Abstract
Purpose
Acute leukemia (AL) and its initial treatment can impair physical functioning and capacity significantly. Exercise as a countermeasure has been investigated in few studies confirming its feasibility and safety during intensive induction chemotherapy, but the relative effects of diverse exercise programs have not been analyzed. Therefore, we aimed to investigate independent effects of endurance and resistance training on physical capacity and quality of life (QOL).
Methods
Twenty-nine adult AL patients were randomly allocated to an endurance (EG), resistance (RG), or control (CG) group. The intervention took place during induction chemotherapy with three exercise sessions per week for 30–45 min each. Endurance capacity at individual anaerobic threshold, maximum knee extension and flexion strength, standardized phase angle (SPA), and QOL were measured at baseline prior to induction chemotherapy and before discharge.
Results
Endurance capacity changed in neither the EG, RG, or CG (P = 0.104); descriptively, the EG (− 0.05 W/kg) and RG (− 0.04 W/kg) exhibited a smaller decrease than CG (− 0.22 W/kg). We noted a significant difference in knee extension strength (P = 0.002); RG improved their maximum strength (+ 0.14 Nm/kg), while the EG’s (− 0.13 Nm/kg) and CG’s (− 0.19 Nm/kg) was significantly reduced. QOL and SPA revealed no change after the intervention.
Conclusions
We conclude that resistance training is a key component when exercising during induction chemotherapy: it improved maximum strength, but also influenced endurance capacity even during intensive treatment. Considering the prognostic value of physical function, we strongly propose integrating exercise, especially resistance-based training, already during induction chemotherapy to preserve AL patients’ physical capacity and functional status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute leukemias (AL) are hematological malignancies characterized by a dramatically fast-growing malignant transformation of hematopoietic stem cells affecting normal hematopoiesis. This alteration causes a wide range of symptoms (e.g., anemia, vulnerability to infections, fever, bleeding, nausea, weight loss, and fatigue) which affect most patients already before diagnosis [1]. Curative treatment requires several cycles of chemotherapy, initiated as soon as possible mainly via intensive induction chemotherapy with lengthy hospitalization lasting 4 to 8 weeks. During this time, aplasia and further treatment-related side effects (e.g., nausea, vomiting, appetite loss, diarrhea, fatigue, toxicity) as well as circumstances-induced inactivity increase the risk of physical deconditioning and impair functional performance. Moreover, psychological stress (e.g., anxiety, depression) reinforces this negative impact and influences health-related quality of life (QOL) directly [2]. Furthermore, the most common type of leukemia in adults is acute myeloid leukemia, with more than a half of diagnoses made in patients above 65 years [3]. Old age is generally associated with a reduced functional status and a higher risk of comorbidity inducing subsequent physical and psychosocial impairments. There are strong indications that a higher physical performance level enabled patients to tolerate more the intensive or repetitive chemotherapies mandatory within a curative approach, thus enhancing their chance for recovery [4].
Physical exercise is known to be an effective strategy to counteract physical and psychosocial deterioration in cancer patients during active treatment [5]. Recent reviews about exercising in hematological malignancies survivors [6,7,8] have confirmed feasibility and safety even during most intensive chemotherapy. Most of those studies focused on endurance exercise, whereas resistance exercises have seldom been assessed. Although it would seem obvious that preventing physical deconditioning requires early interventions, only six studies included patients with acute leukemia during induction chemotherapy [9,10,11,12,13,14]. As already mentioned, this is a very sensitive setting because of the therapeutic burden and urgent need for clinical intervention immediately after diagnosis. The fact that there are so few such trials may be due to both this setting’s organizational challenges and sensitivity. Recent exercise programs included walking only [14] or a multimodal approach containing aerobic, resistance, and flexibility exercises [9,10,11,12,13]. As all those trials focused primarily on feasibility and safety, they also reported mostly weak, but positive effects on functional performance, muscular endurance capacity, fatigue, distress, and health-related QOL [6, 10]. Differentiated analyses of the effects of diverse exercise programs have not been done.
In general, one’s physical performance level depends on aerobic capacity and significantly on muscle strength. Both factors can considerably influence fatigue and functional performance—relevant for daily-living activities and QOL. Especially, resistance training is known to counteract the substantial loss of strength already during acute therapy [15]. However, the relative effect of resistance training in patients with acute leukemia must still be examined. We therefore conducted this randomized pilot study to investigate independent effects of endurance and resistance training on physical capacity and QOL in patients with acute leukemia during induction chemotherapy. We hypothesized that both exercise regimes would be feasible and superior to usual care.
Patients and methods
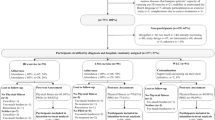
Twenty-nine adult patients diagnosed with AL and before induction chemotherapy were enrolled in this study between June 2010 and February 2013 at the Department of Medicine I, Medical Center—University of Freiburg. Exclusion criteria were Karnofsky performance status < 60, uncontrolled hypertension, cardiac illness (NYHA III-IV), instable bone metastases, or lack of informed consent after screening. All patients gave written informed consent for the study protocol and data collection; this prospective, three-armed, open randomized controlled pilot study (ration 1:1:1) was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Commission of the University of Freiburg, Germany.
Outcome measurements
Demographic and anthropometric data as well as clinical information were documented at baseline. All measurements were taken twice: at baseline prior to induction chemotherapy (PRE) and after leukocyte regeneration prior to discharge (POST) at the Institute for Exercise- and Occupational Medicine, Medical Center.
Endurance capacity was assessed via an incremental exercise test on a cycle ergometer (Ergoline 900, Bitz, Germany) in recumbent position. The exercise test started with a workload of 25 W and increased by 25 W every 3 min. Heart rate (HR) and lactate concentration were determined at rest and after the end of each exercise level. Based on these values submaximum (individual anaerobic threshold (IAT) [16]) and maximum performance levels (Watt/kg, HR) were identified.
Isokinetic measurements (CON-TREX MJ, CMV, Duebendorf, Switzerland) were used to determine maximum voluntary knee extension and flexion strength. Patients did a submaximum warm-up before testing to familiarize themselves with the device and the testing protocol: 5 maximum concentric contractions at 60°/s. Examiners provided standardized encouragement to achieve best possible values (Nm/kg). Highest values for extension and flexion were used for subsequent analyses; values from the right and left leg were pooled as both sides’ results revealed similar trends.
As a factor to diagnose malnutrition, each patient’s standardized phase angle (SPA; °) was measured using a single-frequency bioelectrical impedance analysis at 50 kHz. The phase angle represents one of the raw data obtained from this measurement and is proven to be a good prognostic marker in various diseases including cancer [17,18,19,20]. By transforming the measured phase angle values into standardized values (SPA = ((phase angle-phase angleref)/SDref)) and then calculating age-, sex-, and BMI-independent values, heterogeneous groups can be compared [21].
QOL (%) was assessed by analyzing health-related QOL and the five functional subscales of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ-C30) [22]. Patients were also asked to fill out the Freiburg Questionnaire on physical activity [23] at baseline to determine their physical activity level before hospitalization.
Exercise intervention
Patients were randomly allocated to an endurance (EG), resistance (RG), or control (CG) group. The exercise intervention took place throughout hospitalization and during induction chemotherapy with three sessions per week lasting 30–45 min each. Training sessions were conducted and documented by a certified sports therapist. Training protocols were based on previous studies and exercise guidelines for cancer patients [5, 13, 24, 25].
Patients in the EG underwent individually supervised aerobic endurance training performed on an upright stationary bicycle (ECB Fitness Ergometer, Tunturi, Almere, Netherlands) or a treadmill (quasar 2.0, HP Cosmos, Traunstein, Germany) according to their preference. If continuous method for 30 min was impossible, we applied the interval method to achieve at least 30 min of exercise. Intensity was defined at 60–70% of maximum capacity in HR (and the corresponding Watts) based on their individual exercise test results. Since medication induces HR alterations and patients’ individual physical condition can change daily, the rated perceived exertion (RPE) scale [26] supported the intensity control within endurance training (12–14 on 6–20 rating scale).
Patients in the RG performed individually supervised resistance exercises for the major muscle groups, including bodyweight exercises (e.g., squats, sit-ups) and exercises with small devices (e.g., dumbbells, elastic bands). In case of good physical condition and adequate blood values to leave the ward, patients exercised on resistance machines (leg press, abdominal trainer, rope pull). Our prescribed training protocol included 4–6 varying exercises; intensity, sets and repetitions were adapted using the RPE scale [26] with a target score between 12 and 14.
The CG’s control intervention included a low-intensity mobilization and stretching program primarily to avoid psychosocial bias.
All patients received standard clinical care including regular nutrition counseling by experienced dieticians and physiotherapists. To ensure patients’ safety, blood values and temperature were monitored prior to every training session. Patients omitted exercising when platelets < 10 × 109/L, hemoglobin < 4.96 mmol/L, temperature > 38 °C, acute petechiae, or bleedings occurred or in case of the physician’s advice to avoid exercise. Platelets between 10 × 109/L and 20 × 109/L implied an intensity reduction in resistance exercise. Additionally, blood pressure and HR were controlled during each training session. Adverse events and compliance were monitored and documented throughout the study: adverse events were analyzed by counts, whereas compliance was calculated in percent ((completed training sessions/planned training sessions) ×100).
Sample size and randomization
As there were no previous trials to rely on to establish a sample size, we sized our sample according to recruitment and infrastructural possibilities at participating institutes or departments. We assumed 12 patients per group would be an acceptable number of patients for a pilot study [27]. Owing to low recruitment, our recruitment process was stopped after 2.5 years and 29 enrolled patients. Random patient allocation was based on a computer-generated blocked randomization list with a block size of six. Due to the character of exercise interventions, “blinding” the patients or sports therapists about allocation was impossible. During assessments, examiners were not blinded, but trained in standardized testing procedures.
Statistics
Baseline characteristics are presented as the median and upper and lower quantile for continuous variables and counts (n, %) for categorical variables. All variables were included in non-parametric analysis as the assumption of normal distribution (Shapiro-Wilk test) was not satisfied. Differences within groups were calculated by Wilcoxon signed-rank test. Changes over time were compared among groups by Kruskal-Wallis one-way analysis of variance. In case of significant differences among the three groups, the Mann-Whitney U Test was used for pair-wise comparisons. To estimate the treatment effect, the point estimate and 95% confidence interval of the Hodges-Lehmann’s median differences for paired groups were used. All data is presented as median and upper-lower quartile. A P value < 0.05 was considered statistically significant. Analyses were done using SPSS statistic version 22.0 and figures were generated by GraphPad prism version 5.
Results
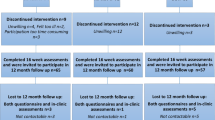
After informing 39 patients about the study, a total of 29 patients were randomly assigned to the EG (n = 10), RG (n = 9), or CG (n = 10), corresponding to an 85% recruitment rate. Overall dropout rate was 24%, and 22 patients underwent PRE and POST assessments; their data served for analysis (Fig. 1). Reasons for prematurely terminating study participation were mental overload (n = 3), change in diagnosis (n = 2), persistent thrombocytopenia < 10/nl (n = 1), or death (n = 1). None of these reasons were associated with the exercise intervention.
Patient characteristics were similar among the groups (Table 1). Median compliance with the training sessions was 68.9%, 76.0%, and 60.0% for EG, RG, and CG, respectively (Table 2). Reasons for not exercising were medical exclusion, nausea/dizziness, fatigue, fever, low blood values, time conflicts, or refusal without a reason. No adverse events due to the study intervention occurred.
PRE and POST results are presented in Table 3. Numbers of patients included in the analyses are also indicated, since not all patients completed all tests due to clinical or organizational conflicts.
Endurance capacity represented by Watt/kg at the IAT changed in neither the intervention groups nor in the control group. We detected no group-by-time effect; descriptively, the EG and RG (EG − 0.05 W/kg; RG − 0.04 W/kg) showed a smaller decrease in IAT than the CG (− 0.22 W/kg). Yet, it must be noted that only 14 of 22 patients could reach IAT pre and post treatment, meaning that this analysis excluded very weak patients especially. Maximum performance (Watt/kg) during cycling exercise test did not change significantly over time, and no intergroup changes were detected.
We observed a significant difference in the thigh musculature’s strength capacity (Nm/kg) in both movement directions—knee extension (P = 0.002) and knee flexion (P = 0.009). Post hoc analysis revealed significant differences between the RG versus the EG and CG. The RG maintained (extension + 0.14 Nm/kg, P = 0.209) or increased strength values (flexion + 0.15 Nm/kg, P = 0.008), while the EG and CG exhibited a significant decrease in extension (EG − 0.13 Nm/kg, P = 0.019; CG − 0.19 Nm/kg P = 0.003) and no change in flexion strength (EG − 0.02 Nm/kg, P = 0.701; CG − 0.12 Nm/kg P = 0.117). Data on maximum extension strength is illustrated in Fig. 2.
Health-related QOL revealed no significant change at POST (Table 3); descriptively, it improved in all three study groups between PRE and POST (median difference across all patients + 8.3%; (CI − 12.5–20.8)). Except for the emotional functioning scale, where all groups improved over time (EG + 14.6% P = 0.012; RG + 41.7% P = 0.043; CG without statistical significance + 10.4% P = 0.128), the remaining scales (physical, role, cognitive, and social functioning) revealed no change.
Statistical analyses revealed no significant differences in the SPA in either intragroup or intergroup analyses. However, RG patients improved their SPA values descriptively, whereas the EG’s and CG’s declined.
Discussion
This randomized pilot study aimed to investigate the feasibility and independent effects of endurance and resistance exercise on physical capacity and QOL in patients with acute leukemia undergoing induction chemotherapy. In line with our hypothesis, both exercise regimes proved to be feasible, safe, and superior to the CG due to positive effects on the maintenance of physical capacity. Impact on overall QOL was inconsistent, but revealed a non-significant tendency to improvement in all three study groups.
Former studies during induction chemotherapy have included endurance exercise only [14] or a multimodal program containing aerobic and resistance exercise as well as flexibility exercise [10,11,12,13]. All five studies detected improvements in various self-reported outcomes and objective physical measures. The largest (n = 81) randomized controlled exercise trial during induction chemotherapy published recently also included a multimodal exercise program [10]. However, the independent effect of endurance or resistance training was not evaluated in this particular clinical setting. Although the present pilot study included relatively few patients, we have been able to show that exercise and in particular resistance training play a key role in avoiding a substantial deterioration in physical capacity. The RG maintained their level of performance in most outcome measures, while individual maximum strength levels even rose. Accordingly, RG showed, albeit not significantly, an improvement in their SPA, which is an indicator for cell membrane function [28] and indirectly for muscle function [29]. These findings reinforce the importance of exercising as a supportive treatment during induction chemotherapy, and resistance training should be an essential component within the exercise regime. Especially, muscular performance is a fundamental aspect of mobility and autonomy for everyday life, highlighting the need to initiate an exercise program as soon as possible. Concerning clinical relevance, patients’ functional status affects therapeutic decision-making, can alter therapeutic options [30], and is a major predictor of survival [31]. This underlines the importance of ongoing exercising throughout the entire treatment process and the need to implement physical exercise as an immediate element of supportive care. However, long-term effects on health-related outcomes and survival due to exercise interventions during IC or during the entire treatment are still unknown and should be investigated in further trials. We will not expect prominent long-term effects following 4–8 weeks of moderate exercise during IC. The objective should rather be to avoid a detrimental loss of physical and psychosocial function in the first weeks of medical treatment. Furthermore, it is well known that those patients additionally suffer from inadequate nutritional intake, increased protein catabolism, and decreased protein synthesis [32, 33], causing intensified muscle loss. Thus, combining exercising with a well-scheduled dietary intervention during leukemia treatment may optimize patients’ physical function.
During this study, 24% of patients dropped out. Our dropout rate was thus higher than the 16% or 18% reported by Alibhai et al. [10, 11], for instance. As the diagnoses of two patients changed after allocation, this dropout rate could be adjusted. We also noted that our patients’ compliance with a mean of nine training sessions throughout hospitalization during induction chemotherapy (39 ± 12 days) resembles that in the study by Alibhai et al. [11], who reported a mean of eight training sessions throughout hospitalization (37 ± 10 days). However, Alibhai et al. [11] reported a lower percentage of supervised exercise days with 46%. During the present study, patients performed 68.3% of three planned training sessions per week. Our results therefore confirm our exercise program’s feasibility. However, these findings require qualification: our prescribed exercise program could not be fulfilled during each training session because of the very intensive therapy and its side effects. As patients’ physical conditions and blood values varied during therapy, the prescribed exercise protocol had to be adjusted daily, including the intensity, number of sets, and repetitions. Thus, the predefined individual training recommendations based on exercise tests can only serve as orientation. During the intervention period, we strove for progression, but due to our patients’ varying therapy tolerance, a rigorous training regime was impossible to maintain.
How should an individual training prescription in such a variable setting be determined? To set the intensity for resistance exercises, we referred to the RPE scale to adjust the exercise program accordingly at each session. However, a common method to define and control the intensity for an appropriate resistance training program is the one-repetition maximum test (1RM) or the hypothetic 1RM based on a multiple repetition maximum test [34] to quantify strength. Those tests, however, are only realizable on strength machines and do not apply to exercises with the small devices used primarily in this pilot study. Furthermore, isokinetic measurements to determine maximum voluntary knee extension and flexion strength are not practicable to determine training prescriptions because they only cover one isolated movement of the lower limb. Therefore, we assume that using a subjective scale like the RPE [26] is legitimate in this specific setting.
The intensity of endurance training seems much easier to prescribe when using data from an incremental exercise test. Maximum values (e.g., HRmax) or HR reserve (HRR) and the particular percentages are commonly used for intensity and training prescription. However, recommendations for cancer patients [5] are adopted from healthy individuals. Kuehl et al. [35] evaluated these exercise intensity classifications in hematological cancer patients before and after allogeneic hematopoietic stem cell transplantation, and showed that the measured values (%HRR, % V̇O2max, %HRmax) differ significantly from the recommended values. This therefore influences the training stimulus in terms of not meeting the targeted intensity class. Kuehl et al. [35] thus provided exercise intensity classifications for cancer patients during intensive treatment that can be used to define more appropriate exercise prescriptions in future exercise trials. Individual training zones (HR and/or Watt) can also be defined by relying on the lactate performance curve [36] and IAT [16]. For this analysis, a minimum of four lactate values are required [37]. Unfortunately, only 14 of 22 patients achieved IAT at PRE and POST in the present study due to a demanding protocol (25 W steps every 3 min). Therefore, individual training prescription based on IAT was not valid for every patient in this study. Consequently, the use of an alternative testing protocol is suggested to ensure the collection of enough lactate values to ensure a thorough performance analysis [38] ideally in combination with spiroergometry. This and possible alterations due to medication and physical weakness in this special setting should be investigated in future trials. Another aspect influencing results is the position in which the test is carried out. Weak and patients at risk often assume a recumbent position to avoid echocardiographic artifacts, but that position negatively influences cycling performances—an aspect to keep in mind when comparing results with other investigations.
The QOL data reveals a non-significant increase during the intervention period in all three study groups. The frightening diagnosis immediately prior to our baseline assessments probably influenced those results. The process of coping with the disease, and regularly scheduled psychological counseling during hospitalization might improve the QOL score post-intervention. In particular, we found that emotional functioning improved significantly—reflecting the disease’s emotional burden especially right after diagnosis. One might assume that exercising is what influenced this trend, since the EG and RG revealed significant improvements. However, direct associations with endurance or resistance training are unclear, because the CG improved also, although not significantly due to their higher values already at baseline. Nevertheless, the intervention’s psychosocial aspects might have had a positive effect on QOL results.
The present pilot study provides data demonstrating the importance of physical exercise in supportive care of patients with acute leukemia. Obviously, there are a lot of open questions regarding the optimal exercise prescription (e.g., modes, duration, frequency, and intensity), the effects on hard outcome parameters, and thus the dose-response effect in this special setting. Investigations with larger sample sizes and randomized controlled designs are therefore needed to confirm these initial results. Nevertheless, in view of the prognostic value of physical function, we strongly recommend integrating physical exercise as supportive therapy already during induction chemotherapy. Especially, resistance-based training is a key component that must be considered in exercise programs for AL patients to preserve their physical capacity and functional status. We conclude that this pilot trial yielded initial and fundamental findings on endurance and resistance training during induction chemotherapy, and provides impulses for future investigations.
References
Deschler B, Ihorst G, Platzbecker U, Germing U, Marz E, de Figuerido M, Fritzsche K, Haas P, Salih HR, Giagounidis A, Selleslag D, Labar B, de Witte T, Wijermans P, Lubbert M (2013) Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica 98:208–216. https://doi.org/10.3324/haematol.2012.067892
Redaelli A, Stephens JM, Brandt S, Botteman MF, Pashos CL (2004) Short- and long-term effects of acute myeloid leukemia on patient health-related quality of life. Cancer Treat Rev 30:103–117. https://doi.org/10.1016/S0305-7372(03)00142-7
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66:271–289. https://doi.org/10.3322/caac.21349
Klepin HD (2016) Definition of unfit for standard acute myeloid leukemia therapy. Curr Hematol Malig Rep 11:537–544. https://doi.org/10.1007/s11899-016-0348-8
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen V, Schwartz AL, American College of Sports Medicine (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42:1409–1426. https://doi.org/10.1249/MSS.0b013e3181e0c112
Smith-Turchyn J, Richardson J (2015) A systematic review on the use of exercise interventions for individuals with myeloid leukemia. Support Care Cancer 23:2435–2446. https://doi.org/10.1007/s00520-015-2752-3
Bergenthal N, Will A, Streckmann F et al (2014) Aerobic physical exercise for adult patients with haematological malignancies. Cochrane Database Syst Rev (11):CD009075
Battaglini CL (2011) Physical activity and hematological cancer survivorship. Recent Results Cancer Res 186:275–304. https://doi.org/10.1007/978-3-642-04231-7_12
Bryant AL, Deal AM, Battaglini CL, Phillips B, Pergolotti M, Coffman E, Foster MC, Wood WA, Bailey C, Hackney AC, Mayer DK, Muss HB, Reeve BB (2017) The effects of exercise on patient-reported outcomes and performance-based physical function in adults with acute leukemia undergoing induction therapy: exercise and quality of life in acute leukemia (EQUAL). Integr Cancer Ther 17:263–270. https://doi.org/10.1177/1534735417699881
Alibhai SMH, Durbano S, Breunis H, Brandwein JM, Timilshina N, Tomlinson GA, Oh PI, Culos-Reed SN (2015) A phase II exercise randomized controlled trial for patients with acute myeloid leukemia undergoing induction chemotherapy. Leuk Res 39:1178–1186. https://doi.org/10.1016/j.leukres.2015.08.012
Alibhai SMH, O’Neill S, Fisher-Schlombs K, Breunis H, Brandwein JM, Timilshina N, Tomlinson GA, Klepin HD, Culos-Reed SN (2012) A clinical trial of supervised exercise for adult inpatients with acute myeloid leukemia (AML) undergoing induction chemotherapy. Leuk Res 36:1255–1261. https://doi.org/10.1016/j.leukres.2012.05.016
Klepin HD, Danhauer SC, Tooze JA, Stott K, Daley K, Vishnevsky T, Powell BL, Mihalko SL (2011) Exercise for older adult inpatients with acute myelogenous leukemia: a pilot study. J Geriatr Oncol 2:11–17. https://doi.org/10.1016/j.jgo.2010.10.004
Battaglini CL, Hackney AC, Garcia R, Groff D, Evans E, Shea T (2009) The effects of an exercise program in leukemia patients. Integr Cancer Ther 8:130–138. https://doi.org/10.1177/1534735409334266
Chang P-H, Lai Y-H, Shun S-C, Lin LY, Chen ML, Yang Y, Tsai JC, Huang GS, Cheng SY (2008) Effects of a walking intervention on fatigue-related experiences of hospitalized acute myelogenous leukemia patients undergoing chemotherapy: a randomized controlled trial. J Pain Symptom Manag 35:524–534. https://doi.org/10.1016/j.jpainsymman.2007.06.013
Padilha CS, Marinello PC, Galvão DA, Newton RU, Borges FH, Frajacomo F, Deminice R (2017) Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta-analysis. J Cancer Surviv 11:339–349. https://doi.org/10.1007/s11764-016-0592-x
Coyle EF, Martin WH, Ehsani AA, Hagberg JM, Bloomfield SA, Sinacore DR, Holloszy JO (1983) Blood lactate threshold in some well-trained ischemic heart disease patients. J Appl Physiol 54:18–23
Gupta D, Lammersfeld CA, Burrows JL, Dahlk SL, Vashi PG, Grutsch JF, Hoffman S, Lis CG (2004) Bioelectrical impedance phase angle in clinical practice: implications for prognosis in advanced colorectal cancer. Am J Clin Nutr 80:1634–1638
Paiva SI, Borges LR, Halpern-Silveira D, Assunção MCF, Barros AJD, Gonzalez MC (2011) Standardized phase angle from bioelectrical impedance analysis as prognostic factor for survival in patients with cancer. Support Care Cancer 19:187–192
Schwenk A, Beisenherz A, Romer K et al (2000) Phase angle from bioelectrical impedance analysis remains an independent predictive marker in HIV-infected patients in the era of highly active antiretroviral treatment. Am J Clin Nutr 72:496–501
Urbain P, Birlinger J, Ihorst G, Biesalski HK, Finke J, Bertz H (2013) Body mass index and bioelectrical impedance phase angle as potentially modifiable nutritional markers are independent risk factors for outcome in allogeneic hematopoietic cell transplantation. Ann Hematol 92:111–119. https://doi.org/10.1007/s00277-012-1573-4
Bosy-Westphal A, Danielzik S, Dörhöfer R-P, Later W, Wiese S, Müller MJ (2006) Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. JPEN J Parenter Enteral Nutr 30:309–316. https://doi.org/10.1177/0148607106030004309
Fayers P, Bottomly A (2002) Quality of life research within the EORTC— the EORTC QLQ-C30. Eur J Cancer 38:125–133
Frey I, Berg A, Grathwohl D, Keul J (1999) Freiburger Fragebogen zur körperlichen Aktivität-Entwicklung, Prüfung und Anwendung. Soz- Präventivmedizin SPM 44:55–64. https://doi.org/10.1007/BF01667127
Dimeo F, Schwartz S, Wesel N, Voigt A, Thiel E (2008) Effects of an endurance and resistance exercise program on persistent cancer-related fatigue after treatment. Ann Oncol 19:1495–1499. https://doi.org/10.1093/annonc/mdn068
Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G (2005) Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. JClinOncol 23:3830–3842
Borg G (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Julious SA (2005) Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 4:287–291. https://doi.org/10.1002/pst.185
Barbosa-Silva MCG, Barros AJD (2005) Bioelectrical impedance analysis in clinical practice: a new perspective on its use beyond body composition equations. Curr Opin Clin Nutr Metab Care 8:311–317
Norman K, Stobäus N, Zocher D, Bosy-Westphal A, Szramek A, Scheufele R, Smoliner C, Pirlich M (2010) Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am J Clin Nutr 92:612–619. https://doi.org/10.3945/ajcn.2010.29215
Rao AV (2016) Fitness in the elderly: how to make decisions regarding acute myeloid leukemia induction. Hematol Am Soc Hematol Educ Program 2016:339–347. https://doi.org/10.1182/asheducation-2016.1.339
Wedding U, Röhrig B, Klippstein A, Fricke HJ, Sayer HG, Höffken K (2006) Impairment in functional status and survival in patients with acute myeloid leukaemia. J Cancer Res Clin Oncol 132:665–671. https://doi.org/10.1007/s00432-006-0115-7
Hickson RC, Marone JR (1993) Exercise and inhibition of glucocorticoid-induced muscle atrophy. Exerc Sport Sci Rev 21:135–167
LaPier TK (1997) Glucocorticoid-induced muscle atrophy. The role of exercise in treatment and prevention. J Cardiopulm Rehabil 17:76–84
Adamsen L, Quist M, Midtgaard J, Andersen C, Møller T, Knutsen L, Tveterås A, Rorth M (2006) The effect of a multidimensional exercise intervention on physical capacity, well-being and quality of life in cancer patients undergoing chemotherapy. Support Care Cancer 14:116–127
Kuehl R, Scharhag-Rosenberger F, Schommer K et al (2015) Exercise intensity classification in cancer patients undergoing allogeneic HCT. Med Sci Sports Exerc 47:889–895. https://doi.org/10.1249/MSS.0000000000000498
Faude O, Kindermann W, Meyer T (2009) Lactate threshold concepts: how valid are they? Sports Med Auckl NZ 39:469–490
Roecker K (2013) Die sportmedizinische Laktatdiagnostik: Technische Rahmenbedingungen und Einsatzbereiche. Dtsch Z Für Sportmed 64:367–371
Scharhag-Rosenberger F, Becker T, Streckmann F et al (2014) Studien zu körperlichem Training bei onkologischen Patienten: Empfehlungen zu den Erhebungsmethoden. Dtsch Z Für Sportmed 65:304–313
Acknowledgements
We thank all patients who participated in this study. We are grateful to Carole Cürten (English Copy Editor) for proofreading our manuscript and we thank Manfred Baumstark for statistical assistance.
Funding
This trial was supported by Baden-Württemberg foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All patients gave written informed consent for the study protocol and data collection; this pilot study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Commission of the University of Freiburg, Germany.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wehrle, A., Kneis, S., Dickhuth, HH. et al. Endurance and resistance training in patients with acute leukemia undergoing induction chemotherapy—a randomized pilot study. Support Care Cancer 27, 1071–1079 (2019). https://doi.org/10.1007/s00520-018-4396-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4396-6