Abstract
Purpose
Hyponatremia is a common and associated with poor clinical outcome in cancer patients. But little is known regarding hyponatremia in terminal cancer patients. The purpose of this study is to investigate the prognostic role of the hyponatremia in terminal cancer patients.
Methods
A retrospective observational study was conducted between January 2010 and December 2012 in a tertiary hospital palliative care unit. Medical records were collected from hospitalized patients who were eligible for obtaining serum sodium level. Hyponatremia was defined as serum sodium <136 mEq/L. And we classified patients into three groups; eunatremia (sodium 136–145 mEq/L), mild to moderate hyponatremia (sodium 126–135 mEq/L), and severe hyponatremia (sodium ≤125 mEq/L). Univariate and multivariate Cox regression analyses were performed to determine factors affecting survival time.
Results
Of the 576 patients, hyponatremia was present in 367 individuals (63.7 %). In the univariate analysis, serum CRP, PPS, and sodium ≤125 mEq/L were associated with survival time (HR = 1.22; p < 0.001, HR = 0.69; p < 0.001, HR = 1.91; p < 0.001). In the multivariate analysis, serum CRP, PPS, sodium 126–135 mEq/L, and sodium ≤125 mEq/L were associated with survival time (HR = 1.16; p < 0.001, HR = 0.70; p < 0.001, HR = 1.19; p = 0.048, HR = 1.75; p < 0.001).
Conclusions
Hyponatremia is an independent prognostic factor in terminal cancer patients and careful clinical concern is needed. In the future, large prospective study is warranted in terminal cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Hyponatremia is an electrolyte abnormality, indicating dilution of serum sodium in relation to excessive total body fluid, and is commonly observed in various medical condition, such as heart failure, liver cirrhosis, renal failure, infectious state (pneumonia and HIV infection), and malignancy [1, 2]. It is known that hyponatremia is associated with longer hospital days, medical costs, and higher mortality compared to eunatremia [2–5]. Although it is hypothesized that long-term hyponatremia might disturb the metabolic and genetic balance in cells, resulting in higher mortality, pathophysiologic effect of hyponatremia on clinical outcome has not yet clearly been identified [6].
Hyponatremia is also commonly seen in cancer patients. Among the diverse causes of hyponatremia in cancer patients, syndrome of inappropriate secretion of antidiuretic hormone (SIADH) is considered to be the most common cause in cancer [7–10]. Berghmans et al. reported that one third of hyponatremia cases were caused by SIADH, followed by a depleted state due to gastrointestinal fluid loss and diuretic use [7]. Pain, the most common symptom in cancer patients, is also a powerful stimulus for ADH secretion, as is physical and emotional stress. Nausea and vomiting also increase the secretion of ADH [8–10]. Morphine and other opioids are known to directly increase ADH secretion [11].
Previous reports also revealed that hyponatremia was associated with increased length of admission day and higher mortality rate in cancer patients [6, 7, 12], though prognostic value of hyponatremia is different depending on the population. Studies have reported that patients with hyponatremia had poor prognosis in small cell lung cancer (SCLC) [13, 14]. On the other hand, some studies showed different results according to the extension of disease. One study found that patients with hyponatremia had shorter survival time only in extended disease (ED), and not in limited disease (LD) [15], whereas another study showed that hyponatremia was associated with poor prognosis in LD [16]. Previous literatures on non-small cell lung cancer (NSCLC) and metastatic renal cell cancer (RCC) patients also showed that hyponatremia was significantly associated with poor survival outcome [17, 18]. However, the prognostic value of hyponatremia exclusive to terminal cancer patients is yet to be examined. Therefore, this study investigated hyponatremia as a prognostic indicator of terminal cancer patients.
Materials and methods
Study population
The database was collected from electronic medical records of hospitalized terminal cancer patients in palliative care unit of Seoul St. Mary’s Hospital, between January 2010 and December 2012. By using the electronic database, information on patients’ age, sex, type of cancer, presence of metastases, comorbidities, survival time, palliative performance scale (PPS), and laboratory results were obtained. Patients who did not have the records of serum sodium level were excluded. Also, patients who had serum sodium level above 145 mEq/L, which is defined as hypernatremia, were not eligible. Among 755 patients admitted between January 2010 and December 2012, 576 patients were available for the final analysis.
The level of sodium and C-reactive protein (CRP) checked on the first day of hospitalization were recorded. Hyponatremia was defined as serum sodium level below 136 mEq/L. For classification of the severity of hyponatremia, serum sodium levels were divided into three groups; eunatremia (serum sodium 136–145 mEq/L), mild to moderate hyponatremia (serum sodium 126–135 mEq/L), and severe hyponatremia (serum sodium ≤125 mEq/L). The survival time was defined as the time from the first day of hospitalization at palliative care unit until death.
The Institutional Review Board at Seoul St. Mary’s Hospital has approved this study (IRB no. KC13RASI0903).
Statistical analysis
Sex, type of cancer, presence of metastases, comorbidities, PPS, and the severity of sodium level were investigated. The median value of survival time and the mean values of age and CRP were recorded. One-way ANOVA analysis was performed to analyze the differences in age, PPS, survival time, and CRP level in respect to groups of sodium level. Univariate and multivariate Cox regression analyses were performed to determine factors affecting survival time. The Kaplan–Meier method was used to measure differences in survival time for each sodium level groups. All analyses were performed with SAS Statistical Package Release 9.3 (SAS Institute, Cary, NC, USA) program. A p value of less than 0.05 was considered to indicate statistical significance.
Results
Demographic and clinical characteristics
A total of 576 patients were examined; 289 (50.2 %) male patients and 287 (49.8 %) female patients. The mean age of the study population was 62.0 (±13.14). Pancreatic/hepatobiliary cancer was the most common type, comprising 128 (22.2 %) patients, followed by 114 (19.8 %) gastric cancer patients, 90 (15.6 %) colorectal cancer patients, and 85 (14.7 %) lung cancer patients. Metastases were present in 496 (85.6 %) patients. In regards to comorbidities, 156 (27.0 %) patients had hypertension and 89 (15.4 %) patients had diabetes mellitus. There were 317 (55.1 %) patients whose PPS was 50 % equal or greater, 231 (40.1 %) patients 30–40 %, and 28 (4.9 %) patients 20 % or less. The mean CRP level was 9.60 (±7.75). Hyponatremia (serum sodium <136 mEq/L) was present in 367 (63.7 %) patients; mild to moderate hyponatremia (serum sodium 126–135 mEq/L) was in 313 (54.3 %) patients; and severe hyponatremia (serum sodium ≤125 mEq/L) in 54 (9.4 %) patients. The median survival time of the study population was 15 days (Table 1).
Comparisons of age, PPS, survival time, and CRP in each groups of sodium level
The serum sodium level was divided into three groups. Each serum sodium group statistically showed significant difference in age, PPS, survival time, and CRP level. The mean age in the serum sodium ≤125 mEq/L group, 126–135 mEq/L group, and 136–145 mEq/L group was 59.3, 61.6, and 63.4 (p = 0.013), respectively. The mean PPS was 42.6, 47.4, and 45.3 % for each group (p = 0.003). Mean survival times were 14.7, 24.8, and 31.2 days (p = 0.024), respectively. The mean CRP levels for each group were 10.66, 10.28, and 8.30 mg/dl (p = 0.003) (Table 2).
Univariate and multivariate Cox proportional hazard model analyses
Univariate and multivariate Cox regression analyses were performed to investigate the factors affecting of survival time. In the univariate analysis, CRP and PPS showed significant associations with survival (HR = 1.22; p < 0.001, HR = 0.69; p < 0.001). Compared to eunatremia group, serum sodium ≤125 mEq/L was significantly associated with poor prognosis (HR = 1.91; p < 0.001). In the multivariate analysis, CRP and PPS also showed significant associations with survival (HR = 1.16; p < 0.001, HR = 0.70; p < 0.001). Serum sodium ≤125 mEq/L and 126–135 mEq/L groups showed statistical significance regarding survival time compared to eunatremia group (HR = 1.75; p < 0.001, HR = 1.19; p = 0.048) (Table 3).
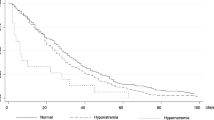
Figure 1 shows survival curves for different serum sodium level groups by using the Kaplan–Meier method. Each group statistically showed significant difference in survival times (Log rank test <0.001). Serum sodium ≤125 mEq/L group showed the shortest survival time compared to eunatremia group.
Discussion
This study confirmed that terminal cancer patients with hyponatremia (serum sodium <136 mEq/L) have a significantly shorter survival time compared to patients with eunatremia (serum sodium 136 ∼ 145 mEq). It also proved hyponatremia as an independent prognostic factor as already shown in various study populations including cancer patients [6, 12–14, 17, 18]. Hansen et al. with 453 SCLC patients receiving chemotherapy reported that patients with hyponatremia (serum sodium <136 mEq/L) showed shorter survival time compared to patients with normal sodium levels [14]. Jabot et al. suggested that hyponatremia was one of the poor prognostic factors in their study with 301 NSCLC patients [17]. In a Danish study with 120 metastatic RCC patients, hyponatremia (serum sodium <136 mEq/L) was identified as an independent prognostic factor [18]. This study demonstrated prognostic value of hyponatremia in different type of cancer patients in the terminal stage who are treated with palliative purpose.
In this study, hyponatremia was categorized into two groups; mild to moderate (serum sodium 126–135 mEq/L) group and severe (serum sodium ≤125 mEq/L) group, and severe hyponatremia statistically showed higher hazard ratio than mild to moderate hyponatremia. However, in a study of 3357 cancer patients, Doshi et al. found that moderate hyponatremia (serum sodium <120–129 mEq/L) was associated with higher hazard ratio (HR) for 90-day mortality and showed shorter survival time than severe hyponatremia (serum sodium <120 mEq/L) [6]. And in another large-scale study including metastatic cancer inpatients, 5-year mortality was significantly higher in patients with mild hyponatremia (serum sodium <130–134 mEq/L), but not significantly different in patients with severe hyponatremia (serum sodium <120 mEq/L) [3]. This difference might be caused by the different classification of hyponatremia. Hyponatremia was divided into mild, moderate, and severe groups in previous studies, whereas mild and moderate hyponatremia was categorized as one group in this study. Different cutoff value of hyponatremia and study population may also lead to different results from preceding studies. Consequently, the linear relationship between the severity of hyponatremia and survival time in cancer patients may not have been clearly concluded at this point.
The prevalence of hyponatremia in cancer patients was known to be 4–47 % in previous studies, depending on the cutoff value for hyponatremia and the study sample [6, 7, 12]. In this study, the frequency of hyponatremia (serum sodium <136 mEq/L) was 63.7 %, which was slightly higher than existing previous studies. The differences in prevalence seem to have been exhibited, because the terminal cancer patients are in more severe condition than patients in the former reports.
Hyponatremia can be managed by both conservative and medical treatment. By hydration, water restriction, or hypertonic saline infusion, most hyponatremic status can be successfully converted into eunatremia. Moreover, a new pharmacological treatment for hyponatremia by AVP-receptor antagonist has been highlighted [19]. The efficacy of AVP-receptor antagonist has already been demonstrated for the treatment of patients with non-cancer patients [20–23]. However, there are no high-quality study outcomes on terminal cancer patients. Waikar et al. demonstrated that the mortality rate is lower in patients, including metastatic cancer patients, with resolved hyponatremia than in those with persistent hyponatremia [3]. Doshi et al. found that cancer patients with corrected serum sodium level during admission were associated with lower mortality than those who did not [6]. Another previous study also concluded that failure to normalize serum sodium was a poor prognostic factor in patients with SCLC receiving chemotherapy [14]. Considering the present result, treatment of hyponatremia, such as conservative treatment or drug administration, may be a considerable interest in the aspect of improving clinical outcome in terminal cancer patients. Consequently, further studies are warranted to determine if interventions for hyponatremia affect the survival time of terminal cancer patients.
This is the first study to demonstrate the relationship between hyponatremia and survival time in terminal cancer patients. Its most notable feature is that the data, from the patients with different types of terminal cancer, were collected over 3 years in a palliative care unit. Furthermore, the PPS and CRP, which were previously recognized as independent prognostic factors, also showed significant prognostic values statistically, adding more reliability to this study [24–27].
However, there are several limitations in this study. First, this study is a retrospective observational study with data from a single center. Second, the data did not consider all the variables that cause hyponatremia, such as the use of diuretics or fluid replacement with free water. Finally, this study did not have follow-up data of serum sodium changes in patients.
In the future, a large-scaled, prospective study with terminal cancer patients should be conducted to better understand the prognostic role of hyponatremia, precisely. Furthermore, additional studies regarding the correction of hyponatremia also need to be examined.
Conclusion
Hyponatremia is an independent prognostic factor in terminal cancer patients and careful clinical concern is needed.
References
Schrier RW (2006) Water and sodium retention in edematous disorders: Role of vasopressin and aldosterone. Am J Med 119:S47–S53. doi:10.1016/j.amjmed.2006.05.007
Upadhyay A, Jaber BL, Madias NE (2009) Epidemiology of hyponatremia. Semin Nephrol 29(3):227–238. doi:10.1016/j.semnephrol.2009.03.004
Waikar SS, Mount DB, Curhan GC (2009) Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 122(9):857–865. doi:10.1016/j.amjmed.2009.01.027
Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE (2010) Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med 170(3):294–302
Zilberberg MD, Exuzides A, Spalding J, Foreman A, Jones AG, Colby C, Shorr AF (2008) Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients. Curr Med Res Opin 24(6):1601–1608. doi:10.1185/03007990802081675
Doshi SM, Shah P, Lei XD, Lahoti A, Salahudeen AK (2012) Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. Am J Kidney Dis 59(2):222–228. doi:10.1053/j.ajkd.2011.08.029
Berghmans T, Paesmans M, Body JJ (2000) A prospective study on hyponatraemia in medical cancer patients: Epidemiology, aetiology and differential diagnosis. Support Care Cancer 8(3):192–197
Raftopoulos H (2007) Diagnosis and management of hyponatremia in cancer patients. Support Care Cancer 15(12):1341–1347. doi:10.1007/s00520-007-0309-9
Sorensen JB, Andersen MK, Hansen HH (1995) Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in malignant disease. J Intern Med 238(2):97–110
Langfeldt LA, Cooley ME (2003) Syndrome of inappropriate antidiuretic hormone secretion in malignancy: Review and implications for nursing management. Clin J Oncol Nurs 7(4):425–430. doi:10.1188/03.CJON. 425-430
Liamis G, Milionis H, Elisaf M (2008) A review of drug-induced hyponatremia. Am J Kidney Dis 52(1):144–153. doi:10.1053/j.ajkd.2008.03.004
Castillo JJ, Vincent M, Justice E (2012) Diagnosis and management of hyponatremia in cancer patients. Oncologist 17(6):756–765. doi:10.1634/theoncologist.2011-0400
Hermes A, Waschki B, Reck M (2012) Hyponatremia as prognostic factor in small cell lung cancer—a retrospective single institution analysis. Respir Med 106(6):900–904. doi:10.1016/j.rmed.2012.02.010
Hansen O, Sorensen P, Hansen KH (2010) The occurrence of hyponatremia in SCLC and the influence on prognosis a retrospective study of 453 patients treated in a single institution in a 10-year period. Lung Cancer 68(1):111–114. doi:10.1016/j.lungcan.2009.05.015
Kawahara M, Fukuoka M, Saijo N, Nishiwaki Y, Ikegami H, Tamura T, Shimoyama M, Suemasu K, Furuse K (1997) Prognostic factors and prognostic staging system for small cell lung cancer. Jpn J Clin Oncol 27(3):158–165
Osterlind K, Andersen PK (1986) Prognostic factors in small cell lung cancer: multivariate model based on 778 patients treated with chemotherapy with or without irradiation. Cancer Res 46(8):4189–4194
Jacot W, Colinet B, Bertrand D, Lacombe S, Bozonnat MC, Daures JP, Pujol JL, network Oh (2008) Quality of life and comorbidity score as prognostic determinants in non-small-cell lung cancer patients. Ann Oncol 19(8):1458–1464. doi:10.1093/annonc/mdn064
Jeppesen AN, Jensen HK, Donskov F, Marcussen N, von der Maase H (2010) Hyponatremia as a prognostic and predictive factor in metastatic renal cell carcinoma. Brit J Cancer 102(5):867–872. doi:10.1038/sj.bjc.6605563
Gross P, Reimann D, Henschkowski J, Damian M (2001) Treatment of severe hyponatremia: Conventional and novel aspects. J Am Soc Nephrol 12(2):S10–S14
Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C (2006) Tolvaptan, a selective oral vasopressin V-2-receptor antagonist, for hyponatremia. New Engl J Med 355(20):2099–2112. doi:10.1056/Nejmoa065181
Gheorghiade M, Niazi I, Ouyang J, Czerwiec F, Kambayashi J, Zampino M, Orlandi C, Tolvaptan I (2003) Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: Results from a double-blind, randomized trial. Circulation 107(21):2690–2696. doi:10.1161/01.CIR.0000070422.41439.04
Gerbes AL, Golberg V, Gines P, Decaux G, Gross P, Gandjini H, Djian J, Grp VS (2003) Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: a randomized double-blind multicenter trial. Gastroenterology 124(4):933–939. doi:10.1053/gast.2003.50143
Saito T, Ishikawa SE, Abe K, Kamoi K, Yamada K, Shimizu K, Saruta T, Yoshida S (1997) Acute aquaresis by the nonpeptide arginine vasopressin (AVP) antagonist OPC-31260 improves hyponatremia in patients with syndrome of inappropriate secretion of antidiuretic hormone (SIADH). J Clin Endocrinol Metab 82(4):1054–1057. doi:10.1210/Jc.82.4.1054
Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, Eychmueller S, Glare P, Nabal M, Vigano A, Larkin P, De Conno F, Hanks G, Kaasa S (2005) Prognostic factors in advanced cancer patients: Evidence-based clinical recommendations—a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol 23(25):6240–6248. doi:10.1200/Jco.2005.06.866
Anderson F, Downing GM, Hill J, Casorso L, Lerch N (1996) Palliative performance scale (PPS): a new tool. J Palliat Care 12(1):5–11
Seow H, Barbera L, Dudgeon D, Howell D, Husain A, Atzema C, Sussman J, Liu Y, Earle C, Sutradhar R (2013) The association of the palliative performance scale and hazard of death in an ambulatory cancer population. J Palliat Med 16(2):156–162. doi:10.1089/jpm.2012.0239
Mahmoud FA, Rivera NI (2002) The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep 4(3):250–255
Conflicts of interests
We do not have any financial relationships to disclose. We have full control over all primary data and agree to allow Supportive Care in Cancer to review the data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, J., Ahn, S.H., Lee, Y.J. et al. Hyponatremia as an independent prognostic factor in patients with terminal cancer. Support Care Cancer 23, 1735–1740 (2015). https://doi.org/10.1007/s00520-014-2522-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2522-7