Abstract
Purpose
Physical activity (PA) during and after cancer treatment is associated with improved cancer- and non-cancer-related outcomes. We assessed for predictors of change in PA levels among cancer survivors.
Methods
Adult cancer survivors from a comprehensive cancer center completed a one-time questionnaire retrospectively assessing PA levels before, during, and after cancer treatment along with their perceptions of PA. Multivariable logistic regression models evaluated the association of clinico-demographics variables and perceptions of PA with changes in whether patients were meeting PA guidelines after cancer diagnosis.
Results
Among the 1003 patients, 319 (32%) met moderate to vigorous PA (MVPA) guidelines before diagnosis. Among those meeting guidelines before diagnosis, 50% still met guidelines after treatment; 12% not meeting MVPA guidelines initially met them after treatment/at follow-up. Among patients meeting guidelines before diagnosis, better ECOG performance status at follow-up, receiving curative therapy, and spending a longer time on PA initially were each associated with meeting guidelines at follow-up. After controlling for other variables, perceiving that PA improves quality of life (adjusted odds ratio, aOR = 11.09, 95%CI [1.42–86.64], P = 0.02) and overall survival (aOR = 8.52, 95%CI [1.12–64.71], P = 0.04) was each associated with meeting MVPA guidelines during/after treatment, in patients who did not meet guidelines initially. Only 13% reported receiving counseling, which was not associated with PA levels. Common reported barriers to PA included fatigue, lacking motivation, and being too busy.
Conclusions
Patient perceptions of PA benefits are strongly associated with improving PA levels after a cancer diagnosis. Clinician counseling should focus on patient education and changing patient perceptions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Through advances in both early cancer detection and therapy improvements, cancer patients are now living longer; over 60% of patients are expected to live beyond 5 years after their cancer diagnosis [1, 2]. Thus, cancer care has expanded to include the post-treatment period of survivorship and secondary prevention. Compared to patients without cancer, many cancer survivors have persistent treatment-related side effects such as fatigue, the psychosocial sequelae of being diagnosed with cancer alongside other comorbidities including cardiovascular disease, diabetes, osteoporosis, functional decline, and increased risk of second primary cancers [1, 3].
A key aspect of cancer survivorship focuses on lifestyle behaviors (i.e., smoking cessation, dietary changes, alcohol moderation, and physical activity promotion) [1, 4]. Prior studies have demonstrated that regular physical activity (PA) among cancer survivors can reduce the risk of cancer recurrence, improve survival, help with treatment-related side effects, reduce the development of cardiovascular complications, and improve overall quality of life [4,5,6,7,8,9,10,11,12,13]. PA has also been found safe for cancer survivors in both the active treatment and post-treatment phases [11,12,13]. Optimal PA levels recommended in guidelines of the American Cancer Society and American College of Sports Medicine is 150 min of moderate to vigorous physical activity (MVPA) per week, even during the treatment phase of cancer [14, 15]. In addition, these guidelines also recommend that PA be spread throughout the week and be conducted in addition to one’s activities for daily living and that cancer survivors to limit sedentary behaviors.
Prior studies have investigated factors associated with PA among cancer survivors. Younger age, male gender, higher socioeconomic status, more social support, fewer disease-related symptoms, and improved perceptions of PA were each associated with higher levels of PA among cancer survivors [16,17,18,19,20]. Few studies have evaluated factors associated with changes in PA levels in cancer survivors. Of these, the following factors were found to be associated with changes in PA after a cancer diagnosis: age, occupation, education, body mass index (BMI), baseline PA levels, receiving specific cancer therapies (chemotherapy and radiation therapies), and participating in a rehabilitation program [21,22,23,24,25,26].
Using the health belief model (HBM) framework [27], patients are thought to be willing to adopt healthy behavior changes if (i) there is sufficient motivation, (ii) patients have a perceived threat of health-related sequelae, and (iii) patients believe that health recommendations can prevent or reduce that threat. By applying the HBM to studying PA changes in cancer survivors, we can use a cancer diagnosis as a “teachable moment” to motivate behavior change. To date, patient perceptions of PA and physician counseling on improving PA levels have not been well studied and it is unclear how much patient perceptions play a role and how often physicians are discussing the benefits of PA with patients.
Our study aims were to (i) measure self-reported PA levels both before and after a diagnosis of cancer in a large sample of cancer survivors; (ii) assess the perceptions in cancer survivors of the benefits of PA on three cancer survivorship domains (i.e., 5-year overall survival, quality of life, and fatigue) and perceptions of safety of PA; and (iii) determine if patient perceptions of PA were associated with change in PA level after a cancer diagnosis. We also explored (iv) if cancer patients received any counseling or information from their healthcare providers (family physician, oncologist) on PA and its association with change in PA levels; and (v) perceived barriers to engaging in PA among cancer survivors who were not meeting PA guidelines. These components will be important to inform how best to incorporate PA programs into cancer survivorship programs.
Patients and methods
Patient recruitment and data collection
Cancer survivors across diverse disease sites were recruited from May 2012 to April 2014 at a single tertiary cancer center, the Princess Margaret Cancer Centre, Toronto, Canada, where the study received institutional ethics approval. Patients aged 19 years or greater, with a histologically confirmed diagnosis of either a solid or hematological malignancy of all stages and treatment intents, were included in the study. Cognitive deficits and language barriers that limited patient understanding of the study or consenting process were exclusion criteria.
All consenting patients completed a one-time, self-administered and self-reported questionnaire at an ambulatory oncology clinic visit, which assessed sociodemographic information, PA levels, perceptions of PA, and functional status (as measured by the Eastern Cooperative Oncology Group (ECOG) performance score and a separate 5-point Likert scale from poor to excellent). Clinicopathological information (including date of diagnosis, stage, treatments received to date, and treatment intent) were obtained from medical chart review.
Given the diversity of cancer subtypes that were included in the study, all forms of systemic therapy were grouped together (chemotherapy, hormonal therapy, targeted agents, and stem cell therapy) and all forms of radiation therapy were grouped together (i.e., external beam, stereotactic beam radiotherapy, radioactive iodine, and brachytherapy). Patients diagnosed with cancer > 10 years prior to the study recruitment date were excluded from further analyses.
Physical activity level and patient perception assessments
Patients retrospectively reported their PA levels at 1 year before their cancer diagnosis (baseline), during treatment, and at follow-up using an adapted validated tool which assesses for and groups types of PA based on their intensity level [28]. Cancer patients were dichotomized based on whether they met regular PA level recommendations of at least 150 min of MVPA per week at each time point [14].
We did not find any previously validated instruments to assess patient perceptions of PA on patient survivorship outcomes. Hence, patient perceptions of the effect of PA on their own health in the three survivorship domains (quality of life, fatigue, and overall survival) were assessed using a 7-point Likert scale (1, makes much worse; 4, no effect; 7, makes much better).
After a mid-study protocol amendment, roughly half of patients also completed additional questions about perceived barriers to PA. These questions included whether or not they felt PA was safe during cancer treatment, what perceived barriers they had regarding being or becoming physically active, and the number of minutes of PA per week that they felt were the recommended PA guidelines [16, 29, 30]. Patients could select as many options as applicable to them from a list of barriers or provide free-text answers, which were then grouped by theme.
Patients were divided into two subgroups based on their baseline physical levels 1 year before diagnosis: those who met MVPA guidelines and those who did not. Within each subgroup, patients were further divided into those who continued to meet MVPA guidelines at follow-up and those who did not, which took into account transient reduction in levels expected during active cancer therapy. As one aim was to assess for factors influencing changes in PA levels after a cancer diagnosis and patients may limit their PA levels during active therapy due to treatment side effects, only patients who were beyond active treatment (i.e., those reporting not undergoing regular intravenous chemotherapy or radiation therapy and were not peri-operative) were included in that particular analysis.
Statistical analysis
Statistical analyses were completed using SAS 9.4 and R (http://CRAN.R-project.org). Descriptive statistics were provided, and comparisons were conducted using Fisher’s exact test, Pearson’s chi-square test, or Kruskal–Wallis test, where appropriate.
Univariable logistic regression models were applied to evaluate the effects of each primary predictor variable and covariates (sociodemographics, clinicopathological variables) for each PA level subgroup as described above. Multivariable logistic regression models were then applied to construct baseline models using backward selection algorithms of all covariates that showed a trend of association with change in PA level (P < 0.10). Each primary predictor variable, namely each perception variable and counseling variable, was then added to each model and its significance evaluated.
Results
Baseline patient characteristics and demographics
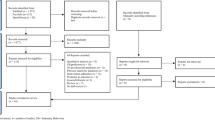
Of 2523 patients approached, 1747 were eligible and 1003 were ultimately recruited and completed the study questionnaire (effective response rate, 57%; Fig. 1). For baseline characteristics (Table 1), almost half were female, with a mean age of 54 years; the majority were married, were English-speaking, had completed at least some form of post-secondary education, worked white-collar jobs, and were relatively asymptomatic from their malignancy. Most patients had localized disease and received surgery and systemic therapy. Our study population represented a broad range of tumor subtypes including breast (16%), gastrointestinal (13%), genitourinary (10%), gynecologic (12%), head and neck (11%), hematologic (19%), lung (6%), and other (11%) cancers.
Physical activity at baseline
Of 1003, 318 (32%) met MVPA guidelines at baseline. When compared to those not meeting MVPA guidelines at baseline, those who met guidelines were more likely to be younger, employed, Caucasian, have completed some post-secondary education, and have better ECOG performance status, and were also more likely to perceive that PA can improve their own quality of life, fatigue, and overall survival (Table 1). There were also slight differences in the eventual treatments that patients received between the groups meeting and not meeting guidelines at baseline, with those meeting guidelines more likely to receive systemic therapy but less likely to receive radiation. The distribution of baseline minutes of PA per week for patients meeting and not meeting guidelines is shown in Fig. 2a, b.
Distributions of patient-reported minutes of moderate to vigorous physical activity (MVPA) per week for various questions. The distributed baseline MVPA minutes for patients: a not meeting MVPA guidelines at diagnosis and b meeting MVPA guidelines at diagnosis. The change in minutes of MVPA per week between baseline and follow-up for c patients not meeting MVPA guidelines at baseline and d patients meeting MVPA guidelines at baseline, whereby negative values in the figures represent a reduction of the minutes of physical activity per week from baseline. The distribution of patient responses to their perceived guideline requirements for the minutes of recommended physical activity per week for cancer survivors (e) and difference between patient’s perceived recommendation and actual minutes of physical activity per week they are currently performing; the red vertical line represents equivalence between perceived recommendation and actual minutes of physical activity (f). Negative values in the panel f represent values where patient’s actual level of physical activity performed was less than their perceived minutes recommended per week. Max maximum, Min minimum, MVPA moderate to vigorous physical activity, N number, Qu quartile, SD standard deviation
Changes in physical activity levels
Among patients who met guidelines at diagnosis (n = 319), 25% (80/319) of patients reported still meeting guidelines during treatment, and 50% (129/259) met guidelines at follow-up. Among patients who were not meeting MVPA guidelines at baseline (n = 685), 3% (22/685) reported improving their PA level to meet MVPA guidelines during treatment, and 8% (57/579) reported meeting guidelines at follow-up. Patients were excluded from the follow-up count if they were still undergoing active treatment. Overall, for those not meeting MVPA guidelines at baseline, only 12% (69/579) improved to meeting MVPA guidelines at any time point after their cancer diagnosis. Figure 2c, d shows the distribution in the absolute change in the minutes of PA stratified by meeting/not meeting MVPA guidelines.
Univariable and multivariable analysis results of significant covariates associated with change in PA level stratified by meeting/not meeting MVPA guidelines at baseline are shown in Supplementary Tables 1 and 2, respectively. For patients who met MVPA guidelines at baseline, patients with better ECOG performance status at follow-up (aOR = 5.56), those who were treated for cure (aOR = 5.00), and those who reporting exercising longer at baseline (aOR = 1.004 per minute) were more likely to keep meeting guidelines after cancer treatment. Among patients not meeting MVPA guidelines at baseline, those completing post-secondary studies (aOR = 2.34), or who had better self-rated health (aOR = 3.52) were more likely to improve to meet guidelines during their cancer care, while those who were older (aOR = 0.97 per year) and who spoke English at home (aOR = 0.39) were less likely to meet guidelines during follow-up.
Association between patient perceptions and physician counseling and changes in physical activity level
Table 2 summarizes the results of our primary predictor variables (patient perceptions and receiving counseling) on change in PA levels after diagnosis. Among all patients, about 90% perceived that PA improves quality of life and 5-year overall survival while 76% perceived that PA improves fatigue. Only 13% of patients recalled receiving counseling from their physicians. Among patients meeting MVPA guidelines at baseline, none of the primary predictors were associated with maintenance of PA after diagnosis, (P > 0.05). For patients not meeting MVPA guidelines before diagnosis, perceiving that PA improves quality of life (aOR = 11.09) and overall survival (aOR = 8.52) was individually associated with increased PA (to meet guidelines) after diagnosis. In addition, patients who perceived that PA is always safe were more likely (aOR = 2.57) to increase their PA levels to meet guidelines after diagnosis.
Cancer patient-perceived barriers towards physical activity
Supplementary Table 3 lists the common barriers our patients reported facing which prevented them from undergoing regular PA. Among all patients in general (n = 489) and those who were not currently meeting MVPA guidelines (n = 299), the top three reported barriers were (1) feeling too tired, (2) not having enough motivation, and (3) being too busy. Another potential barrier may be patient perceptions on what are the true recommended exercise guidelines. The distribution of responses to perceived recommended minutes of moderate to vigorous PA per week is shown in Fig. 2e. The median number of minutes estimated by patients was 120 (range 3–510). Figure 2f shows the distribution of the difference between the actual number of minutes of exercise performed by cancer patients and their perceived number of minutes recommended per week (where negative values are cases whereby the patient perceived the recommendation to be higher than the time they themselves actually exercised). In general, patients tended to exercise at MVPA levels about 50 min per week less than what they perceived as the recommended guidelines.
Discussion
Engaging in PA after a cancer diagnosis is associated with both improved quality of life and mortality among cancer survivors [7, 14]. We demonstrate that among patients not meeting MVPA guidelines prior to diagnosis, perceiving that PA improves quality and quantity of life was strongly associated with improving PA levels (to meet guidelines) after diagnosis. Among those meeting guidelines at diagnosis, patients who exercised more minutes at baseline, those undergoing curative treatments, and those who reported better ECOG performance status at follow-up were each associated with maintaining PA at follow-up. Regardless, patients rarely recalled receiving PA counseling from healthcare providers. The most common barriers to participating in PA by cancer survivors were feeling tired, lack of motivation, or time. Taken together, these results suggest that routine PA counseling should be integrated into cancer care, with patients being educated about the benefits of PA and being provided with practical exercise plans.
To date, studies evaluating factors associated with PA level among cancer survivors have focused on different isolated time points (diagnosis, after treatment, or during long-term follow-up), with the majority limited to breast and colorectal cancer survivors (and a few in other sites) [4, 29, 31, 32]. Multiple prior studies have evaluated factors associated with PA levels and changes in PA levels among cancer patients [17, 18, 20,21,22,23,24,25,26, 29, 33]. In comparison to existing studies, our study not only evaluated absolute changes in PA levels, but additionally compared levels to specific guideline recommendations and evaluated the impact of patient perceptions and counseling on behavior change.
The application of health behavior theories helps suggest solutions: using the HBM, cancer patients are motivated by their cancer diagnosis and can perceive a lack of PA as potentially harmful to their quality of life or overall survival [27]. Through proper counseling and education, we hypothesize that patients may adjust their behavior to improve their PA levels. Similarly, using the theory of reasoned action, cancer patients may improve their PA levels in response to them perceiving it to improve their quality of life/survival [34].
Maintaining good levels of PA after diagnosis was associated with being treated for cure, greater PA level (in min/week) prior to diagnosis, and better current ECOG performance status; each of which has predictive with future exercise levels in cancer and/or non-cancer populations [17, 29, 35,36,37,38,39]. Although being treated for cure may provide greater motivation than being palliated, there are multiple benefits of PA among those receiving palliative care [38, 39].
The peri-diagnosis window is a potential “teachable moment” for many lifestyle factors [3, 40], though there are always concerns about patients being overwhelmed during the initial diagnostic, staging, and early treatment phases. Our data suggests that this teachable moment is not fully taken advantage of, given that half of active patients are no longer active later, while only 12% of inactive patients become active as a cancer survivor. Although important factors include incorrect patient perceptions and/or lack of counseling [3, 41], other pragmatic factors include cancer symptoms and treatment toxicities [4, 42, 43]. Education tools should explain how even some toxicities (e.g., fatigue, arthralgia, and bone loss) may improve with PA and also educate patients that PA is safe during and after active cancer treatment [4, 11, 12, 37, 44,45,46].
Multiple barriers affecting the integration of a standard PA program into routine cancer care include cancer and treatment-related side effects (i.e., neuropathy, lymphedema, ostomies, fatigue) and lack of rehabilitation physiatrists, kinesiologists, or physiotherapists with cancer specialization [4]. Despite about half of clinicians agreeing with the benefits of PA in cancer patients, most are reluctant to take additional clinic time to discuss this and other lifestyle matters with their patients [4, 43, 47]; thus, PA screening in clinic is uncommon [48]. We thus identify the need to develop validated rapid screening and brief interventions (e.g., short but effective home-based exercise programs) teachable quickly in the ambulatory setting. The outpatient waiting room can be a fertile ground for testing such interventions [48].
There are limitations to our study. As this study is a one-time questionnaire, our data is subject to recall bias and biases of social desirability, which may lead patients to report their lifestyle behavior more favorably toward investigators. We tried to limit recall bias by limiting follow-up to 10 years post-diagnosis. Furthermore, most patients had short follow-up times with a median of 23 months; longer follow-up is required to determine if the changes in PA levels observed are persistent. Longitudinal collection of this data in future studies, including assessing patient perceptions at both baseline and follow-up, would help provide useful information for further studies. Our assessment of patient perceptions was exploratory in nature in this study and used single-item measures to assess perceptions in each domain of cancer survivorship; validation of their psychometric properties is warranted in future studies. Although many perceptions towards survivorship outcomes could be assessed, given the exploratory nature of the study, we chose to focus on these three outcomes which represent different areas of survivorship. Furthermore, there was significant heterogeneity in our cancer patient population as we included patients of multiple disease sites, stages, treatment types, and treatment intents. Some of these factors (i.e., stage of disease, metastatic sites, and possibility of lymphedema from breast cancer surgery) can influence whether patients participate in PA and how much PA they engage in. However, this heterogeneity has its own advantage given the strength of the associations identified in this population. Future validation of findings in specific tumor disease sites is warranted and may help to identify further unique needs and barriers to undergoing PA for each disease site. This, in turn, may help with development of specialized PA programs for each unique tumor site [15].
In summary, many patients who initially met MVPA guidelines reduced their PA levels after diagnosis, while patients who were not initially meeting MVPA guidelines rarely improved their activity level to meeting guidelines at follow-up. Patients perceiving PA to be beneficial and safe were more likely to improve to meeting MVPA guidelines if they were not initially meeting guidelines. Perceptions could potentially be altered through improved counseling; our data suggests that currently, very few patients recalled receiving counseling from their physicians. Examining strategies to integrate PA programs and counseling into routine cancer care should be a priority.
References
Stull VB, Snyder DC, Demark-Wahnefried W (2007) Lifestyle interventions in cancer survivors: designing programs that meet the needs of this vulnerable and growing population. J Nutr 137:243S–248S
Sabatino SA, Coates RJ, Uhler RJ, Pollack LA, Alley LG, Zauderer LJ (2007) Provider counseling about health behaviors among cancer survivors in the United States. J Clin Oncol 25:2100–2106
Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM (2005) Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol 23:5814–5830
Vijayvergia N, Denlinger CS (2015) Lifestyle factors in cancer survivorship: where we are and where we are headed. J Pers Med 5:243–263
Burnham TR, Wilcox A (2002) Effects of exercise on physiological and psychological variables in cancer survivors. Med Sci Sports Exerc 34:1863–1867
Demark-Wahnefried W, Morey MC, Sloane R, Snyder DC, Miller PE, Hartman TJ, Cohen HJ (2012) Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol 30:2354–2361
Garcia DO, Thomson CA (2014) Physical activity and cancer survivorship. Nutr Clin Pract 29:768–779
Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA (2005) Physical activity and survival after breast cancer diagnosis. JAMA 293:2479–2486
Lee IM, Wolin KY, Freeman SE, Sattlemair J, Sesso HD (2014) Physical activity and survival after cancer diagnosis in men. J Phys Act Health 11:85–90
Zeng Y, Huang M, Cheng AS, Zhou Y, So WK (2014) Meta-analysis of the effects of exercise intervention on quality of life in breast cancer survivors. Breast Cancer 21:262–274
Loughney L, West MA, Kemp GJ, Grocott MP, Jack S (2016) Exercise intervention in people with cancer undergoing neoadjuvant cancer treatment and surgery: a systematic review. Eur J Surg Oncol 42:28–38
Segal R, Zwaal C, Green E, Tomasone JR, Loblaw A, Petrella T, Exercise for People with Cancer Guideline Development Group (2017) Exercise for people with cancer: a systematic review. Curr Oncol 24:e290–e315
Toohey K, Pumpa K, McKune A, Cooke J, Semple S (2018) High-intensity exercise interventions in cancer survivors: a systematic review exploring the impact on health outcomes. J Cancer Res Clin Oncol 144:1–12
Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T, American Cancer Society 2010 Nutrition and Physical Activity Guidelines Advisory Committee (2012) American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 62:30–67
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL, American College of Sports Medicine (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42:1409–1426
Rogers LQ, Courneya KS, Shah P, Dunnington G, Hopkins-Price P (2007) Exercise stage of change, barriers, expectations, values and preferences among breast cancer patients during treatment: a pilot study. Eur J Cancer Care (Engl) 16:55–66
Courneya KS, Friedenreich CM, Reid RD, Gelmon K, Mackey JR, Ladha AB, Proulx C, Vallance JK, Segal RJ (2009) Predictors of follow-up exercise behavior 6 months after a randomized trial of exercise training during breast cancer chemotherapy. Breast Cancer Res Treat 114:179–187
Hair BY, Hayes S, Tse CK, Bell MB, Olshan AF (2014) Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer 120:2174–2182
Pinto BM, Trunzo JJ (2005) Health behaviors during and after a cancer diagnosis. Cancer 104:2614–2623
Lynch BM, Cerin E, Newman B, Owen N (2007) Physical activity, activity change, and their correlates in a population-based sample of colorectal cancer survivors. Ann Behav Med 34:135–143
Naik H, Qiu X, Brown MC, Eng L, Pringle D, Mahler M, Hon H, Tiessen K, Thai H, Ho V, Gonos C, Charow R, Pat V, Irwin M, Herzog L, Ho A, Xu W, Jones JM, Howell D, Liu G (2016) Socioeconomic status and lifestyle behaviours in cancer survivors: smoking and physical activity. Curr Oncol 23:e546–e555
Huy C, Schmidt ME, Vrieling A, Chang-Claude J, Steindorf K (2012) Physical activity in a German breast cancer patient cohort: one-year trends and characteristics associated with change in activity level. Eur J Cancer 48:297–304
Hackshaw-McGeagh LE, Penfold CM, Walsh E, Donovan JL, Hamdy FC, Neal DE, Jeffreys M, Martin RM, Lane JA, ProtecT Study Group (2015) Physical activity, alcohol consumption, BMI and smoking status before and after prostate cancer diagnosis in the ProtecT trial: opportunities for lifestyle modification. Int J Cancer 137:1509–1515
Bock C, Schmidt ME, Vrieling A, Chang-Claude J, Steindorf K (2013) Walking, bicycling, and sports in postmenopausal breast cancer survivors—results from a German patient cohort study. Psychooncology 22:1291–1298
Fassier P, Zelek L, Partula V, Srour B, Bachmann P, Touillaud M, Druesne-Pecollo N, Galan P, Cohen P, Hoarau H, Latino-Martel P, Menai M, Oppert JM, Hercberg S, Deschasaux M, Touvier M (2016) Variations of physical activity and sedentary behavior between before and after cancer diagnosis: results from the prospective population-based NutriNet-Sante cohort. Medicine (Baltimore) 95:e4629
Littman AJ, Tang MT, Rossing MA (2010) Longitudinal study of recreational physical activity in breast cancer survivors. J Cancer Surviv 4:119–127
Rosenstock IM, Strecher VJ, Becker MH (1988) Social learning theory and the Health Belief Model. Health Educ Q 15:175–183
Godin G, Shephard RJ (1985) A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 10:141–146
Rogers LQ, Courneya KS, Robbins KT, Malone J, Seiz A, Koch L, Rao K (2008) Physical activity correlates and barriers in head and neck cancer patients. Support Care Cancer 16:19–27
Ottenbacher AJ, Day RS, Taylor WC, Sharma SV, Sloane R, Snyder DC, Kraus WE, Demark-Wahnefried W (2011) Exercise among breast and prostate cancer survivors—what are their barriers? J Cancer Surviv 5:413–419
Coups EJ, Park BJ, Feinstein MB, Steingart RM, Egleston BL, Wilson DJ, Ostroff JS (2009) Correlates of physical activity among lung cancer survivors. Psychooncology 18:395–404
Szymlek-Gay EA, Richards R, Egan R (2011) Physical activity among cancer survivors: a literature review. N Z Med J 124:77–89
Rabin C, Pinto B (2006) Cancer-related beliefs and health behavior change among breast cancer survivors and their first-degree relatives. Psychooncology 15:701–712
Fishbein M, Ajzen I (1975) Belief, attitude, intention, and behavior: an introduction to theory and research. Addison-Wesley, Reading
Trost SG, Owen N, Bauman AE, Sallis JF, Brown W (2002) Correlates of adults’ participation in physical activity: review and update. Med Sci Sports Exerc 34:1996–2001
Lynch BM, Owen N, Hawkes AL, Aitken JF (2010) Perceived barriers to physical activity for colorectal cancer survivors. Support Care Cancer 18:729–734
Peddle CJ, Au HJ, Courneya KS (2008) Associations between exercise, quality of life, and fatigue in colorectal cancer survivors. Dis Colon Rectum 51:1242–1248
Titz C, Hummler S, Thomas M, Wiskemann J (2016) Physical exercise in advanced cancer patients undergoing palliative treatment 1:433–442
Eyigor S, Akdeniz S (2014) Is exercise ignored in palliative cancer patients? World J Clin Oncol 5:554–559
Ganz PA (2005) A teachable moment for oncologists: cancer survivors, 10 million strong and growing! J Clin Oncol 23:5458–5460
Demark-Wahnefried W, Pinto BM, Gritz ER (2006) Promoting health and physical function among cancer survivors: potential for prevention and questions that remain. J Clin Oncol 24:5125–5131
Denlinger CS, Engstrom PF (2011) Colorectal cancer survivorship: movement matters. Cancer Prev Res (Phila) 4:502–511
Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR (2009) Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol 10:598–605
Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, Snyder C (2012) Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev (8):CD007566
Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, Cerin E, Chan WY, Leung IP, Lam SH, Taylor AJ, Cheng KK (2012) Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ 344:e70
Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, Wittink HM (2010) The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of randomised controlled trials. Clin Oncol (R Coll Radiol) 22:208–221
Peeters C, Stewart A, Segal R, Wouterloot E, Scott CG, Aubry T (2009) Evaluation of a cancer exercise program: patient and physician beliefs. Psychooncology 18:898–902
Santa Mina D, Alibhai SM, Matthew AG, Guglietti CL, Steele J, Trachtenberg J, Ritvo PG (2012) Exercise in clinical cancer care: a call to action and program development description. Curr Oncol 19:e136–e144
Funding
This research was supported in part by the Alan B Brown Chair in Molecular Genomics, the CCO Chair in Experimental Therapeutics and Population Studies and the Posluns Family Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The institutional (University Health Network) research ethics board approved the study. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Eng, L., Pringle, D., Su, J. et al. Patterns, perceptions, and perceived barriers to physical activity in adult cancer survivors. Support Care Cancer 26, 3755–3763 (2018). https://doi.org/10.1007/s00520-018-4239-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4239-5