Abstract
Context
There are no prospective pediatric trials evaluating olanzapine for chemotherapy-induced nausea and vomiting (CINV) prevention.
Objective
This study evaluated the feasibility of a trial of olanzapine to evaluate the contribution of olanzapine to CINV control in pediatric oncology patients.
Methods
Patients < 18 years receiving CINV prophylaxis with ondansetron/granisetron/palonosetron ± dexamethasone ± aprepitant were eligible to participate in this prospective, single-arm, open-label study. All patients received olanzapine (0.14 mg/kg/dose; max 10 mg/dose) once daily orally starting before the first chemotherapy dose and continuing for up to four doses after the last chemotherapy administration. A future trial was considered feasible if mean time to enroll 15 patients was ≤ 12 months/site, ≥ 12/15 took at least half of the planned olanzapine doses, and ≤ 3/15 experienced significant sedation or dizziness despite dose reduction. The proportion of children who experienced complete CINV control (no nausea, vomiting, or retching) was described.
Results
Fifteen patients (range 4.1–17.4 years) participated; mean recruitment period was 9.3 months/site. All patients took at least half of the planned olanzapine doses. Six patients experienced sedation which resolved with olanzapine dose reduction (N = 5) or bedtime administration (N = 1). Olanzapine was stopped in one patient with blurry vision and in another with increased plasma GGT values. In both the acute and delayed phases, eight patients experienced complete control of vomiting but almost all (14/15) had nausea.
Conclusion
A pediatric trial of olanzapine for CINV control is feasible. Our findings will inform the design of a future study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) continues to be problematic in pediatric oncology patients despite use of recommended antiemetics [1]. New antiemetic strategies are required to improve CINV control. Olanzapine, an atypical antipsychotic, improves CINV control in adult oncology patients [2,3,4]. Together with a 5-HT3 antagonist, dexamethasone, and aprepitant, olanzapine is now recommended for CINV prophylaxis in adults receiving highly emetogenic chemotherapy (HEC) [5, 6]. Although olanzapine is approved in the USA for the treatment of adolescents with mental illness [7], olanzapine has not been evaluated in prospective trials of CINV prevention in children. Before initiation of a pediatric comparative trial, it is important to determine whether patients and families would participate in such a trial and if participants are able to take the study drug orally as prescribed.
This study aimed to determine the feasibility of conducting a future trial to evaluate the contribution of olanzapine to CINV control in children. CINV control and adverse effects of olanzapine in these children were also described.
Methods
This multi-center, prospective, open-label study was approved by Health Canada and the Research Ethics Board of each participating institution (SickKids; Children’s Hospital, London Health Sciences Centre (CH-LHS); and Children’s Hospital of Eastern Ontario (CHEO)). Children or their guardian provided informed consent or assent to participate as appropriate. Each patient participated only once. This study was registered on clinicaltrials.gov (NCT02129478).
Patients
English-speaking children, 4 to 18 years old, weighing at least 14 kg, able to complete assessments, and with an English-speaking parent were eligible to participate. Eligible patients were planned to receive moderately emetogenic chemotherapy or HEC [8] on at least 1 day during the study chemotherapy block. At baseline, eligible patients had serum total bilirubin ≤ 50 μmol/L and ALT and AST ≤ 3 times the upper limit of normal for age. Post-pubertal females were confirmed not to be pregnant. Sexually active participants consented to use adequate contraception or remain abstinent on each day olanzapine was given and for 5 days afterward.
Patients with a history of any of the following were excluded: neuroleptic malignant syndrome, seizure disorder, cardiac arrhythmias including prolonged QT interval, low left ventricular ejection fraction, uncontrolled diabetes mellitus, or hypersensitivity to olanzapine. Patients with a brain tumor or uncontrolled hypertension, who had received olanzapine within 14 days or another antipsychotic agent within 30 days prior to enrollment or who were planned to receive amifostine, citalopram, antipsychotic agents other than olanzapine, quinolone antibiotics, or CYP1A2 inducers or inhibitors while receiving olanzapine were also excluded.
CINV prophylaxis
All patients received ondansetron, granisetron, or palonosetron with or without dexamethasone or aprepitant/fosaprepitant at doses as ordered by their clinical team. Use of dexamethasone and aprepitant/fosaprepitant was not standardized since there is a great deal of variability in their use among pediatric cancer centers [9]. Planned administration of any other antiemetic agent on a scheduled basis or administration of scopolamine patches, phenothiazines, acupressure, or acupuncture was not permitted. Other antiemetics were permitted on an “as needed” basis.
Patients received olanzapine 0.14 mg/kg/dose (maximum 10 mg/dose) as a single daily oral dose rounded to the nearest 2.5 mg starting just before the first dose of chemotherapy of the study chemotherapy block. This dose was derived using pediatric dose scaling methods [10] given that the pharmacokinetic disposition of olanzapine is similar in children and adults [7, 11]. The initial olanzapine dose chosen for study was at the lower end of the derived pediatric olanzapine dose range (0.12 to 0.26 mg/kg/day). Olanzapine was given once daily until discharge from hospital for a maximum of four doses after the last dose of chemotherapy of the study chemotherapy block. Olanzapine tablets were disintegrated in water immediately prior to administration if patients were unable to swallow tablets.
When patients experienced undesirable sedation potentially attributable to olanzapine, administration was either moved to bedtime, the dose was decreased by 2.5 mg/day (minimum 1.25 mg (¼ tablet)/day), or olanzapine was discontinued. Decisions were made after discussion with the study team, the attending physician, and the family.
Clinical data
Plasma glucose concentration, glucosuria, and maximum and minimum blood pressure were determined once daily on each day of olanzapine administration. Body weight and AST, ALT, plasma prolactin, and triglyceride concentrations were obtained prior to chemotherapy and on the last day of olanzapine administration.
CINV severity assessment
Patients or parents recorded nausea severity and vomiting/retching episodes on a structured CINV diary. A vomit was defined as expulsion of stomach contents via the mouth separated by at least 1 min from another vomit or retch. Retching was defined as an effort to vomit which did not produce stomach contents.
The patient rated the severity of his/her present nausea using the Pediatric Nausea Assessment Tool [12] (PeNAT) twice daily (upon rising in the morning and at bedtime), as well as whenever he/she felt nauseated, requested PRN antiemetic agents or the child’s caregiver believed the child was nauseated. The PeNAT is reliable and valid in children with cancer and consists of three elements: determination of the term for nausea used within each child’s family, a script to center the child on the subjective symptom of nausea, and a facial nausea severity scale. PeNAT scores correspond to no nausea (1), mild nausea (2), moderate nausea (3), and severe nausea (4). Caregivers serve only as the transcriber of the nausea severity score provided by the child.
CINV diary completion started just before the study chemotherapy block and continued through the acute and delayed phases. The acute phase was defined as starting with the first dose of chemotherapy of the study chemotherapy block and ending 24 h after the last chemotherapy dose of the study chemotherapy block. The delayed phase was defined as beginning at the end of the acute phase and continuing until the first chemotherapy dose of the next chemotherapy block was administered to a maximum of 7 days.
Adverse events
Each child’s health record was reviewed for 30 days for CTCAE v4.03(11) grade 3 to 5 non-hematological adverse events after administration of the last olanzapine dose [13]. In addition, seizure, dizziness, or unexplained somnolence/depressed level of consciousness were noted, irrespective of grade. The association between olanzapine and adverse events (definite, probable, possible, or doubtful) was evaluated using the Naranjo probability scale [14].
Patients’ mood and behavior were assessed by a parent/guardian using the Side Effects Rating Scale (SERS) [15, 16] prior to administration of the first olanzapine dose and again within 72 h after the last dose. The SERS, a parent-report scale, ranks symptom severity from 0 (absent) to 9 (severe); any side effect rated at 7 or higher indicates the need for additional assessment [16].
Patients who experienced any serious adverse event thought to be possibly, probably, or definitely attributable to olanzapine as determined by their primary physician were withdrawn from the study. If more than three patients experienced grade 3 or higher sedation/depressed level of consciousness or dizziness which was probably or definitely associated with olanzapine and which persisted or recurred despite dose reduction, recruitment into the study was to be suspended and the initial olanzapine dose reevaluated.
Study endpoints
Feasibility of a future, pediatric trial to evaluate the contribution of olanzapine to CINV control was the primary endpoint of this study. All feasibility endpoints were decided a priori. Feasibility was first defined as enrollment of 15 patients across three sites with a mean recruitment period of ≤ 12 months/site. The mean enrollment time was used since study activation was expected to be staggered across sites. We planned to stop enrollment once 15 patients had been enrolled. On enrollment of 15 patients, other feasibility endpoints were as follows: receipt of at least half of the olanzapine doses as per the study protocol by ≥ 12 patients and observation of grade ≥ 3 sedation/depressed level of consciousness or dizziness which was probably or definitely associated with olanzapine and persisted or recurred despite olanzapine dose reduction in ≤ 3 patients.
Chemotherapy-induced vomiting (CIV), chemotherapy-induced nausea (CIN), and CINV control during the acute, delayed, and overall (acute plus delayed) phases were described as secondary endpoints. Complete CIV control and complete CIN control were defined as no vomiting or retching and as no nausea (maximum PeNAT score of 1), respectively. Complete CINV control was defined as no vomiting, retching, or nausea. The mean proportion of calendar days that were free of vomiting and retching or nausea was described for each phase.
Statistical analysis
A sample size of 15 evaluable patients was chosen to permit assessment of the feasibility of study procedures and a preliminary description of adverse effects attributable to olanzapine in children receiving chemotherapy. We believed this to represent the accrual rate that would allow completion of a definitive trial with a reasonable number of centers over a realistic time frame. Patients who took at least one olanzapine dose and whose parent provided both SERS assessments were considered evaluable.
The secondary safety (incidence and severity of adverse effects, laboratory abnormalities, weight gain, and SERS scores) and efficacy endpoints (CIV, CIN, and CINV control in the acute, delayed, and overall phases) were described as were reasons for early discontinuation of olanzapine or dose reduction. Statistics were conducted using SAS Enterprise Guide 6.100, SAS Institute Inc., Cary, NC, USA.
Results
Feasibility
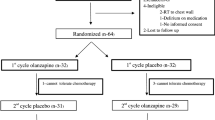
Fifteen patients (mean age 11.1 years; range 4.1–17.4) were enrolled across three sites (SickKids, 9; CH-LHS, 5; and CHEO, 1; Table 1; Fig. 1). Eligible patients who declined participation did not differ from participants in terms of age and sex (median age 11.1 years (interquartile range (IQR) = 7.6 to 14.6) vs. 9.5 years (IQR = 6.5 to 13.3); p = 0.29; male 50 vs. 40%; p = 0.55).
Feasibility endpoints were met. The mean time to recruit 15 patients across three sites was 9.3 months/site. All patients took at least half of the planned olanzapine doses. No patient experienced sedation or dizziness of grade 3 or higher. The mean initial olanzapine dose administered was 0.12 ± 0.03 mg/kg/dose.
CINV control
The mean acute phase length was 98 h (range 24 to 201 h). Seven patients received hematopoietic stem cell transplant conditioning as the study chemotherapy block. One patient included in the analysis applied acupressure bands on the last day of the acute phase. The use of acupressure bands has not been planned at the outset of the chemotherapy block; thus, this patient was included in the analysis. Nausea severity assessments were not completed on 2 days by one patient who was too unwell to do so. Delayed phase diaries were missing for 3 days from one patient. Based on the data that were submitted, these patients did not experience complete CIN or CIV control.
The CINV prophylaxis received and the proportions of children who experienced acute and delayed complete CINV, CIV, and CIN control are presented in Table 2. On average, patients were free of vomiting and retching on 86 ± 21% of acute phase days. All but one patient experienced nausea during the acute phase (maximum PeNAT score 2, 4/15; 3, 7/15; or 4, 3/15). The mean proportion of the acute phase days where patients were free of nausea was 40 ± 39%.
During the delayed phase, seven patients received no olanzapine; others received from 1 to 4 olanzapine doses. On average, patients were free of vomiting and retching on 75 ± 32% and free of nausea on 50 ± 33% of delayed phase days.
Safety
No patient experienced ≥ CTCAE grade 3 sedation/depressed level of consciousness or dizziness. Six patients reported mild sedation prompting olanzapine dose reduction in five and bedtime administration in another. The mean reduced olanzapine dose administered to the five patients where the dose was reduced was 0.08 ± 0.02 mg/kg/dose. Two of these patients also reported mild dizziness which resolved with dose reduction; however, one later experienced mild orthostatic hypotension at the reduced olanzapine dose.
Olanzapine was stopped in two patients: one with blurred vision (CTCAE grade 1; Naranjo score = 3; doubtful association with olanzapine) and another with isolated increased plasma GGT concentrations (CTCAE grade 3; Naranjo score = 4: possibly associated with olanzapine). Body weight was obtained both before and at the end of the olanzapine course in 13 children. The mean change in body weight over the study period was 0.1 kg (range − 5 to + 3.1 kg). End-of-therapy laboratory values are presented in Table 3. Other than one patient who experienced grade 4 hypertriglyceridemia with no clinical sequelae, no grade 3 or higher adverse events occurred.
The median number of symptoms with a SERS score of 1 or more (i.e., were present to any degree) was 6 (range 2 to 16) at the start of the study period and 7 (range 2 to 17) at the end of the study (Table 4). This represented an increase in symptom severity over the study period from “not serious” to “serious” for 12 symptoms in eight patients. Conversely, a decrease in symptom severity from “serious” to “not serious” was observed for four symptoms in three patients over the study period.
Discussion
We have determined that a future trial to evaluate acute CINV control in children receiving olanzapine is feasible. Patient recruitment was timely, patients were able to take olanzapine and no grade 3 or higher sedation or dizziness occurred.
Although recruitment goals were met, a high proportion (75%) of eligible patients/parents declined study participation. Many were reluctant to assume the additional risk, however small, of an unfamiliar drug. We also speculate that parents and patients were skeptical that CINV control could be improved, regardless of the intervention offered. During the consenting process of a future pediatric trial, it may be worthwhile to discuss the goal of complete CINV control with patients and families.
The most commonly reported adverse effects associated with olanzapine in adult oncology patients, fatigue and drowsiness, are dose-related [17]. The optimal olanzapine dose for CINV control in adults is under discussion. Similar CIV control rates and lower somnolence rates have been reported in adult cancer patients receiving olanzapine 5 vs. 10 mg/day [18]. In the present study, six children experienced mild but nevertheless undesirable sedation following the first olanzapine dose. When allometric scaling is used to derive a pediatric dose based on the 5-mg adult dose, a pediatric dose of 0.06 to 0.13 mg/kg/day is predicted. Thus, an initial olanzapine dose of 0.1 mg/kg/day (max 10 mg/day) may merit investigation in a future pediatric trial.
The SERS scores allay concerns regarding the potentially negative influence of olanzapine on mood and behavior. However, it is not possible to attribute the SERS score changes to the use of olanzapine since they may have been provoked by other factors such as hospitalization or administration of chemotherapy or other medications. Nevertheless, since parents did not raise concerns regarding their ability to complete the SERS, its inclusion in a future trial is not likely to be a logistical barrier.
Other than olanzapine, the CINV prophylaxis received by our patients was not standardized. This and the lack of a comparator group make it impossible to discern the contribution of olanzapine to CINV control. However, the measurement of CINV control as a binary endpoint (complete/incomplete) may lack the discriminatory ability to identify clinically meaningful improvements in CINV control in patients receiving multiple-day chemotherapy. An intervention which increases the number of CINV-free days but does not completely control CINV may still benefit patients. It may be wise for future studies of CINV control in patients receiving multiple-day chemotherapy, as is typical in pediatric oncology, to adopt a different metric of efficacy as the primary study endpoint. Nevertheless, the low number of children who experienced complete acute and delayed CINV control points to the need for improved screening to identify children with breakthrough or refractory CINV, the provision of evidence-based CINV prophylaxis [1, 19] and more effective antiemetic and antinauseant strategies.
The olanzapine dose studied here may not be optimal. Our study methods included a dose reduction plan to manage dose-related toxicity but no dose escalation plan to manage uncontrolled CINV. A future pediatric trial should therefore include plans for both dose reduction and dose escalation to be sure that both dose-related toxicity and efficacy are addressed.
The strengths of this study are its multi-center design, careful titration of the olanzapine dose against sedation, and the use of a validated, pediatric self-report nausea severity assessment tool. Interpretations of the secondary outcomes regarding the contribution of olanzapine to CINV control and of its safety in the setting of pediatric oncology must be extremely cautious due to the non-comparative study design, variability in the CINV prophylaxis administered, inclusion of patients who were not chemotherapy-naïve, and small sample size.
In conclusion, we have shown that a trial to determine the contribution of olanzapine to CINV control in children receiving standard CINV prophylaxis is feasible. The design of a future trial will be informed by our findings.
References
Dupuis LL, Boodhan S, Holdsworth M, Robinson PD, Hain R, Portwine C, O'Shaughnessy E, Sung L (2013) Guideline for the prevention of acute nausea and vomiting due to antineoplastic medication in pediatric cancer patients. Pediatr Blood Cancer 60:1073–1082
Chiu L, Chow R, Popovic M, Navari R, Shumway N, Chiu N, Lam H, Milakovic M, Pasetka M, Vuong S, Chow E, DeAngelis C (2016) Efficacy of olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis. Support Care Cancer 24:2381–2392
Yoodee J, Permsuwan U, Nimworapan M (2017) Efficacy and safety of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: a systematic review and meta-analysis. Crit Rev Oncol Hematol 112:113–125. https://doi.org/10.1016/j.critrevonc.2017.02.017
Yang T, Liu Q, Lu M, Ma L, Zhou Y, Cui Y (2017) Efficacy of olanzapine for the prophylaxis of chemotherapy-induced nausea and vomiting: a meta-analysis. Br J Clin Pharmacol 83(7):1369–1379. https://doi.org/10.1111/bcp.13242
Antiemesis Version 2.2016. (2016) National Comprehensive Cancer Network https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed December 27 2016
Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla R, Bruera E, Clark-Snow R, Dupuis L, Einhorn L, Feyer P, Hesketh P, Jordan K, Olver I, Rapoport B, Roscoe J, Ruhlmann C, Walsh D, Warr D, van de Wetering M (2016) 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27(suppl 5):v119–v133. https://doi.org/10.1093/annonc/mdw270
Eli Lilly and Company (2010) Zyprexa prescribing information. Eli Lilly and Co., Indianapolis
Dupuis L, Boodhan S, Sung L, Portwine C, Hain R, McCarthy P, Holdsworth M (2011) Guideline for the classification of the acute emetogenic potential of antineoplastic medication in pediatric cancer patients. Pediatr Blood Cancer 57:191–198
Patel P, Robinson P, Orsey A, Freedman J, Langevin A, Woods D, Sung L, Dupuis L (2016) Chemotherapy-induced nausea and vomiting prophylaxis: practice within the Children’s Oncology Group. Pediatr Blood Cancer 63:856–871. https://doi.org/10.1002/pbc.25915
Johnson TN (2008) The problems in scaling adult drug doses to children. Arch Dis Child 93:207–211
Grothe D, Calis K, Jacobsen L, Kumra S, DeVane C, Rapoport J, Bergstrom R, Kurtz D (2000) Olanzapine pharmacokinetics in pediatric and adolescent inpatients with childhood-onset schizophrenia. J Clin Psychopharmacol 20:220–225
Dupuis LL, Taddio A, Kerr EN, Kelly A, MacKeigan L (2006) Development and validation of a pediatric nausea assessment tool (PeNAT) for use by children receiving antineoplastic agents. Pharmacotherapy 26:1221–1231
US Department of Health and Human Services (2010) Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. National Institutes of Health, Bethesda, Maryland
Naranjo C, Busto U, Sellers E, Sandor P, Ruiz I, Roberts E, Janacek E, Domecq C, Greenblatt D (1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30:239–245
Conklin HM, Khan RB, Reddick WE, Helton S, Brown R, Howard SC, Bonner M, Christensen R, Wu S, Xiong X, Mulhern R (2007) Acute neurocognitive response to methylphenidate among survivors of childhood cancer: a randomized, double-blind, cross-over trial. J Pediatr Psychol 32(9):1127–1139
Barkley RA (1981) Hyperactive children: a hand-book for parents. Guilford Press, New York, NY
Chow R, Chiu L, Navari R, Passik S, Chiu N, Popovic M, Lam H, Pasetka M, Chow E, DeAngelis C (2016) Efficacy and safety of olanzapine for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV) as reported in phase I and II studies: a systematic review. Support Care Cancer 24(2):1001–1008
Hashimoto H, Yanai T, Nagashima K, Tsuda N, Horinouchi H, Takiguchi T, Ohyanagi F, Nakao M, Takeda K, Nakayama T, Sakai H (2016) A double-blind randomized phase II study of 10 versus 5 mg olanzapine for emesis induced by highly emetogenic chemotherapy with cisplatin. ASCO Annual Meet Proc 34(sppl (15)):10111
Dupuis L, Sung L, Molasiotis A, Orsey A, Tissing W, van de Wetering M (2017) 2016 updated MASCC/ESMO consensus recommendations: prevention of acute chemotherapy-induced nausea and vomiting in children. Support Care Cancer 25(1):323–331. https://doi.org/10.1007/s00520-016-3384-y
Acknowledgements
This research was supported by the Pediatric Oncology Group of Ontario Research Unit. We are sincerely grateful to the children who participated in this study and their parents. We also thank the clinical staff of each participating institution for their support of the study and Ms. Mila Khanna, Clinical Research Coordinator, SickKids, for logistical support.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This multi-center, prospective, open-label study was approved by Health Canada and the Research Ethics Board of each participating institution (SickKids; Children’s Hospital, London Health Sciences Centre (CH-LHS); and Children’s Hospital of Eastern Ontario (CHEO)).
Rights and permissions
About this article
Cite this article
Flank, J., Schechter, T., Gibson, P. et al. Olanzapine for prevention of chemotherapy-induced nausea and vomiting in children and adolescents: a multi-center, feasibility study. Support Care Cancer 26, 549–555 (2018). https://doi.org/10.1007/s00520-017-3864-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3864-8