Abstract
Purpose
The purpose of the present study is to describe the incidence and intensity of chemotherapy-induced nausea and vomiting (CINV) and patterns of symptom change after chemotherapy among Korean cancer patients for whom antiemetic guidelines were widely utilized and guideline-consistent antiemetics were available. The study also aimed to determine the contribution of known risk factors for CINV to the incidence and intensity of CINV, as well as patterns of symptom change.
Methods
A prospective observational descriptive study was conducted. A total of 332 adult cancer patients starting their first adjuvant chemotherapy participated in this study. Items of the Multinational Association of Supportive Care in Cancer Antiemesis Tool were utilized to generate a symptom diary. Descriptive statistics, logistic regression analyses, repeated measures ANOVA, and hierarchical generalized linear models were applied to analyze the data.
Results
Vomiting occurred, on average, less than once in the acute and delayed phases, and its frequency remained similar throughout 5 days after chemotherapy infusion in the first and second cycles. A quadratic pattern of nausea change was found. Nausea intensity increased to a peak on the third day after chemotherapy infusion (first-cycle incidence rate ratio (IRR) = 1.40 and second-cycle IRR = 1.27, both p < .001) and then changed gradually (first-cycle IRR = 0.69 and second-cycle and IRR = 0.76, both p < .001). Nausea experience in the previous cycle contributed to the subsequent nausea intensity (IRR = 2.78, p < .001). Younger age, consuming less alcohol, and expecting nausea were identified as risk factors for chemotherapy-induced nausea that needed to be considered from the start of the chemotherapy.
Conclusions
Nausea control, especially in the delayed phase, has room for improvement. As the first chemotherapy-induced nausea experience contributes to subsequent symptom experience, intense control from the start of chemotherapy is necessary while considering patient-related risk factors. Future studies should evaluate the contribution of risk factors when antiemetic prophylaxis is fully provided in multiple settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) has been considered among the most distressful side effect of chemotherapy, affecting the functional status and quality of life of cancer patients. Poorly controlled CINV leads to unscheduled clinic visits, emergency room visits, and even hospitalization [1]. An increased understanding of the mechanisms underlying CINV and the corresponding development of antiemetics and their guidelines [2–4] have significantly improved symptom control. Following antiemetic guidelines when chemotherapy begins is recommended for the prevention of CINV [2–4].
Actual clinical practice and subsequent symptom control are not currently optimal. An investigation of community-hospital-based clinical practices during 2005–2007 in the Netherlands [5] found that acute and delayed nausea occurred in 39 and 68 % of patients, respectively, with lower rates of acute (12 %) and delayed (23 %) vomiting. Suboptimal antiemetic coverage, with only 15 % receiving a triple-antiemetic regimen after highly emetogenic chemotherapy (HEC), might have contributed to the poor symptom control observed in the study. An investigation of CINV in clinical practices in the Asia-Pacific region during 2011 and 2012 [6] also found a high occurrence rate of nausea (61.6 %), while the rate of emesis was relatively low (25.2 %) among patients receiving HEC. Again, the proportion of patients receiving a triple-antiemetic regimen for HEC was limited to 38.7 % [1]. Improved nausea control after moderately emetogenic chemotherapy (MEC) was demonstrated by occurrence rates of acute and delayed nausea of 23.3 and 38.5 %, respectively [7]. This study reflected improvements in clinical practice during 2012 and 2013, although more than half of the participants did not receive antiemetic prophylaxis for the delayed phase, which limited the generalizability of the findings. These studies used clinical data through 2012 and then data limited to CINV after MEC from 2012, demonstrating nonoptimal antiemetic prophylaxis. Clinical data from 2012, reflecting symptoms after both HEC and MEC, could provide information about the current status of CINV management and areas that require further attention.
Patients who suffer from symptoms despite the continuous improvements in antiemetic prophylaxis require special attention. Patients with personal risk factors reportedly have a higher risk of nausea even when advanced symptom control is applied with antiemetics, including aprepitant, extended-duration dexamethasone, or olanzapine [8]. Previously reported key risk factors, such as chemotherapy emetogenicity, are no longer risk factors when sufficient antiemetics are applied for prophylaxis [9]. Clinical data regarding CINV experiences when the best available antiemetic prophylaxis is administered would facilitate risk factor analysis of CINV.
Antiemetic guidelines are well adapted, and antiemetics are fully available through reimbursement in the Republic of Korea, making an evaluation of current antiemetic practice appropriate. This study investigated the current status of CINV management among Korean cancer patients in a setting in which antiemetic guidelines were widely utilized and guideline-consistent antiemetics were available. This prospective observational study aimed to describe the actual experiences with CINV of patients receiving prophylactic, as well as p.r.n. (as needed) antiemetics. Whether the known risk factors for CINV remained influential risk factors with current antiemesis was also evaluated.
Purpose
The purpose of this study was to describe the incidence and intensity of CINV and the patterns of symptom changes after chemotherapy among Korean cancer patients for whom antiemetic guidelines were widely utilized and guideline-consistent antiemetics were available. The study also aimed to determine the contribution of known risk factors for CINV to the incidence and intensity of CINV, as well as patterns of symptom changes.
Methods
Design
This study was a prospective, observational, descriptive study.
Sample
A total of 332 adult cancer patients diagnosed as having stomach, lung, breast, or colorectal cancer and who were starting their first adjuvant chemotherapy participated in this study. The patients were recruited from outpatient clinics and inpatient wards of a university hospital in Seoul, Republic of Korea. Patients who were expected to receive at least three cycles of HEC or MEC in single-day chemotherapy were eligible for inclusion. Patients with colorectal cancer were included if they were receiving continuous infusion of 5-FU for 2 days. The exclusion criteria were receiving concurrent radiotherapy or the presence of health issues that could cause nausea or vomiting (e.g., bowel obstruction), cognitive problems, or a history of psychiatric problems. Of the 332 participants, 313 and 284 patients provided data regarding CINV experiences during the first and second cycles of chemotherapy, respectively. Data were available at all data collection points for 274 cancer patients (77.6 % retention rate) (Table 1). Those subjects who dropped out of the study before completing the first symptom diary (n = 40) were significantly older than the remaining participants (t = −2.914, p = .004). The types of cancer were evenly distributed among the dropouts (8–12 patients for each cancer), while a large proportion of the remaining participants had breast cancer. There were no differences in terms of sex, cancer stage, ECOG status, emetogenicity of the chemotherapy regimen, or treatment setting.
Measurements
Items of the Multinational Association of Supportive Care in Cancer Antiemesis Tool [10] were utilized to generate a symptom diary. The participating patients were asked to log incidences of vomiting, the severity of nausea, and the use of p.r.n. antiemetics. A list of CINV risk factors was included as survey questions. Demographic characteristics were obtained from the patients using structured questionnaires, while clinical characteristics, such as the type of cancer diagnosis, chemotherapy regimen, and antiemetic prescription, were retrieved from electronic medical records.
Procedures
The study was approved by the Institutional Review Board (IRB approval number 4-2012-0504). The purpose and details of the study protocol were explained to the patients, who then provided written informed consent. Patient data were collected between December 2012 and February 2015. Consistency with inclusion and exclusion criteria was confirmed by research nurses. The participants were asked to log their CINV experiences in the symptom diary for 5 days, including the frequency of vomiting, intensity of nausea, and use and effects of p.r.n. antiemetics. The participants were asked to return their diaries when they next visited the hospital. Research nurses reviewed the diaries to confirm the use of p.r.n. antiemetics and to find any erroneous remarks. CINV risk factors were inquired about at the end of the study because some of the risk factors for CINV, such as expectations regarding symptoms, might evoke CINV. This was an observational study and thus did not involve providing or changing the chemotherapy or antiemetic regimen.
Analysis
Standard statistical software (SPSS 22 and STATA 14) was used to analyze the data. Descriptive statistics were utilized to provide general information about the characteristics of the participants and key values of CINV. To describe the overall symptom experience of CINV, patients receiving both HEC and MEC were included in the analyses. The evaluation of antiemetic use included the entire group while incorporating emetogenicity-specific criteria (Table 2). For example, the use of a triple antiemetic regimen (5HT3 RA + NK1 RA + dexamethasone) was considered to adhere to HEC, whereas it was considered to utilize additional antiemetics (+ alpha) for MEC. In the evaluation of risk factors for CINV, the emetogenicity of chemotherapy regimens (HEC or MEC) was considered one of the risk factors for CINV; thus, the whole patient group was evaluated. Exceptions were the analyses that yielded results specific to the emetogenicity of the chemotherapy regimen: incidence of CINV and rates of emetogenicity-specific antiemetic adherence (Table 3 and the “Results” section). When specific patient groups were utilized for the analysis, subgroup membership was identified with “HEC” or “MEC” in the sentence. Risk factors were identified through logistic regression analyses. For the analysis of age as a risk factor, age as a continuous variable as well as a dichotomized variable (age <55 vs age ≥55) was utilized based on previous studies [11–13]. Repeated measures ANOVA evaluted CINV change over time. Hierarchical generalized linear models (HGLMs) involving multilevel negative binomial regression and Poisson regression analyses were applied to analyze the experience of chemotherapy-induced nausea (CIN) over two cycles of chemotherapy while considering individual variance, as well as risk factors for CIN.
Results
General characteristics
The participants were aged 52.12 ± 9.96 years, 67.2 % of them were female, and they had the following types of cancer: breast (46.4 %), colorectal (18.7 %), stomach (18.1 %), and lung (16.9 %). All of the patients were cared for by medical oncologists, and most of them received chemotherapy on an outpatient basis (71 %). More than half of the patients were receiving HEC (63.6 %). In the second cycle of chemotherapy, 14 patients received a reduced dose of the chemotherapy regimen, mainly due to CINV. One patient started chemotherapy with a reduced dose but received the standard dose in the second chemotherapy cycle (Table 1).
Antiemetic use
During the first cycle of chemotherapy, most of the patients received 5HT3RA (99.4 %) and dexamethasone (81.8 %). NK1RA was prescribed to 63.9 % of the patients. A triple-antiemetic regimen consisting of 5HT3RA + NK1RA + dexamethasone was prescribed to 62.9 % of the patients, while a two-drug regimen of 5HT3RA + dexamethasone was provided to 18.2 % of the patients. In terms of guideline adherence, 78.6 % of the patients received guideline-recommended antiemetics, although only 11.8 % strictly adhered to the type and dose of the antiemetics without taking additional or prn antiemetics. A major change in the antiemetic prescription occurred in the second cycle in 18.0 % of the patients. The most frequent changes were adding aprepitant + dexamethasone (4.9 %), excluding dexamethasone (4.6 %), or adding aprepitant (2.8 %). Four patients did not receive antiemetic prophylaxis during the second cycle of chemotherapy. Metoclopramide was frequently prescribed as an additional and/or prn antiemetic (Table 2).
Among the patients who received HEC in the first cycle, 99 % received a guideline-recommended antiemetic regimen (5HT3RA + NK1RA + dexamethasone), whereas 50 % received the recommended 5HT3RA + dexamethasone after MEC. In the second cycle, 98.9 % received guideline-recommended antiemetics for HEC, whereas only 31.7 % received them after MEC, and 18.3 % received a triple regimen for MEC in the second cycle.
CINV incidence
During the first cycle, 74.4 % of the patients did not vomit. However, nausea (defined as a nausea intensity of ≥1 out of 10) was experienced by 81.5 % of participants, with 64.9 % experiencing significant nausea (nausea intensity of ≥3 out of 10) [14]. In the second cycle, fewer patients experienced vomiting (76.8 %), and more patients experienced nausea (86.3 %) and significant nausea (71.1 %) (Table 3).
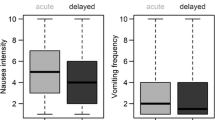
Frequency of chemotherapy-induced vomiting and intensity of CIN
The vomiting frequency and nausea intensity of each cycle are depicted in Fig. 1. Vomiting occurred, on average, less than once during the acute and delayed phases, and its frequency remained similar throughout 5 days after chemotherapy infusion in both cycles (p = .363 for the first cycle and p = .174 for the second cycle). The nausea intensities in the acute and delayed phases during the first cycle were 1.65 ± 2.43 and 2.91 ± 2.50, respectively; the corresponding ratings during the second cycle were 2.11 ± 2.59 and 3.23 ± 2.48. A quadratic pattern of nausea change was found in repeated measures ANOVA (p < .001 for both cycles). The HGLM analysis predicted the change in the CIN intensity (estimated nausea intensity in Fig. 1) accounting for individual variance in the CIN experience; the nausea intensity increased to a peak on the third day after chemotherapy infusion (first-cycle incidence rate ratio [IRR] = 1.40 and second-cycle IRR = 1.27, both p < .001) and then gradually changed (first-cycle IRR = .69 and second-cycle IRR = .76, both p < .001).
Prevalence of known risk factors for CINV
A history of morning sickness was reported in 30.7 % (n = 65) of the female participants. Approximately one quarter of the patients (n = 77, 24.6 %) had a history of motion sickness, and 20.4 % (n = 64) reported a history of nausea and vomiting (NV) with stress. More than half (61.3 %, n = 192) consumed fewer than four glasses of alcoholic beverages per week. Approximately three quarters (70.6 %, n = 221) of the patients expected nausea with chemotherapy (with an intensity of 4.10 ± 3.23, range 0–10), and 62.3 % (n = 195) expected to vomit (with a frequency of 3.57 ± 3.30, range 0–10).
Logistic regression analyses: risk factors for NV and significant NV
The only risk factor that contributed to NV and significant NV during the first cycle was less alcohol consumption. Consuming fewer than four drinks per week increased the odds for NV (OR = 2.27, p = .010) and significant NV (OR = 1.87, p = .016). NV in the first cycle was a contributing factor to NV in the second cycle (OR = 7.45, p < .001). First-cycle significant NV (OR = 8.99, p < .001) and expecting nausea (OR = 2.57, p = .006) were contributing factors to significant NV in multiple logistic regression analysis (Table 4 and Appendix).
Logistic regression analyses: risk factors for vomiting, nausea, and significant nausea overall and in the acute and delayed phases
In the first cycle, having a history of morning sickness increased the odds for overall vomiting (OR = 2.39, p = .017) and delayed vomiting (OR = 2.93, p = .005), whereas receiving HEC decreased the odds for overall vomiting (OR = .35, p = .035) and delayed vomiting (OR = .33, p = .037) among female patients in multiple logistic regression analysis. Being younger than 55 years old (OR = 1.90, p = .018), consuming less alcohol (OR = 1.85, p = .029), and expecting nausea (OR = 2.45, p = .003) were the factors with greater odds for acute nausea.
In the second cycle, experiencing symptoms in the previous cycle was the strongest predictor of vomiting, nausea, and significant nausea, overall and in the acute and delayed phases (all p < .05). In multiple logistic regression analysis, HEC decreased the odds for overall, acute, and delayed vomiting (OR = .31, p = .005; OR = .18, p = .015; and OR = .38, p = .005, respectively). A history of morning sickness increased the odds for overall vomiting (OR = 2.49, p = .035) and delayed vomiting (OR = 2.61, p = .034) among female patients. Expecting nausea increased the odds for acute nausea (OR = 2.21, p = .011), overall significant nausea (OR = 2.49, p = .009), and delayed significant nausea (OR = 2.95, p = .001). Not adhering to antiemetic guidelines increased the odds for acute significant nausea (OR = 3.02, p = .014) (Table 4 and Appendix).
HGLM analysis of risk factors for nausea intensity
There was no significant change in vomiting frequency for 5 days in either cycle. Single risk factor analysis identified aging (as well as age ≥55) as a protective factor for nausea intensity in both cycles. Expecting nausea contributed to nausea intensity in both cycles. In multiple risk factor analyses, being younger than 55 years old and expecting nausea contributed to the nausea intensity of the first cycle (IRR = 1.36, p = .033 and IRR = 1.40, p = .038) and the second cycle (IRR = 1.12, p < .001 and IRR = 1.20, p < .001). Nausea experience in the previous cycle (IRR = 2.78, p < .001) was the most important factor contributing to nausea in the second cycle. Other risk factors were a history of motion sickness (IRR = 0.89, p = .004), a history of NV associated with stress (IRR = 1.18, p < .001), and receiving MEC (IRR = 1.23, p < .001). For female patients, a history of morning sickness (IRR = 1.14, p = .001) was the main risk factor, while histories of motion sickness and NV associated with stress were not significant (Table 5).
Discussion
Vomiting in the acute phase was better controlled in the present study than in previous studies [5, 6]. The 23 % incidence rate of delayed vomiting was similar to that found by Hilarius et al. but higher than that reported by Heish et al. (19.2 % after HEC and 16.1 % after MEC). Half of the patients received the recommended 5HT3RA + dexamethasone regimen after MEC in the first cycle, which might explain the less satisfactory symptom control. CINV after HEC has long been the focus of symptom management. However, an improved understanding of the mechanisms underlying emesis after HEC and the adaptation of the triple-antiemetic regimen have changed CINV experiences. Vomiting occurred, on average, less than once over 5 days during the two cycles of chemotherapy in this study. As depicted in Fig. 1, the traditional biphasic pattern of emesis after cisplatin or a gradual peak in vomiting incidence after cyclophosphamide/carboplatin [15] was no longer observed. Notably, antiemetic prophylaxis according to antiemetic guidelines also improved the control of CINV after MEC [16]. Although the emetogenic potential of MEC is lower than that of HEC, poor antiemetic prophylaxis, especially in the delayed phase, can increase the symptoms experienced. In an era when a triple-antiemetic regimen provides good control of CINV after HEC [13, 17, 18], patients might suffer more CINV from MEC when antiemetic prophylaxis continues to be less satisfactory.
The control of nausea, especially in the delayed phase, continues to be problematic [19–21], including in the current study, because more than 65 % of the patients experienced significant nausea. The mechanisms underlying nausea are poorly understood; thus, controlling CINV has been largely focused on vomiting, based on the belief that vomiting and nausea are closely related. However, as depicted in Fig. 1, NV demonstrated different patterns of change with the applied antiemetic prophylaxis. Reported patterns of nausea changes have remained similar despite the evolution of antiemetics over several decades. Nausea gradually increased up to day 3 in an evaluation of CINV using the Index of Nausea Vomiting and Retching among breast cancer patients [22]. This pattern of nausea has also been observed among breast cancer patients using a numeric rating scale [23]. The rate of nausea occurrence in the current study was higher than previously reported [21] for patients receiving 5HT3RA + dexamethasone after MEC in the acute phase while not receiving antiemetic prophylaxis in the delayed phase, which resulted in 54 % of patients experiencing delayed nausea. Acute-phase symptom control might have contributed to delayed nausea control. In an investigation of CINV after MEC [7], most patients (95.3 %) received 5HT3RA + corticosteroids in the acute phase, and approximately half did not receive antiemetics in the delayed phase. With satisfactory antiemetic prophylaxis in the acute phase, nausea was experienced by 23.3 and 38.5 % in the acute and delayed phases, respectively. However, great variations remain in delayed-phase antiemetic prophylaxis after MEC, and corticosteroids are often underprescribed (37.3 %).
Clinicians are concerned about the potential side effects of steroids [1]. A regimen of single-day corticosteroid + palonosetron could be considered for controlling delayed nausea after MEC [24–27]. Olanzapine has promising effects in nausea control after HEC [28, 29], and a recent phase III trial found that nausea control was significantly improved by adding olanzapine (74 and 43 % with no acute and delayed nausea, respectively) [30]. There is a greater room for further improving delayed nausea than acute nausea. Adhering to the current antiemetic guideline (5HT3RA + corticosteroids) in the acute phase, combining single-dose corticosteroid + palonosetron (considering the side effects of steroids) after MEC and utilizing olanzapine (as an adjunct to the antiemetic regimen) after HEC, could improve symptom control. Evidence-based nonpharmacological approaches, such as progressive muscle relaxation, could also improve symptom control [31].
Different risk factors for CINV have been identified for NV [5, 11] and for acute and delayed symptoms [9, 32], with the results also being inconsistent across different patient groups [5, 9, 11, 33]. These differences could be due to differences in the antiemetic coverage for chemotherapy and to the risk factors included in the analyses. Warr [34] suggested that the risk factors for emesis found in at least two clinical trials of substantial size were vomiting in the previous cycle, receiving HEC, not receiving guideline-recommended antiemetics, being younger, being female sex, drinking less alcohol, and having a history of pregnancy-associated emesis and of motion sickness. Experiencing symptoms in the previous cycle, not using antiemetics in accordance with international guidelines, younger age, and nausea before chemotherapy were found to be key factors for CINV [9]. Clinically significant nausea in the previous cycle and younger age were also previously found to be important predictors of clinically significant nausea [35]. The risk factors for CIN identified in the current study (i.e., symptom experience in the previous cycle, younger age, and less alcohol intake) were congruent with these previous reports.
Preventing CINV is the goal of antiemesis because symptom experience serves as a key risk factor for further symptom experience [4], especially given that experiencing CINV in the previous cycle resulted in patient characteristics no longer playing a major role in determining the subsequent risk of CINV [34]. The appropriate use of antiemetics provides good control of the risk of NV from emetogenic chemotherapy agents. Compared to emetogenicity itself [9], adhering to antiemetic guidelines is more important for controlling CINV [1, 36]. Receiving MEC was a risk factor for CINV in the current study in which antiemetic prophylaxis was unsatisfactory. This finding indicates the need to optimize antiemetic prophylaxis.
Younger age is considered a risk factor for CIN. Age is reportedly a significant predictor of the intensity [23] and the frequency and duration [37] of nausea. Lee et al. [23] and the current study evaluated age as a continuous variable contributing to CIN, whereas various age cutoffs (50, 55, and 65 years old) have also been identified as a risk factor for CINV [33, 35, 37–39], vomiting [11], and nausea [12]. Future studies should attempt to determine the optimal cutoff age.
Drinking less alcohol increased the odds for experiencing nausea and significant nausea in this study. Chronic alcohol intake exceeding 100 g/day has been reported as a protective factor against CINV [40, 41]. Consuming five or more alcoholic drinks per week was also found to be significantly associated with improved complete response (no vomiting and no rescue medication) [33]. Alcohol consumption therefore should be considered in risk assessments. Expecting nausea also increases the risk of developing nausea [42], although nausea expectations were significant only in certain phases and cycles [9]. Managing the expectations of patients is a viable approach for controlling CIN [43, 44]. The data analyzed in this study were collected in a single institution, which could have limited the generalizability of the findings. Considering that the institution was one of the five hospitals that treat 20 to 30 % of cancer patients in the Republic of Korea [45] and that guideline-recommended antiemetics were fully utilized, the results of this study should reflect the current CINV experience of Korean patients. To include colorectal cancer patients, patients receiving multiday chemotherapy were included. Although administered 5-FU was considered to have low emetogenic potential, including multiday chemotherapy might have influenced symptom control.
Conclusion
Advances in antiemetics targeting emesis mechanisms allow for good control of vomiting, especially after HEC. However, the control of CINV after MEC requires further improvement, with an emphasis on antiemetic prophylaxis. Nausea control also has room for further improvement, especially in the delayed phase. The first chemotherapy-induced experience of nausea contributes to subsequent symptom experience, making intense control from the start of chemotherapy necessary when considering patient-related risk factors. Being younger, consuming less alcohol, and expecting nausea were identified risk factors for CIN, which should be considered at the start of chemotherapy. Future studies should evaluate the contribution of risk factors when antiemetic prophylaxis is fully provided in multiple settings.
References
Yu S, Burke TA, Chan A, Kim HK, Hsieh RK, Hu X, Liang JT, Banos A, Spiteri C, Keefe DM (2015) Antiemetic therapy in Asia Pacific countries for patients receiving moderately and highly emetogenic chemotherapy—a descriptive analysis of practice patterns, antiemetic quality of care, and use of antiemetic guidelines. Support Care Cancer 23:273–282
Basch E, Hesketh PJ, Kris MG, Prestrud AA, Temin S, Lyman GH (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract 7:395–398
Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 21(Suppl 5):v232–v243
NCCN (2013) Antiemesis guidelines. In: Editor (ed)^(eds) Book Antiemesis guidelines, City, pp. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf
Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK (2012) Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer 20:107–117
Hsieh RK, Chan A, Kim HK, Yu S, Kim JG, Lee MA, Dalen J, Jung H, Liu YP, Burke TA, Keefe DM (2015) Baseline patient characteristics, incidence of CINV, and physician perception of CINV incidence following moderately and highly emetogenic chemotherapy in Asia Pacific countries. Support Care Cancer 23:263–272
Escobar Y, Cajaraville G, Virizuela JA, Alvarez R, Munoz A, Olariaga O, Tames MJ, Muros B, Lecumberri MJ, Feliu J, Martinez P, Adansa JC, Martinez MJ, Lopez R, Blasco A, Gascon P, Calvo V, Luna P, Montalar J, Del Barrio P, Tornamira MV (2015) Incidence of chemotherapy-induced nausea and vomiting with moderately emetogenic chemotherapy: ADVICE (Actual Data of Vomiting Incidence by Chemotherapy Evaluation) study. Support Care Cancer 23:2833–2840
Dranitsaris G, Mazzarello S, Smith S, Vandermeer L, Bouganim N, Clemons M (2016) Measuring the impact of guideline-based antiemetic therapy on nausea and vomiting control in breast cancer patients with multiple risk factors. Support Care Cancer 24:1563–1569
Molassiotis A, Aapro M, Dicato M, Gascon P, Novoa SA, Isambert N, Burke TA, Gu A, Roila F (2014) Evaluation of risk factors predicting chemotherapy-related nausea and vomiting: results from a European prospective observational study J Pain Symptom Manage 47:839–848.e834
Molassiotis A, Coventry PA, Stricker CT, Clements C, Eaby B, Velders L, Rittenberg C, Gralla RJ (2007) Validation and psychometric assessment of a short clinical scale to measure chemotherapy-induced nausea and vomiting: the MASCC antiemesis tool. J Pain Symptom Manage 34:148–159
Warr DG, Street JC, Carides AD (2011) Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of phase 3 trial of aprepitant in patients receiving adriamycin-cyclophosphamide-based chemotherapy. Support Care Cancer 19:807–813
Dibble SL, Luce J, Cooper BA, Israel J, Cohen M, Nussey B, Rugo H (2007) Acupressure for chemotherapy-induced nausea and vomiting: a randomized clinical trial. Oncol Nurs Forum 34:813–820
Saito H, Yoshizawa H, Yoshimori K, Katakami N, Katsumata N, Kawahara M, Eguchi K (2013) Efficacy and safety of single-dose fosaprepitant in the prevention of chemotherapy-induced nausea and vomiting in patients receiving high-dose cisplatin: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Ann Oncol 24:1067–1073
Keefe DM, Chan A, Kim HK, Hsieh RK, Yu S, Wang Y, Nicholls RJ, Burke TA (2015) Rationale and design of the Pan Australasian chemotherapy-induced emesis burden of illness study. Support Care Cancer 23:253–261
Martin M (1996) The severity and pattern of emesis following different cytotoxic agents. Oncology 53(Suppl 1):26–31
Aapro M, Molassiotis A, Dicato M, Pelaez I, Rodriguez-Lescure A, Pastorelli D, Ma L, Burke T, Gu A, Gascon P, Roila F, investigators P (2012) The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol 23:1986–1992
Stiff PJ, Fox-Geiman MP, Kiley K, Rychlik K, Parthasarathy M, Fletcher-Gonzalez D, Porter N, Go A, Smith SE, Rodriguez TE (2013) Prevention of nausea and vomiting associated with stem cell transplant: results of a prospective, randomized trial of aprepitant used with highly emetogenic preparative regimens. Biol Blood Marrow Transplant 19:49–55 e41
Aapro MS, Schmoll HJ, Jahn F, Carides AD, Webb RT (2013) Review of the efficacy of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in a range of tumor types. Cancer Treat Rev 39:113–117
Molassiotis A, Saunders MP, Valle J, Wilson G, Lorigan P, Wardley A, Levine E, Cowan R, Loncaster J, Rittenberg C (2008) A prospective observational study of chemotherapy-related nausea and vomiting in routine practice in a UK cancer centre. Support Care Cancer 16:201–208
Olver I, Molassiotis A, Aapro M, Herrstedt J, Grunberg S, Morrow G (2011) Antiemetic research: future directions. Support Care Cancer 19(Suppl 1):S49–S55
Hesketh PJ, Sanz-Altamira P, Bushey J, Hesketh AM (2012) Prospective evaluation of the incidence of delayed nausea and vomiting in patients with colorectal cancer receiving oxaliplatin-based chemotherapy. Support Care Cancer 20:1043–1047
Lee J, Dibble SL, Pickett M, Luce J (2005) Chemotherapy-induced nausea/vomiting and functional status in women treated for breast cancer. Cancer Nurs 28:249–255
Lee J, Dibble S, Dodd M, Abrams D, Burns B (2010) The relationship of chemotherapy-induced nausea to the frequency of pericardium 6 digital acupressure. Oncol Nurs Forum 37:E419–E425
Celio L, Frustaci S, Denaro A, Buonadonna A, Ardizzoia A, Piazza E, Fabi A, Capobianco AM, Isa L, Cavanna L, Bertolini A, Bichisao E, Bajetta E (2011) Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: a randomized, multicenter, phase III trial. Support Care Cancer 19:1217–1225
Komatsu Y, Okita K, Yuki S, Furuhata T, Fukushima H, Masuko H, Kawamoto Y, Isobe H, Miyagishima T, Sasaki K, Nakamura M, Ohsaki Y, Nakajima J, Tateyama M, Eto K, Minami S, Yokoyama R, Iwanaga I, Shibuya H, Kudo M, Oba K, Takahashi Y (2015) Open-label, randomized, comparative, phase III study on effects of reducing steroid use in combination with Palonosetron. Cancer Sci 106:891–895
Aapro M, Fabi A, Nole F, Medici M, Steger G, Bachmann C, Roncoroni S, Roila F (2010) Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 21:1083–1088
Celio L, Bonizzoni E, De Braud F, Agustoni F, Aapro M (2016) Should clinicians always administer dexamethasone beyond 24 h after chemotherapy to control delayed nausea and vomiting caused by moderately emetogenic regimens? Insight from the re-evaluation of two randomized studies. Support Care Cancer 24:1025–1034
Abe M, Hirashima Y, Kasamatsu Y, Kado N, Komeda S, Kuji S, Tanaka A, Takahashi N, Takekuma M, Hihara H, Ichikawa Y, Itonaga Y, Hirakawa T, Nasu K, Miyagi K, Murakami J, Ito K (2016) Efficacy and safety of olanzapine combined with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy in gynecological cancer: KCOG-G1301 phase II trial Support Care Cancer 24:675–682
Mizukami N, Yamauchi M, Koike K, Watanabe A, Ichihara K, Masumori N, Yamakage M (2014) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly or moderately emetogenic chemotherapy: a randomized, double-blind, placebo-controlled study. J Pain Symptom Manag 47:542–550
Navari R, Qin R, Ruddy KJ, Liu H, Powell SF, Bajaj M. et al. (2015) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC): Alliance A221301, a randomized, double-blind, placebo-controlled trial.. In: Editor (ed)^(eds) Book Olanzapine for the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC): Alliance A221301, a randomized, double-blind, placebo-controlled trial., City.
Molassiotis A, Yung HP, Yam BM, Chan FY, Mok TS (2002) The effectiveness of progressive muscle relaxation training in managing chemotherapy-induced nausea and vomiting in Chinese breast cancer patients: a randomised controlled trial. Support Care Cancer 10:237–246
Dranitsaris G, Bouganim N, Milano C, Vandermeer L, Dent S, Wheatley-Price P, Laporte J, Oxborough KA, Clemons M (2013) Prospective validation of a prediction tool for identifying patients at high risk for chemotherapy-induced nausea and vomiting. J Support Oncol 11:14–21
Hesketh PJ, Aapro M, Street JC, Carides AD (2010) Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin-based chemotherapy. Support Care Cancer 18:1171–1177
Warr D (2014) Prognostic factors for chemotherapy induced nausea and vomiting. Eur J Pharmacol 722:192–196
Kim HK, Hsieh R, Chan A, Yu S, Han B, Gao Y, Banos A, Ying X, Burke TA, Keefe DM (2015) Impact of CINV in earlier cycles on CINV and chemotherapy regimen modification in subsequent cycles in Asia Pacific clinical practice. Support Care Cancer 23:293–300
Aapro M, Molassiotis A, Dicato M, Pelaez I, Rodriguez-Lescure A, Pastorelli D, Ma L, Burke T, Gu A, Gascon P, Roila F (2012) The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol: Off J Eur Soc Med Oncol/ESMO 23:1986–1992
Molassiotis A, Yam BM, Yung H, Chan FY, Mok TS (2002) Pretreatment factors predicting the development of postchemotherapy nausea and vomiting in Chinese breast cancer patients. Support Care Cancer 10:139–145
Pirri C, Katris P, Trotter J, Bayliss E, Bennett R, Drummond P (2011) Risk factors at pretreatment predicting treatment-induced nausea and vomiting in Australian cancer patients: a prospective, longitudinal, observational study. Support Care Cancer 19:1549–1563
Sekine I, Segawa Y, Kubota K, Saeki T (2013) Risk factors of chemotherapy-induced nausea and vomiting: index for personalized antiemetic prophylaxis. Cancer Sci 104:711–717
D’Acquisto RW, Tyson LB, Gralla RJ et al. (1986) The influence of a chronic high alcohol intake on chemotherapy-induced nausea and vomiting. In: Editor (ed)^(eds) Book The influence of a chronic high alcohol intake on chemotherapy-induced nausea and vomiting., City, pp. 257.
Sullivan JR, Leyden MJ, Bell R (1983) Decreased cisplatin-induced nausea and vomiting with chronic alcohol ingestion. N Engl J Med 309:796
Roscoe JA, Bushunow P, Morrow GR, Hickok JT, Kuebler PJ, Jacobs A, Banerjee TK (2004) Patient expectation is a strong predictor of severe nausea after chemotherapy: a University of Rochester Community Clinical Oncology Program study of patients with breast carcinoma. Cancer 101:2701–2708
Roscoe JA, O’Neill M, Jean-Pierre P, Heckler CE, Kaptchuk TJ, Bushunow P, Shayne M, Huston A, Qazi R, Smith B (2010) An exploratory study on the effects of an expectancy manipulation on chemotherapy-related nausea. J Pain Symptom Manag 40:379–390
Shelke AR, Roscoe JA, Morrow GR, Colman LK, Banerjee TK, Kirshner JJ (2008) Effect of a nausea expectancy manipulation on chemotherapy-induced nausea: a university of Rochester cancer center community clinical oncology program study. J Pain Symptom Manag 35:381–387
Hong SC, Jang SN, Whang SS, Lim JY (Seogang University Research Foundation) (2014) Health service utilization by patients diagnosed with severe illness. Final report, Health Insurance Review and Assessment Service (Korea). http://www.dbpia.co.kr/SKnowledge/ArticleDetail/NODE06276150
Acknowledgments
The current study was supported in part by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science, and Technology (2012R1A1A1010107 and 2015R1A1A1A05001342), and in part by the Public Welfare & Safety Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2010-0020841).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest. They state that they have full control of all of the primary data and that they agree to allow the journal to review their data if requested.
Electronic Supplementary Material
ESM 1
(PDF 14 kb)
Rights and permissions
About this article
Cite this article
Rha, S.Y., Park, Y., Song, S.K. et al. Controlling chemotherapy-induced nausea requires further improvement: symptom experience and risk factors among Korean patients. Support Care Cancer 24, 3379–3389 (2016). https://doi.org/10.1007/s00520-016-3146-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3146-x