = 22 older CIPN patients and were able to show motor improvements (balance: sway of hip, ankle, and center of mass). Thus, the type of exercise is essential and training parameters also play a role as the neuromuscular system adapts specifically and progressively to the initial training volume and the applied training intensity. Within this study, we therefore isolated SMT in order to investigate its effects on the relevant symptoms of CIPN.
Targeting similar mechanisms as SMT, we also considered whole-body vibration [WBV] as a further potential training modality. Previous studies investigating WBV have shown a positive impact on parameters which can be affected by neuropathies: It has been shown that the elderly improve their gait [18] and that WBV has a positive impact on pain reduction [19] and deconditioned skeletal muscle [20], improved isometric strength [19], improved postural sway [21], and reduced fall frequency [22]. In a recent study by Schönsteiner et al. [23], N = 131 CIPN patients benefited from a multimodal exercise program containing WBV. Patients experienced less symptoms and pain (P < .001) and improved in the chair-rise test. In order to investigate the properties of WBV for CIPN patients, it is essential to solely investigate WBV as well as focus on targeting the sensory symptoms.
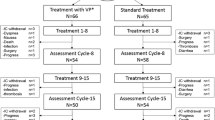
SMT and WBV require very little time and effort, but potentially have high impact. Especially for cancer patients, this aspect plays an important role, as patients are usually severely compromised after completion of medical therapy. We therefore conducted a randomized, controlled, pilot study assessing cancer patients with neurologically confirmed CIPN who were randomized to either SMT (N = 10) or WBV (N = 10) or a control group with no intervention (N = 10) and matched by age and gender with a healthy control (N = 10).
Patients and methods
Our prospective, single-center, four-armed RCT was approved by the ethics committee of the German Sport University. All patients gave written informed consent in accordance with the Declaration of Helsinki.
Patient characteristics
Fifty-seven patients in the aftercare of the University Hospital of Cologne, aged ≥ 18 years and self-reported symptoms of CIPN (since 1–5 years), reacted to the advertisements and were screened for exclusion criteria: peripheral neuropathy of other derivation, contraindications for WBV (osteosynthesis, unstable osteolysis, fracture of the lower extremity in the past 2 years, foot ulcers, acute thrombosis). Fifty-two primarily eligible patients underwent neurophysiological assessment to neurologically confirm the CIPN (nerve conduction velocity < 40 m/s, amplitude < 5 mV/μV: sural nerve/tibial nerve). Thirty eligible patients were randomized into either one of the intervention groups (SMT or WBV) or the control group. Due to neurophysiological deterioration from 60 years onward, and lacking references, an age- and gender-matched healthy control group (N = 10) was also recruited. All patients were recruited from the oncological training site in the University Hospital of Cologne. They had previously performed endurance and strength training (standardized protocol, equipment-based circuit, twice a week for 45 min at moderate intensity) for the past 6 months to 2 years with no self-reported effect on the neuropathy.
Training interventions
Training was performed for 6 weeks, twice a week, supervised by a qualified and trained personnel.
Sensorimotor training consisted of progressively more difficult balance exercises on progressively unstable surfaces. Each patient performed four exercises per session following a standardized protocol. Each exercise was performed three times for 20 s, with a rest of 40 s between each set and 1 min between each exercise, to avoid neuronal fatigue.
Vibration training was performed on a side-alternating vibration platform (Milbration, Milon™). Each training session consisted of four progressing sets of 30-s to 1-min vibration exercises, with a frequency from 18 to 35 Hz and amplitude of 2–4 mm. One-minute rest was kept between exercises to avoid neuronal fatigue. Patients were asked to stand on the platform in tight anti-slip socks or gymnastic shoes with thin soles on their forefoot (or if too unstable, with an 80/20% distribution).
Measurements
All assessments were carried out by blinded, qualified examiners, twice: pre (T0) and post (T1) the 6-week intervention.
Since CIPN comprises an entire symptom pattern, it is not possible to assess it with only one objective parameter. The primary endpoint of the study was therefore the standardized clinical test battery, for the objective assessment of CIPN-induced symptoms. It contains five parameters:
-
1.
Achilles and patella tendon reflexes were assessed symmetrically with a reflex hammer and rated on a 3-point scale (1 = agile, 2 = weak, 3 = missing).
-
2.
Peripheral deep sensitivity was evaluated with a Rydel-Seiffer tuning fork (128 Hz) on a graduating scale from 0 (no sensitivity) to 8 (highest sensitivity). Pathological values are 0–5 for patients < 60 years and 0–4 for patients ≥ 60 years [24]. The measurement was carried out bilaterally, at the first and fifth toes and the malleolus medialis.
-
3.
Light-touch perception was investigated by symmetrically stroking the outsides of the patients’ legs and feet in order to detect reduced or altered sensation (symmetrical or impaired) [25].
-
4.
Sense of position was determined by the investigator changing the position of the first toe while the patient remained with eyes closed. The examiner classified the perception to 1 = position recognized, 2 = only position of knee recognized, and 3 = no recognition.
-
5.
Lower leg strength (knee extension and ankle dorsiflexion) was assessed by requesting the patient to actively move their legs against the resistance of the examiner’s arm. The examiner then graded the strength on a 6-point scale (0 = no activity to 5 = normal force).
Secondary endpoints
Patient-reported reduction of CIPN-related symptoms
In accordance with related pharmaceutical studies, we assessed the patient-reported outcome for neuropathic symptoms with the FACT GOG-Ntx. This questionnaire has been validated and contains 11 items assessing the extent of PNP symptoms—from “not at all” to “very much” [22].
Nerve conduction velocity and amplitude
We further assessed compound muscle action potentials (CMAP), distal motor latency, and conduction velocity from the tibial nerve. The tibial nerve was stimulated at the ankle and popliteal fosse. Antidromic sensory nerve conduction studies were performed in the sural nerve. Sensory nerve action potentials (SNAPs) were recorded from the lateral malleolus with surface electrodes. Skin temperature was monitored and maintained above 32 °C.
Balance control was assessed on a force plate (Leonardo, Novotec, Pforzheim, Germany). Each stance (mono- and bipedal) was performed three times for 20 s with a 20-s rest between exercises. The cumulative sway path (cm) of all sets was averaged to minimize any learning effect. Measurements were regarded as failed attempts, whenever patients needed support to maintain balance. These were also assessed.
Questionnaires
Health-related quality of life was assessed via the EORTC-QLQ-C30 questionnaire. In addition to a scale for “global quality of life,” the questionnaire contains five functional scales (physical, emotional, social, and cognitive functions, and role function), three symptoms scales (fatigue, pain, nausea/vomiting), and single-item scales of respiratory distress, insomnia, loss of appetite, constipation, diarrhea, and financial problems. The questionnaire is internationally regarded as reliable [26].
Neuropathic pain was evaluated with the Pain-DETECT, a validated questionnaire which consists of 12 questions regarding the intensity, progression, and distribution of neuropathic pain. The questions are answered on a Likert scale ranging from “not at all” to “very much,” which accumulates in a total score reflecting the neuropathic pain status [27].
Sample size
Sample size calculation was conducted for potential interaction between-subjects factor group and within-subjects factor time-point indicating different changes over time between groups. An effect of f = .3 which corresponds to a medium effect following Cohen’s classification [28] was used for this study’s sample size calculation. Type II error rate (β) was set at β = .2 and, accordingly, test power as 1 − β was set at .8. Type I error rate and level of significance level (α), respectively, were set at α = .05. Under the presuppositions made, sample size calculation revealed that 30 participants should be included in the study equally distributed to the three groups.
Randomization
Randomization was performed by an independent, blinded person using an adaptive biased-coin randomization method (urn randomization) (e.g., [29]).
Statistical analysis
Potential baseline differences between treatment groups were investigated using separate one-way analyses of variance (ANOVAs). Parametric statistical models’ assumption of normality was explored for all variables through histograms and normality plots and confirmed with Kolmogorov-Smirnov tests. ANOVA assumption of homogenous variances for between-subjects factor levels was tested using Levene test and, in the case of inhomogeneous variances, F-test was adjusted using Brown-Forsythe F (FBF). Partial eta-square (η2p) values are reported as effect size estimates. Potential baseline differences regarding distribution of gender were investigated using separate Fisher’s exact tests.
To determine the effect of treatment on dependent measures, pre- to post-assessment difference values (delta values) were calculated at first. Delta values were always calculated by subtracting baseline values from post-assessment values. Accordingly, delta values represent mean of change in participants’ values from t0 to t1. To look for statistically significant differences in changes from t0 to t1 between groups for dependent measures, separate ANOVAs were applied. Assumptions were tested as stated above and violation of homogeneity of variances between-subjects factor levels was corrected using FBF.
In the case of significant symptomatic improvements from t0 to t1 in the sensorimotor/vibration group compared to the oncologic control group, training group patients’ data at t1 was compared to data of the healthy control group to look for still persisting differences in symptoms compared to a healthy norm.
For all inferential statistical analyses, significance was defined as P value less than .05. All descriptive and inferential statistical analyses were conducted using SPSS 22® (IBM®, Armonk, NY, USA). Two-tailed probability tests were used throughout all inferential statistical testing. Test power calculations were conducted using free of charge available statistical test power computation software GPower 3 [30].
Results
Overall, 30 patients and 10 age- and gender-matched healthy controls were assessed (N = 40). Patient characteristics at baseline (Table 1) revealed no significant intergroup differences (P = .851) (Table 2 in ESM).
Average compliance for all time points and interventions was 97.5%. We had one dropout in the control group due to progress of the disease, making in-hospital treatment necessary. No adverse events occurred.
Primary endpoint
A significant intergroup difference was detected for the (a) Achilles tendon reflex F(2,26) = 4.791; (b) the patellar tendon reflex F(2,25) = 4.564, P = .020, P = .017; and (c) peripheral deep sensitivity (malleolus medialis) F(2,25) = 5.548, P = .010 (Fig. 1).
Intergroup results of the Achilles tendon reflex (ASR) (a), the patella tendon reflex (PSR) (b), and peripheral deep sensitivity (c). Diagrams show boxes of the 25th to 75th percentile with whiskers from minimum to maximum. Horizontal line marks the median and means are indicated as “+.” Significances are given as *P = .050–.010, **P = .009–.001, and ***P < .001
Secondary endpoints
We found significant intergroup changes in the Pain-DETECT score F(2,25) = 3.575, P = .043 as well as tendencies regarding the subjective improvement of symptoms (FACT) F(2,26) = 2.874, P = .075 as well as in two subscales of the EORTC-QLQ-C30 questionnaire: pain score (EORTC-QLQ-C30) F(2,25) = 3.278, P = .054 and dyspnoea score (EORTC-QLQ-C30) F(2,25) = 3.294, P = .054.
Taking a look at the two interventions separately
In comparison to the CG and the respective second intervention, we found that the SMT group showed significantly higher effects regarding the improvement of the patella tendon reflexes (SMT vs CG P = .020, d = 1.514) and Achilles tendon reflex (SMT vs CG P = .042, d = 0.978/SMT vs WBV P = .036, d = 0.978) as well as a tendency regarding the subjective improvement of neuropathic symptoms (FACT score) (SMT vs CG P = .096, d = 1.079), while we found a tendency for the WBV group regarding the subscale of the EORTC-QLQ-C30 for pain (WBV vs CG, P = .056, d = 0.955) and the Pain-DETECT (WBV vs CG, P = .076, d = 0.938). The CG had one failed attempt more on average in balance exercises than the intervention groups.
In comparison to age- and gender-matched healthy adults (HG)
CIPN patients differed significantly in peripheral deep sensitivity (P = .007), pain (P = .004), and the tendon reflexes (P = .033) in comparison to age- and gender-matched healthy controls at baseline, though not at T1. They were therefore able to reach a “normal value” for their age group after the intervention, in comparison to the oncological CG.
No significant differences were found for all other parameters (nerve conduction velocity or amplitude (N. tibialis/N. suralis) perception of touch, sense of position, balance control, and the other subscales of the EORTC-QLQ-C30 questionnaire).
Discussion
Consistent with our hypothesis, patients experienced improvements in sensory and associated motor symptoms, which is promising as it could behold a treatment option. Especially the sensory symptoms, such as loss of sensibility, absent reflexes, or pain for instance, are clinically highly relevant. They are not only debilitating but also responsible for treatment alterations. To date, they have neither been targeted by pharmacological nor by other supportive measures, besides our previous RCT in lymphoma patients.
In line with these findings, the properties of SMT and the hypothesis generated from a systematic review: that balance exercises seem crucial to target motor and sensory symptoms of CIPN, our hypothesis is confirmed. SMT has the potential to improve symptoms of CIPN. Additionally, WBV also has the potential to target symptoms of CIPN.
Interestingly though, both interventions seem to behold different sensory qualities, targeting slightly different symptoms. WBV appears to target the more superficial nerves such as pain receptors for instance, while SMT appears to activate deeper structures. It seems necessary to challenge the body to maintain or reactivate neuromuscular functions such as sensitivity, light-touch perception, or reflexes, in the sense that the body in its complexity will only regard as essential and worth maintaining what is stimulated sufficiently. This may also explain why supportive measures that do not activate those structures may not have the desired effect. Possibly, we will even have to differ as to which sensory qualities we wish to address and in future also investigate the potential of combining both interventions.
Although associated motor symptoms such as balance or leg power are also very debilitating, they are rarely the cause for treatment alterations. Winters-Stone et al. [7], for instance, showed that CIPN had a functional impact on cancer survivors’ gait (slower and shorter steps), caused more disability and a 1.8 times higher risk of falling.
Associated dysfunctions such as absent tendon reflexes, impaired dorsiflexion, and loss of sensitivity are crucial parameters for balance and gait. Therefore, the improvement of these parameters could potentially contribute to reduce disability and falls.
The underlying mechanisms must still be elucidated. One possibility could lie in the regenerative effect of SMT on nerve fibers. A further possibility is attributed to the nervous system’s plasticity: (i) an increase in the density of receptors, (ii) activating deafferented neurons [31] by increasing the metabolism, (iii) lowering the threshold for excitability [32], or (iv) inducing supraspinal learning effects [33]. Due to the short time period between intervention and observed improvement, learning effects mediated by neurotransmitter activation/ modulation appear to be a particular likely explanation as underlying mechanism.
WBV presumably exerts mechanostimulation in the periphery, targeting the axon terminals and inducing the release of local mediators in the peripheral tissue that can influence the receptors/stimulating the nerve endings in the periphery.
Our finding that SMT alters stretch reflex responses and peripheral deep sensitivity can be explained by regeneration of muscle spindles, sensory afferents (Ia, Ib), or both. Preclinical models have demonstrated that physical exercise can prevent degeneration and improve nerve regeneration in animals which is associated with increased release of neurotrophins [34, 35].
We can furthermore confirm that both interventions can be considered feasible and safe for patients with CIPN.
Regarding the other secondary endpoints, possibly, the intervention was not long enough or the assessments not sensitive enough to detect changes in such short time. Alterations to the nerve, apparent in nerve conduction studies for instance, may require more time. Analyzing the screening procedure, 52 patients were primarily eligible and had self-reported symptoms, though only 30 proved eligible after electrophysiology, it is questionable whether this state of the art assessment method, is the most appropriate. Possibly first symptoms of CIPN are on accord of small-fiber neuropathies, not detectable by standard electrodiagnostic. Potential changes on this level due to the exercises may then also remain undiscovered. In this case, we may need alternative assessment methods and it may possibly be sufficient to solely use the short clinical test battery for short, low-intensity exercise interventions such as this one, in order to reduce assessment time for patients.
Furthermore, we saw a tendency for the subjective report of CIPN-related symptoms. We assume it did not reach significance due to the sample size as we also evaluated a visual analogue scale, asking patients on a scale from 0 (worst decline) to 10 (highest possible improvement), whether they felt the exercises had helped them and their symptoms had improved. All patients, except for one from the WBV group, indicated an improvement (5 to 8.2 and an average of 6.66). In the SMT group, the average improvement was 6.75 (range 5–8.2) and in the WBV group 6.53 (range 2.4–8.1).
The strength of our trial includes being the first RCT investigating specific exercise interventions for patients with CIPN of diverse entities that are feasible and show a meaningful outcome, also on sensory symptoms of CIPN. Furthermore, regarding the translation into practice and daily life, they are feasible with low intensity though high impact, ideal for oncological patients in all phases of therapy. Potentially, we have found a feasible treatment option for CIPN, though larger RCT studies and further reference values are necessary to verify this.
This study also has limitations. Due to the small sample size in each group, individuals weigh higher and achieving statistically significant results is more difficult. Furthermore, study design was challenging as tasks had to be feasible for patients exhibiting very different performance levels. Regarding the balance exercises for instance, this resulted in additional failed attempts rather than high sway paths. Though the endurance and strength training all patients had previously performed was equipment based and followed a standardized protocol, a potential contributing effect cannot be outruled. The assessment of neuropathic pain with the Pain-DETECT questionnaire also proved difficult, as patients often do not experience the discomfort as pain, resulting in missing data. Furthermore, even though patients were told the questionnaire was solely related to their CIPN, we found that some nevertheless indicated pain related to arthritis or similar. In future, we will use a visual analogue scale in accordance with related medication studies. Furthermore, EORTC-QLQ-C-30 may not be sensitive enough for this very short type and intensity of intervention. Many of the health-related questions such as health status or financial problems will most likely still be relevant 6 weeks later for an oncological patient.
In summary, we provide evidence that SMT and WBV behold a potential to treat relevant symptoms of CIPN with no further side effects, are feasible and safe for patients with CIPN, and should therefore be taken seriously as a possible measure in supportive therapy to reduce symptoms of CIPN. The results are of clinical relevance as the sensory symptoms are decisive limiting factors for patients to receive their planed therapy regimen, optimizing cancer control. Further research with a larger sample size is necessary.
References
Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M (2014) Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 155:2461–2470
Wonders KY, Reigle BS, Drury DG (2010) Treatment strategies for chemotherapy-induced peripheral neuropathy: potential role of exercise. Oncol Rev 4:117–125
Pietrangeli A, Leandri M, Terzoli E, Jandolo B, Garufi C (2006) Persistence of high-dose oxaliplatin-induced neuropathy at long-term follow-up. Eur Neurol 56:13–16
Lehky TJ, Leonard GD, Wilson RH, Grem JL, Floeter MK (2004) Oxaliplatin-induced neurotoxicity: acute hyperexcitability and chronic neuropathy. Muscle Nerve 29:387–392
Kneis S, Wehrle A, Freyler K et al (2015) Balance impairments and neuromuscular changes in breast cancer patients with chemotherapy-induced peripheral neuropathy. Clin Neurophysiol. (2):1481–1490
Kolb NA, Smith AG, Singleton JR, Beck SL, Stoddard GJ, Brown S, Mooney K (2016) The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol 73:860–866
Winters-Stone KM, Horak F, Jacobs PG, Trubowitz P, Dieckmann NF, Stoyles S, Faithfull S (2017) Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol 35:2604–2612
Stubblefield MD, Burstein HJ, Burton AW et al (2009) NCCN Task Force report: management of neuropathy in cancer. J Natl Compr Canc Netw 7(Suppl 5):S1–S26 quiz S27–28
Ang CD, Alviar MJ, Dans AL et al (2008) Vitamin B for treating peripheral neuropathy. Cochrane Database Syst Rev; CD004573
Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL (2014) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32:1941–1967
Streckmann F, Kneis S, Leifert JA, Baumann FT, Kleber M, Ihorst G, Herich L, Grüssinger V, Gollhofer A, Bertz H (2014a) Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol 25:493–499
Streckmann F, Zopf EM, Lehmann HC, May K, Rizza J, Zimmer P, Gollhofer A, Bloch W, Baumann FT (2014b) Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports Med 44:1289–1304
Gruber M, Gruber SB, Taube W et al (2007) Differential effects of ballistic versus sensorimotor training on rate of force development and neural activation in humans. J Strength Cond Res 21:274–282
Freeman MA, Dean MR, Hanham IW (1965) The etiology and prevention of functional instability of the foot. J Bone Joint Surg. British volume; 47: 678–685
Verhagen E, van der Beek A, Twisk J, Bouter L, Bahr R, van Mechelen W (2004) The effect of a proprioceptive balance board training program for the prevention of ankle sprains: a prospective controlled trial. Am J Sports Med 32:1385–1393
Granacher U, Gollhofer A, Strass D (2006) Training induced adaptations in characteristics of postural reflexes in elderly men. Gait Posture 24:459–466
Schwenk M, Grewal GS, Holloway D, Muchna A, Garland L, Najafi B (2016) Interactive sensor-based balance training in older cancer patients with chemotherapy-induced peripheral neuropathy: a randomized controlled trial. Gerontology 62:553–563
Kawanabe K, Kawashima A, Sashimoto I, Takeda T, Sato Y, Iwamoto J (2007) Effect of whole-body vibration exercise and muscle strengthening, balance, and walking exercises on walking ability in the elderly. Keio J Med 56:28–33
Rittweger J (2010) Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol 108:877–904
Blottner D, Salanova M, Puttmann B et al (2006) Human skeletal muscle structure and function preserved by vibration muscle exercise following 55 days of bed rest. Eur J Appl Physiol 97:261–271
Spiliopoulou SI, Amiridis IG, Tsigganos G, Economides D, Kellis E (2010) Vibration effects on static balance and strength. Int J Sports Med 31:610–616
Bogaerts A, Delecluse C, Boonen S, Claessens AL, Milisen K, Verschueren SMP (2011) Changes in balance, functional performance and fall risk following whole body vibration training and vitamin D supplementation in institutionalized elderly women. A 6 month randomized controlled trial. Gait Posture 33:466–472
Schonsteiner SS, Bauder Missbach H, Benner A et al (2017) A randomized exploratory phase 2 study in patients with chemotherapy-related peripheral neuropathy evaluating whole-body vibration training as adjunct to an integrated program including massage, passive mobilization and physical exercises. Exp Hematol Oncol 6:5
Oyer DS, Saxon D, Shah A (2007) Quantitative assessment of diabetic peripheral neuropathy with use of the clanging tuning fork test. Endocr Pract 13:5–10
Cochrane DJ (2011) Vibration exercise: the potential benefits. Int J Sports Med 32:75–99
Aaronson NKAS, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB dHJ, Kaasa S, Klee MC, Osoba D, Razavi D, Rofe PB, Schraub S, SM SKCA, Takeda F (1993) The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Bennett MI, Attal N, Backonja MM, Baron R, Bouhassira D, Freynhagen R, Scholz J, Tölle TR, Wittchen HU, Jensen TS (2007) Using screening tools to identify neuropathic pain. Pain 127:199–203
Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences, Erlbaum, Mahwah, NJ, USA
Wei LJ (1978) The adaptive biased coin design for sequential experiments. The Annals of Statistics; 6(1): 92–100
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods; 39:175–191
Gollhofer A (2003) Proprioceptive training: considerations for strength and power production. In: P.V.K. (ed) Strenght and Power in Sport, 2nd edn. Blackwell Publishing, Oxford, pp 331–342
Sjostrom PJ, Rancz EA, Roth A, Hausser M (2008) Dendritic excitability and synaptic plasticity. Physiol Rev 88:769–840
Taube W, Gruber M, Gollhofer A (2008) Spinal and supraspinal adaptations associated with balance training and their functional relevance. Acta Physiol (Oxf) 193:101–116
Park JS, Kim S, Hoke A (2015) An exercise regimen prevents development paclitaxel induced peripheral neuropathy in a mouse model. J Peripher Nerv Syst 20:7–14
Park JS, Hoke A (2014) Treadmill exercise induced functional recovery after peripheral nerve repair is associated with increased levels of neurotrophic factors. PLoS One 9:e90245
Acknowledgements
We acknowledge the contributions of Julia Rizza and Kathrin May as well as the advisory support of Prof. Wolfgang Potthast and Dr. Angela Höhne regarding the balance assessment. We are furthermore grateful to the patients and especially the healthy matched participants for their support and volunteering.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Our prospective, single-center, four-armed RCT was approved by the ethics committee of the German Sport University. All patients gave written informed consent in accordance with the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Relevance/Novelty of manuscript
Chemotherapy-induced peripheral neuropathy (CIPN) is a highly prevalent and clinically relevant side effect of chemotherapy. The symptoms severely diminish patients’ quality of life and represent a decisive limiting factor for medical therapy, consequently affecting the clinical outcome. To date, effective treatment options are lacking. Specific exercise interventions have now proven promising to reduce sensory symptoms of CIPN, revealing a high potential as treatment option, thus impacting supportive therapy in oncology.
Electronic supplementary material
ESM 1
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Streckmann, F., Lehmann, H., Balke, M. et al. Sensorimotor training and whole-body vibration training have the potential to reduce motor and sensory symptoms of chemotherapy-induced peripheral neuropathy—a randomized controlled pilot trial. Support Care Cancer 27, 2471–2478 (2019). https://doi.org/10.1007/s00520-018-4531-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4531-4