Summary
Objective
To investigate prevalence of vitamin D deficiency and its relation to clinical, anthropometrical, and biochemical findings in polycystic ovary syndrome (PCOS) and controls.
Design
Case-control prospective observational study.
Settings
Department of Internal medicine, L.P. University hospital.
Patient(s)
99 PCOS women and 66 controls.
Main outcome measure(s)
25-hydroxyvitamin D level (25(OH)D), anthropometric, endocrine, and metabolic parameters in both groups.
Results
There was no significant difference in 25(OH)D levels between PCOS women and controls (24.79 ± 10.77 vs 25.07 ± 10.14 ng/ml, p = 0.868) and also in the prevalence of 25(OH)D deficiency in both groups (80 vs 70 %; p = 0.138). Vitamin D-deficient PCOS patients had significantly higher body mass index (BMI), fasting insulin, and homeostasis model assessment-insulin resistance (median [quartiles]: 2.24 [1.38; 3.51] vs 1.23 [0.79; 1.66]; p< 0.05, age-and BMI-adjusted p = 0.036) and borderline higher glycemia (4.7 ± 0.5 vs 4.5 ± 0.4 mmol/l; p = 0.05; p_adj = 0.95) compared with vitamin D-deficient controls. PCOS women with metabolic syndrome (MS) had lower serum 25(OH)D compared with those without MS (20.6 ± 8.3 vs 25.9 ± 11.3 ng/ml, p = 0.049). 25(OH)D correlated positively with high-density lipoprotein cholesterol in all subjects (r = 0.159, p = 0.043) and negatively with luteinizing hormone/follicle-stimulating hormone ratio (r = − 0.211, p = 0.037).
Conclusion
Insulin resistance and other metabolic abnormalities in PCOS women seem to be related to PCOS rather than to vitamin D deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is the most common female endocrine disorder characterized by chronic anovulation and menstrual dysfunction, lower pregnancy success and infertility, biochemical and clinical signs of hyperandrogenism, and the presence of polycystic ovaries on ultrasound. It is typically identified during the early reproductive age or adolescence, and its prevalence is as high as 15 % when the broader Rotterdam criteria are applied and approximately 10 % in conditions of criteria Androgen Excess Society (AES) [1]. There is an evidence that patients with PCOS suffer from various metabolic disturbances such as visceral obesity, insulin resistance (IR), impaired glucose tolerance (IGT), dyslipidemia, type 2 diabetes mellitus (T2DM), and metabolic syndrome (MS) with the several-fold higher risk of arterial hypertension and coronary heart disease [2, 3].
Accumulating data suggest that vitamin D deficiency has been shown to be associated with impaired glucose metabolism, especially with impaired insulin secretion and failure of insulin action in both human and animal models. Low vitamin D status is suspected to be a risk factor for IGT, IR, and T2DM, which are also cardinal findings in PCOS [4].
A number of studies demonstrated the relationship between vitamin D levels and PCOS symptoms such as IR, hirsutism, menstrual cycle dysfunction, and infertility [5–7]. However, a potential role of vitamin D in the pathogenesis of PCOS is still unclear. Vitamin D is considered to influence the PCOS development via regulation of gene transcription and hormonal modulation of insulin metabolism as well as reproductive function [8]. Low levels of vitamin D might have potential role in the phenotype expression of PCOS. Vitamin D deficiency can lead to the manifestation of IR through the failure of insulin secretion and a decrease in insulin receptors’ expression. In addition, it can contribute to the development of follicular arrest and results in menstrual and fertility dysfunction in PCOS via calcium dysregulation. Reduction of sex hormone-binding globulin (SHBG) levels and an increased parathyroid hormone (PTH) production in vitamin D deficiency can lead to the worsening of hyperandrogenemia and its clinical manifestation as hirsutism, acne, and androgenic alopecia [9].
Current evidence about the role of 25-hydroxyvitamin D level (25(OH)D) in the development of reproductive and metabolic abnormalities, especially MS, typically found in PCOS has been still limited. The aim of this prospective study was to determine the prevalence of vitamin D deficiency in a selected group of Slovak PCOS women in comparison with control group and to assess the relationship between serum vitamin D levels and clinical and biochemical findings in PCOS.
Materials and methods
Study sample
This prospective study analyzed 99 women with PCOS, which were primarily identified in an outpatient department of gynecology, endocrinology, and diabetology and consequently examined in the 1st Department of Internal medicine L. Pasteur University Hospital, Košice, Slovakia over a period between May 2010 and March 2013. The average age of PCOS women was 29 ± 5 years (range 20–41, median 29). The diagnosis of PCOS was based on the Rotterdam criteria [10], i.e., two out of three of the following features are required for diagnosis confirmation: (1) oligoovulation and/or anovulation, (2) clinical and/or biochemical signs of hyperandrogenism, and (3) presence of polycystic ovaries on ultrasound examination (defined as the presence of ≥ 12 follicles in each ovary measuring 2–9 mm in diameter and/or increased ovarian volume > 10 cm3). Oligo-and/or anovulation were considered by the presence of oligomenorrhea or amenorrhea. Hyperandrogenism was determined by the clinical presence of hirsutism (modified Ferriman–Gallwey score ≥ 6), acne, or alopecia and/or elevated androgen levels. MS was defined according to the National Cholesterol Education Program (NCEP) and the Adult Treatment Panel III (ATP III) guidelines in all subjects [11]. The exclusion criteria were Cushing’s syndrome, congenital adrenal hyperplasia, hyperprolactinemia, androgen-secreting tumors, and severe comorbidities, which were ruled out by careful examination.
Control group consisted of 66 healthy regularly menstruating randomly selected age-matched women without clinically apparent hyperandrogenism. The average age was 28 ± 4 years (range 21–44, median 28). The study participants did not take any medication known to affect endocrine parameters, carbohydrate metabolism, or serum lipid profile. The study design was approved by the local ethics committee and fulfilled the ethical guidelines of the most recent declaration of Helsinki. Written informed consent was signed by each patient.
Standard anthropometric data were obtained from each subject (height, weight, waist, and hip circumference). Waist circumference was measured in a standing position two centrimeters above umbilicus, and hip circumference was measured in a standing position at the maximum circumference over the buttocks. The body mass index (BMI) was calculated as the weight/height2 (kg/m2). According to body weight and BMI, 61 % of PCOS patients had normal BMI (18–24.9 kg/m2), 14 % were overweight (BMI 25–29.9 kg/m2), and 25 % were obese (BMI > 30 kg/m2). Blood samples for hormonal (total testosterone, free testosterone, dehydroepiandrosterone sulphate (DHEAS), SHBG, PTH, luteinizing hormone (LH), follicle-stimulating hormone (FSH), LH/FSH ratio, 25(OH)D), and metabolic (fasting glucose, fasting insulin, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol and triglycerides) parameters were obtained in the morning between 7:00 to 9:00 a.m.after overnight fasting during the early follicular phase of each patient’s spontaneous or progestin/induced menstrual cycle. IR was estimated using the homeostasis model assessment-insulin resistance (HOMA-IR). The free androgen index (FAI) was calculated as testosterone (nmol/L)/SHBG (nmol/L) × 100. According to consensus in clinical praxis, vitamin D deficiency was defined as serum level of 25(OH)D level < 30 ng/ml [12].
Biochemical analysis
Serum 25(OH)D levels were measured using a commercially available radioimmunoassay kit (IDS Ltd.) with intra- and inter-assay coefficients of variation of 5.0 vs 7.3 %, respectively. Hormonal levels of LH, FSH, testosterone, DHEAS, and SHBG were determined using Chemiluminescent Microparticle Immunoassay (analyzer Architect, module C). Free testosterone was detected by radioimmunoassay using commercially available kits of DIA Source. Fasting insulin was assessed radioimmunologically using kits Immunotech France. Fasting glucose, PTH, total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides were measured by routine methods.
Statistical analysis
Data are presented as mean ± standard deviation or count (percentages), unless stated otherwise. Variables with non-normal distribution were logarithmically transformed before analysis. Comparisons between PCOS women and control group were performed by Student’s t-test. Correlations between variables were evaluated using Pearson’s correlation coefficient. p Values of < 0.05 were considered statistically significant. Statistical analyses were performed with SPSS software version 15 (IBM, USA).
Deseasonalized vitamin 25(OH)D levels were calculated by regressing the measured 25(OH)D level on the periodic sinusoid function and then adding the residuals to the seasonal average to create a deseasonalized vitamin D level for each individual as described previously [13]. Given the assumptions and limitations inherent in deseasonalized analysis, we performed and report analyses using unadjusted 25(OH)D values. Importantly, results from analyses using deseasonalized data did not materially differ from unadjusted analyses (data not shown).
Results
The prevalence of vitamin D deficiency was high and similar in both groups, 79 of 99 PCOS women (80 %) and 46 of 66 control group subjects (70 %) (p = 0.138). Distribution of subcategories of decreased 25(OH)D levels between 20–29.9 ng/ml, 10–19.9 ng/ml, and <10 ng/ml in PCOS women was 42 %, 34 %, and 3 %, respectively. Only 20 of 99 PCOS women (20 %) and 20 of 66 controls (30 %) presented with sufficient 25(OH)D levels. There was no significant difference in mean 25(OH)D concentration between patients with PCOS and control group (24.79 ± 10.77 vs 25.07 ± 10.14 ng/ml, p = 0.868). Comparison of anthropometrical and biochemical findings in vitamin D-deficient PCOS women and control group subjects is shown in Table 1.
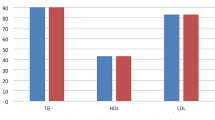
In subjects with low vitamin D status, patients with PCOS had significantly higher weight, waist and hip circumference, BMI, fasting insulin levels, HOMA-IR index (median [quartiles]: 2.24 [1.38–3.51] vs 1.23 [0.79–1.66]; p< 0.001, age- and BMI-adjusted p_adj = 0.036), borderline higher glycemia (mean ± SD: 4.7 ± 0.5 vs 4.5 ± 0.4 mmol/l; p = 0.05, age- and BMI-adjusted p_adj = 0.95), higher serum lipids (total cholesterol, LDL cholesterol, and triglycerides), and lower HDL cholesterol as well as higher serum androgen level (total testosterone, free testosterone) compared with vitamin D-deficient control group subjects.
Comparison of anthropometric and biochemical findings in PCOS subjects according to 25(OH)D status is shown in Table 2. Vitamin D-deficient PCOS women had higher serum triglycerides levels with borderline significance compared to nondeficient PCOS (1.44 ± 0.93 vs 1.03 ± 0.46 mmol/l; p = 0.051). Vitamin D-deficient PCOS women tended to have higher HOMA-IR compared with vitamin D-sufficient PCOS women, however without significance. They did not differ in other variables. Comparison of HOMA-IR index between PCOS and control group in subgroup of vitamin D-deficient subjects (p< 0.001; adjusted for age, BMI: p_adj = 0.036) and vitamin D nondeficient subjects (p = 0.015; p_adj = 0.748) is shown in Fig. 1.
A total of 21 of 99 PCOS women (21.2 %) presented with MS according to NCEP ATPIII guidelines. Patients with PCOS and MS had significantly lower 25(OH)D levels compared to PCOS without MS (20.6 ± 8.3 vs 25.9 ± 11.3 ng/ml, p = 0.049).
25(OH)D levels correlated positively with HDL cholesterol in all subjects (Pearson’s r = 0.159, p = 0.043). Negative correlation between 25(OH)D and LH/FSH was found in PCOS group (Pearson’s r = -0.211, p = 0.037) (Fig. 2). There was no significant correlation between 25(OH)D and serum androgen levels, SHBG and other metabolic and anthropometric parameters (data not shown).
a Positive correlation between 25-hydroxyvitamin D level (25(OH)D) levels and serum high-density lipoprotein cholesterol in all subjects (r = 0.159, p = 0.043). b Negative correlation between 25(OH)D levels and luteinizing hormone/follicle-stimulating hormone ratio in polycystic ovary syndrome (r = − 0.211, p = 0.037)
Assessment of hirsutism according to Ferriman–Gallwey score was performed in a subgroup of 89 PCOS women. Hirsutism was evident in 51 PCOS women (57.3 %), 38 subjects (42.7 %) presented without clinically manifested hirsutism. PCOS women with and without hirsutism had comparable vitamin D levels (24.8 ± 10.8 vs 26.0 ± 11.2 ng/ml, p = 0.60). Hirsute PCOS women tended to have higher BMI (28.70 ± 6.66 vs 25.81 ± 6.94 kg/m2, p = 0.050) as well as higher HOMA-IR (2.86 [1.35–3.83] vs 1.81 [1.08–3.18], p = 0.052) than those without hirsutism. The difference in HOMA-IR lost significance after adjustment for age and BMI (p_adj = 0.332).
In all, 89 of 99 PCOS women (89.9 %) presented with manifest menstrual cycle disturbance such as oligomenorea or amenorea. Vitamin D deficiency was observed in 71 oligomenorrhoic PCOS women (79.8 %). There was no significant difference in the prevalence of irregular menstrual cycle between vitamin D-deficient and nondeficient PCOS women (90 vs 89.9 %, p = 0.987).
Discussion
The prevalence of vitamin D deficiency was high in both patients and controls. We did not observe any significant difference in serum 25(OH)D levels between PCOS women and controls. Data on the prevalence of vitamin D status in PCOS women are limited and differ among studies. Some studies showed that vitamin D levels are similar in women with and without PCOS [14–16], what is in agreement with our results. However, Wehr et al. [17] reported lower serum vitamin D levels in PCOS women compared with controls (25.7 vs 32 ng/ml). In contrast, Mahmoudi et al. [18] described significantly higher serum vitamin D levels in PCOS women compared to BMI- and age-matched controls (29.3 vs 19.4 ng/ml).
Vitamin D deficiency is very common worldwide, and according to previous data 10–60 % of adults have 25(OH)D levels lower than 20 ng/ml [19]. The recommendations for serum 25(OH)D levels are, in general population, at least 30 ng/ml [12]. In our study, 80 % of PCOS and 70 % of control group subjects were below this value. Wehr et al. [6] described vitamin D deficiency in 72.8 % of PCOS women, but this study did not contain a control group. Another observational study on 52 women (25 in PCOS group and 27 in control group) has found severe vitamin D deficiency (25(OH) < 10 ng/ml)) in 44.0 and 11.2 % of subjects in the PCOS and the control group, respectively (p = 0.047) [14]. Vitamin D deficiency has been linked to an increased risk of many chronic diseases such as cardiovascular, autoimmune, infectious diseases, T2DM, cancer, and psychological disorders such as depression and chronic pain and may affect the function of all systems of the body, indicating the importance of sufficient vitamin D status [20].
In the current study, vitamin D deficiency in PCOS women was presented with significantly higher body weight (BMI, waist circumference), higher IR and serum lipids (total cholesterol, LDL cholesterol, triglycerides (TAG), and higher serum androgen levels compared with vitamin D deficiency controls. PCOS women with MS had lower serum 25(OH)D compared with those without MS (p = 0.049). PCOS women with vitamin D deficiency had marginally higher serum triacylglycerides levels (p = 0.051) and tended to have higher HOMA-IR compared to PCOS with sufficient vitamin D levels. 25(OH)D correlated positively with HDL cholesterol in all subjects (r = 0.159, p = 0.043) and negatively with LH/FSH ratio (r = − 0.211, p = 0.037).
Recently published observational studies investigated the relationship between 25(OH)D levels and abdominal obesity and IR. There was found negative correlation between 25(OH)D and BMI, waist circumference, fasting glucose, fasting insulin, HOMA-IR index, and triglycerides [6, 7]. Similarly, Hahn et al. [5] described that increased body weight in PCOS negatively correlated with 25(OH)D levels. Wehr et al. demonstrated in 206 PCOS women a significant association of low 25(OH)D levels with components of the MS. In this study, 25(OH)D levels were significantly lower in patients with PCOS and MS vs PCOS women without features of MS, what is in agreement with our results. 25(OH)D levels were also a significant and independent predictor of IR and insulin sensitivity in a multivariate regression analysis in both PCOS and control women [6]. Patra et al. [21] observed in 60 PCOS women, divided into 3 groups according to vitamin D status, that the patients with vitamin D deficiency demonstrated the highest HOMA-IR values compared with those with vitamin D insufficiency and sufficiency; vitamin D was the best predictor of IR. In study of Ngo et al. [22], 25(OH)D levels directly correlated with Quantitative Insulin Sensitivity Check Index (QUICKI) and vitamin D deficiency was associated with low QUICKI in entire cohort of young women, including a PCOS subgroup. Several studies showed negative correlation between vitamin D levels and HOMA-IR and obesity in PCOS women [5–7, 15], but in two of them this relation disappeared when HOMA-IR values were adjusted for BMI [6, 15, 23]. Tsakova et al. observed that almost 2/3 of the women with PCOS and/or obesity appeared to be vitamin D-deficient. Women with obesity, especially visceral (with or without PCOS), had significantly lower levels of 25(OH)D compared with lean PCOS subjects. Women with and without MS however did not differ significantly in 25(OH)D levels. Women with normal BMI had higher 25(OH)D levels compared to overweight and obese (p = 0.028) [24]. Otherwise, authors Sahin et al. demonstrated presence of IR in 30 % of lean PCOS women, and BMI was not found to correlate with IR. Authors did not observe an association between vitamin D levels and IR in lean women with PCOS, so it is supposed that other intrinsic mechanism and genetic predisposition are related to IR in lean women with PCOS, independently of obesity and vitamin D deficiency [25].
Thus, it is not clear if vitamin D deficiency is related to the presence of IR or to the presence of obesity. It has been suggested that obesity may play a confounding role in the association between vitamin D and IR in PCOS. A recent study found that low levels of 25(OH)D were significantly determined by the degree of adiposity and were not directly affected by the development of IR [26]. It has been shown that in obese individuals there is a higher proportion of vitamin D, which is fat-soluble, sequestred in adipose tissue and thereby the bioavailability of vitamin D is low [23]. However, in study of Li et al. [14], HOMA-IR was significantly higher in subgroup of vitamin D-deficient PCOS even after adjustment for age and BMI. Despite the strong association between obesity and IR, multiple studies have demonstrated that women affected by PCOS have more severe IR than expected on the basis of their body weight, and therefore vitamin D status has been designed as a possible contributing factor for IR in PCOS women [23]. In our sample, vitamin D-deficient PCOS women tended to have higher HOMA-IR compared with vitamin D nondeficient PCOS, but it was not significant. Statistically significant difference in HOMA-IR after adjustment for age and BMI was present between PCOS and control group in subgroup of vitamin D-deficient women.
Hyperinsulinemia, IR, and abdominal obesity may have a complementary effect on the pathogenesis of PCOS. The exact mechanism explaining the relationship between vitamin D and IR is not entirely understood, but some studies showed that biologically active form of vitamin D may enhance insulin action supporting insulin secretion and release, increase insulin receptor expression, and may suppress the overproduction of proinflammatory cytokines that are suggested to mediate IR [27]. Vitamin D deficiency decreases insulin biosynthesis and release, and it is supposed that the low 25(OH)D concentration could be a triggering factor of IR, which is a common feature in PCOS [28]. Furthermore, vitamin D receptor gene polymorphism has been associated with an increased risk of PCOS or related metabolic and endocrine phenotype, suggesting the role of vitamin D in the pathogenesis of PCOS [29].
Few intervention trials had been carried out bringing controversial findings about the effect of vitamin D supplementation on metabolic abnormalities especially on IR typically found in women with PCOS. Ardabili et al. in randomized, placebo-controlled, double-blinded trial evaluated 50 PCOS women with vitamin D deficiency. Authors did not observe any significant decrease in fasting serum insulin, glucose levels, BMI, and HOMA-IR after 2 months supplementation of 3 doses of 50,000 IU vitamin D3 or placebo. Authors demonstrated a significant decrease of total cholesterol, triacylglyceroles, and very low-density lipoprotein cholesterol [30]. Bonakdaran et al. carried out a randomized control trial among 51 PCOS women and compared efect of calcitriol, metformin, or placebo therapy on metabolic components and ovulation evidence in PCOS. Authors did not demostrated effect of calcitriol therapy on BMI, fasting glucose, and insulin levels, HOMA/IR tended to decrease, but not significantly [31]. Similarly, Raja-Khan et al. did not determine the effect of high-dose vitamin D supplementation on insulin sensitivity measured as QUICKI. The authors observed a trend toward lower 2-h insulin and lower 2-h glucose level in oral glucose tolerance test and protective effect of vitamin D supplementation on blood presure [32]. The study of Selimoglu et al. has brought contrasting results. The authors observed in 11 obese PCOS women a significant decrease in HOMA-IR after the administration of a single dose of 300,000 IU cholekalciferol [33].
Low serum levels of 25(OH)D have also been associated with an unfavorable lipid profile, which could possibly explain the relationship between vitamin D status and an increased risk of cardiovascular diseases and cardiovascular mortality [14, 34]. In cross-sectional studies, serum 25(OH)D was positively associated with HDL cholesterol resulting in a favorable LDL cholesterol (or total cholesterol) to HDL cholesterol ratio. There was also a uniform agreement between studies about negative relation between serum 25(OH)D and triglycerides [35]. Studies in PCOS women have brought similar results. Authors demonstrated negative correlation of 25(OH)D levels with total cholesterol, triglycerides, total cholesterol/HDL cholesterol, and leptin [5, 6]. 25(OH)D level positively correlated with HDL cholesterol level [5, 6, 14], that is, in consistency with our results. In one study, the association of vitamin D deficiency with HDL cholesterol was independent of BMI and waist-to-hip ratio [14]. It supports the increasing evidence that vitamin D deficiency is associated with multiple metabolic risk factors in PCOS women.
We also confirmed negative correlation between vitamin D and LH/FSH ratio; however, this finding has not yet been described in the literature. It could be related to anovulation and luteal insuficiency; however, we were not able to demonstrate its relation to LH only.
In our study, we did not detect significant differences in vitamin D levels between hirsute and non-hirsute PCOS women. Hirsute PCOS women were more obese than non-hirsute and both groups had comparable HOMA-IR. Only few observational studies confirmed the relationship between markers of hyperandrogenism and vitamin D status. Hirsute PCOS women presented lower vitamin D levels in comparison with BMI-matched controls (17 vs 29 ng/ml) [36], they also had lower vitamin D levels compared with those without hirsutism (21.4 vs 26.8 ng/ml) [6]. 25(OH)D levels negatively correlated with a degree of hirsutism [5, 6], total testosterone, DHEAS [7], and FAI [5, 14] and positively with SHBG [5, 14]. Correlation between vitamin D and hyperandrogenism in PCOS may be due to the reduction of SHBG levels that result from presence of visceral obesity and IR [5, 14].
In this study, we did not observe any relationship between 25(OH)D levels and serum androgen levels (total testosterone, free testosterone, DHEAS, and FAI) as well as SHBG. A similar result was published in the observational study by Panidis et al. [15], who did not find any differences between PCOS and healthy control women with respect to vitamin D status.
Accumulating data from animal and human studies suggest that vitamin D is involved in many functions of reproductive system in both genders. Vitamin D deficiency is associated with calcium dysregulation, which can influence foliculogenesis and contribute to the development of follicular arrest and may result in menstrual and fertility dysfunction in PCOS [9]. In PCOS patients, we did not detect differences in the prevalence of irregular menstrual cycle according to vitamin D status as well as differences in serum 25(OH)D levels between oligomenorheic PCOS and PCOS with normal menstrual cycle. The observational study of Pal et al. [37] has demonstrated that infertile women with PCOS had lower levels of vitamin D than infertile women with normal ovulation and each unit of vitamin D level reduced the likelihood of PCOS by 96 %.
This is the comprehensive study providing complex assessment of relation between vitamin D deficiency and metabolic abnormalities, hirsutism and menstrual cycle disturbances in PCOS including large group of PCOS women as well as control group subjects. We did not demonstrate any difference in serum vitamin D concentration between PCOS patients and control group subjects. Low vitamin D status was associated with MS, but other metabolic and reproductive abnormalities seem to be related to PCOS rather than to vitamin D deficiency. It was already described that both vitamin D deficiency and PCOS are associated with features of MS, but it is still unclear whether vitamin D deficiency may contribute to the metabolic disturbances commonly found in PCOS women. To prove these findings and to evaluate the effect of vitamin D on metabolic and endocrine disturbances in PCOS large interventional studies are needed in PCOS women.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
Informed consent was obtained from all patients for being included in the study.
References
March WA, Moore VM, Willsen KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–51.
Alvarez-Blasco F, Botella-Carretero JI, San Millan JL, Escobar-Morreale HF. Prevalence and characteristics of the polycystic ovary syndrome in overweight and obese women. Arch Intern Med. 2006;166(19):2081–6.
Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev. 2003;24(3):302–12.
Ortlepp JR, Metrikat J, Albrecht M, von Korff A, Hanrath P, Hoffmann R. The vitamin D receptor gene variant and physical activity predicts fasting glucose levels in healthy young men. Diabet Med. 2003;20(6):451–4.
Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114(10):577–83.
Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR, et al. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol. 2009;161(4):575–82.
Yildizhan R, Kurdoglu M, Adali E, Kolusari A, Yildizhan B, Sahin HG, et al. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch Gynecol Obstet. 2009;280(4):559–63.
Mahmoudi T. Genetic variation in the vitamin D receptor and polycystic ovary syndrome risk. Fertil Steril. 2009;92(4):1381–3.
Thomson RL, Spedding S, Buckley JD. Vitamin D in aetiology and management of polycystic ovary syndrome. Clin Endocrinol. 2012;77(3):343–50.
Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.
National Cholesterol Education Program (NCEP). Expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment panel III). Circulation. 2002;106:3143–421.
Bouillon R, Van Schoor NM, Gielen E, Boonen S, Mathieu C, Vanderschueren D, et al. Optimal vitamin D status: a critical analysis on the basis of evidence-based medicine. Clin Endocrinol Metab. 2013;98(8):1283–304.
Weinstock-Guttman B, Zivadinov R, Ramanathan M. Inter-dependence of vitamin D levels with serum lipid profiles in multiple sclerosis. J Neurol Sci. 2011;311(1–2):86–91.
Li HWR, Brereton RE, Anderson RA, Wallace AM, Co HK. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism. 2011;60(10):1475–81.
Panidis D, Balaris C, Farmakiotis D, Rousso D, Kourtis A, Balaris V, et al. Serum parathyroid hormone concentrations are increased in women with polycystic ovary syndrome. Clin Chem. 2005;51(9):1691–7.
Kim JJ, Choi YM, Chae SJ, et al. Vitamin D deficiency in women with polycystic ovary syndrome. Clin Exp Reprod Med. 2014;41(2):80–5.
Wehr E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B. Vitamin D associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol. 2011;164(5):741–9.
Mahmoudi T, Gourabi H, Ashrafi M, Yazdi RS, Ezabadi Z. Calciotropic hormones, insulin resistance, and the polycystic ovary syndrome. Fertil Steril. 2010;93(4):1208–14.
Lips P. Worldwide status of vitamin D nutrition. J Steroid Bioch Mol Biol. 2010;121(1–2):297–300.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81.
Patra SK, Nasrat H, Goswami B, Jain A. Vitamin D as a predictor of insulin resistance in polycystic ovarian syndrome. Diabetes Metab Syndr. 2012;6(3):146–9.
Ngo DT, Chan WP, Rajendran S, et al. Determinants of insulin responsiveness in young women: impact of polycystic ovarian syndrome, nitric oxide, and vitamin D. Nitric Oxide. 2011;25(3):326–30.
Krul-Poel YH, Snackey C, Louwers Y, et al. The role of vitamin D in metabolic disturbances in polycystic ovary syndrome: a systematic review. Eur J Endocrinol. 2013;169(6):853–65.
Tsakova AD, Gateva AT, Kamenov ZA. 25(OH) vitamin D levels in premenopausal women with polycystic ovary syndrome and/or obesity. Int J Vitam Nutr Res. 2012;82(6):399–404.
Sahin S, Eroglu M, Selcuk S, et al. Intrinsic factors rather than vitamin D deficiency are related to insulin resistance in lean women with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2014;18(19):2851–6.
Muscogiuri G, Policola C, Prioletta A, Sorice G, Mezza T, Lassandro A, et al. Low levels of 25(OH)D and insulin-resistance: 2 unrelated features or a cause-effect in PCOS? Clin Nutr. 2012;31(4):476–80.
Teegarden D, Donkin SS. Vitamin D: emerging new roles in insulin sensitivity. Nutr Res Rev. 2005;22(1):85–92.
Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813–8.
Wehr E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol. 2011;164(5):741–9.
Ardabili HR, Gargari BP, Farzadi L. Vitamin D supplementation has no effect on insulin resistance assessment in women with polycystic ovary syndrome and vitamin D deficiency. Nutr Res. 2012;32(3):195–201.
Bonakdaran S, Mazloom Khorasani Z, Davachi B, Mazloom Khorasani J. The effects of calcitriol on improvement of insulin resistance, ovulation and comparison with metformin therapy in PCOS patients: a randomized placebo-controlled clinical trial. Iran J Reprod Med. 2012;10(5):465–72.
Raja-Khan N, Shah J, Stetter CM, et al. High-dose vitamin D supplementation and measures of insulin sensitivity in polycystic ovary syndrome: a randomized, controlled pilot trial. Fertil Steril. 2014;101(6):1740–6.
Selimoglu H, Duran C, Kiyici S, et al. The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J Endocrinol Invest. 2010;33(4):234–8.
Killkinen A, Knekt P, Aro A, Rissanen H, Marniemi J, Heliövaara M, et al. Vitamin D status and the risk of cardiovascular disease death. Am J Epidem. 2009;170(8):1032–9.
Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res. 2011;50(4):303–12.
Glintborg D, Andersen M, Hagen C, Hermann AP. Higher bone mineral density in Caucasian, hirsute patients of reproductive age. Positive correlation of testosterone levels with bone mineral density in hirsutism. Clin Endocrinol. 2005;62(6):683–91.
Pal L, Shu J, Zeitlian G. Vitamin D insufficiency in reproductive years may be contributory to ovulatory infertility and PCOS. Fertil Steril. 2008;90:14.
Acknowledgments
We thank the study participants whose continued dedication and commitment made this work possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Figurová, J., Dravecká, I., Javorský, M. et al. Prevalence of vitamin D deficiency in Slovak women with polycystic ovary syndrome and its relation to metabolic and reproductive abnormalities. Wien Klin Wochenschr 128, 641–648 (2016). https://doi.org/10.1007/s00508-015-0768-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-015-0768-9