Abstract
Thermal stress has a direct effect on various types of DNA damage, which depends on the stage of the cell cycle when the cell is exposed to different climate conditions. A literature review was conducted to systematically investigate and assess the overall effect of heat stress and DNA damage following heat exposure. In this study, electronic databases including PubMed, Scopus, and Web of Science were searched to find relevant literature on DNA damage in different ambient temperatures. Outcomes included (1) measurement of DNA damage in heat exposure, (2) three different quantification methods (comet assay, 8-hydroxy-2-deoxyguanosine (8-OHdG), and γ-H2AX), and (3) protocols used for moderate (31) and high temperatures (42). The evidence shows that long exposure and very high temperature can induce an increase in DNA damage through aggregate in natural proteins, ROS generation, cell death, and reproductive damage in hot-humid and hot-dry climate conditions. A substantial increase in DNA damage occurs following acute heat stress exposure, especially in tropical and subtropical climate conditions. The results of this systematic literature review showed a positive association between thermal stress exposure and inhibition of repair of DNA damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effect of global warming on climate change has been noticeable throughout the increase in heat strain among humans. Health risks due to heat stress have become a major concern for experts (Golbabaei et al. 2020). The direct effects of thermal stress on human health include fatigue (Kjellstrom et al. 2014), reduced psychomotor performance (Xiang et al. 2014), reduced alertness, increased core body temperature (Habibi et al. 2015), increased sweat rate (Vanos et al. 2019), and dehydration. It is well known that thermal stress at the workplace can cause occupational health hazards, and climate change can aggravate the effects of these hazards and create new risks (Habibi et al. 2021a). Thus, maintaining safety and health promotion in warm workplaces will keep being an important challenge (Habibi et al. 2021b). Heat stress (heat shock, hyperthermia) is considered an important factor for biological effects, damage to cellular structures, interference with important functions, and disturbance of vital enzymes activity (Lepock and Borrelli 2005). Cell injury such as genotoxicity, oxidative stress, and DNA damage is induced by hot-dry and hot-wet environments in several workplaces including steel industry and agriculture. Cellular responses to thermal stress such as various cellular compartments and enzymes such as activating signaling pathways induce the transient heat shock proteins (Hsps) expression (Nasr et al. 2019; Richter et al. 2010). Heat radiosensitization is a physical hazard in which cells are exposed to thermal stress and ionizing radiation (IR) that can increase double-stranded DNA breaks (DSBs) and other agents. In addition, heat stress can inhibit some DNA repair pathways including base excision repair (BER) and nucleotide excision repair (NER) (Kantidze et al. 2016). The damaged DNA, the opposite of lipids and proteins, cannot be removed through cell mechanisms and must be repaired, otherwise, it can lead to lethal mutations and cancer cells (Evans et al. 2004). Physical (heat stress and radiation (ultraviolet (UV) and IR radiation)) and chemical agents can inhibit DNA repair mechanisms and act as exogenous DNA damage factors (Mohammadi et al. 2021). In summary, high and moderate temperature directly results in DNA damage and effect on cellular macromolecules functions, respectively (Milani and Horsman 2008). Interestingly, the type of the heat stress-induced DNA damage depends on the stage of the cell cycle, such as S phase (which leads to top 1 dependent single-stranded DNA breaks (SSBs)) or G1–G2 stage (which induces DSB formation), when the cell is exposed to different climate conditions (Kantidze et al. 2016). Although some studies have investigated the effect of heat stress on DNA damage, no comprehensive study has been done to evaluate the effect of different temperatures and humidity on DNA damage. DNA damage in response to exposure to thermal stress and whole-body hyperthermia (WBH) in mammals has not been investigated thoroughly in a systematic literature review. The aim of this study was to systematically investigate data reporting DNA damage following heat stress exposure, and explore their relationships. There are inconsistencies about thermal stress-induced oxidative DNA damage in exposure to very hot and warm climate conditions, and this review will aim to clear this subject. Furthermore, the possible physiological and pathological responses to heat stress-induced DNA damage need to be investigated in different climate conditions.
Materials and methods
Bibliography search strategy
A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews (PRISMA) statement (Shamseer et al. 2015). Databases such as PubMed, Scopus, and Web of Science were searched for articles published from 2000 to 18.04.2020. All the search terms related to “heat stress” were found by the PubMed Mesh system, and also a specialist’s opinion about synonyms of terms in a combination with “DNA damage.” The search syntax was produced using keywords and synonyms searched in the title, abstract, or keyword fields in the databases. In addition, to find relevant studies, the reference lists of the included studies were manually searched. Databases were investigated using the following search syntaxes to find the relevant studies.
Scopus
TITLE-ABS-KEY(“Heat shock” OR “heat stress” OR “IR radiation” OR Hyperthermia OR “DNA injury” OR (DNA AND injury*)) AND TITLE-ABS-KEY(“DNA damage” OR (DNA AND damage*) OR “DNA repair” OR (DNA AND repair*) OR “DNA replication” OR (DNA AND replicate*)).
At first, all titles and abstracts were checked for inclusion by two reviewers (PH and AH). Then, the full texts of the articles were reviewed (n = 15). Two articles were included from the reference lists and one article was included from an additional search in the Google Scholar.
Eligibility criteria
The “PICO” strategy for systematic exploratory review was: P (humans and animals), I (heat stress), C (DNA), and O (DNA damage). The inclusion/exclusion criteria are shown in Table 1. In addition, the full-text articles and conference papers that were not available were excluded from the study.
Inclusion/exclusion criteria
All published studies were checked for the following criteria: (1) the study assessed humans and animals; and (2) combining keywords used heat stress, heat shock, heat strain, IR radiation, DNA damage, DNA repair, and DNA replication. Note that for this review, we used the term DNA damage to encompass SSBs, DSBs, and nucleotide base oxidation. One investigator initially reviewed records extracted from all databases and applied the inclusion/exclusion criteria to identify eligible studies for inclusion in agreement with at least three authors. The inclusion/exclusion criteria are shown in Table 1. The ambient temperature was defined as hot temperature (over 41) and moderate temperature (around 31–33) (Golbabaei et al. 2020; Prandini et al. 2005). To minimize the limitation of various biological samples, studies using urine samples, RBC count, and muscle cells were also excluded. In addition, mathematical models and dynamics of the heat stress response of the cells were excluded. We concentrated on studies with clear and direct effects of thermal stress exposure on DNA damage and did not include the studies in which physical and chemical hazards were a byproduct of exposure to other agents (e.g., air pollution, cancer therapy, and acute injury) (Table 1).
Data extraction and quality assessment
After conducting screening and selection, two checklists were applied for data extraction. The first checklist included the characteristics of the studies such as the first author’s name, publication year, location, participants, biomarkers, protocols, techniques, findings, and quality scores. Two independent reviewers (PH and AH) assessed the methodological quality of included studies using the 16-item quality assessment tool for studies with diverse designs (QATSDD) (Sirriyeh et al. 2012). The characteristics of the participants (humans and animals such as the sample size, age, and sex) for in vivo studies, type of cells for in vitro studies, climate conditions (ambient temperature, relative humidity, etc.), assayed biomarkers, and methods of DNA quantification were extracted by two investigators.
The outcome measure of DNA damage was expressed using multiple descriptors, and about the comet assay technique including DNA in the tail (%), tail moment, and the length of DNA migration (tail length) (Evans et al. 2004). In this study, 8-OHdG, γ-H2AX, and HSPs were as considered biomarkers and tRNAs sensors. In addition, the analytical approach including high-performance liquid chromatography (HPLC), enzyme-linked immunosorbent assay (ELISA), fluorescent in situ hybridization (FISH), 8-OHdG (pg/ml), and 8-OHdG/105 dG was also reported [8-OHdG (ng/ml) or (pg/ml) and 8-OHdG/105 dG correspond to HPLC and ELISA methods, respectively].
Results
Search results
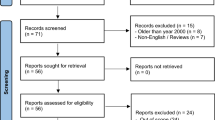
Figure 1 presents a detailed list of search results. The initial electronic search yielded 36,902 papers. Then, after removing duplicate records, 31,683 papers were screened through reviewing their titles and abstracts. In total, 47 articles were excluded. After title and abstract screening, 15 articles were considered eligible for full-text evaluation. Subsequently, 17 articles were included in the qualitative analysis. Table 2 summarizes the characteristics of the included studies.
Descriptive analysis
Our review identified 17 relevant studies out of 36,902 were eligible for data extraction, of which 12 presented in vitro studies in different climate conditions and cells including human HeLa cells, human fibroblasts, and human ESC. Five were in vivo studies in animal and human cells including lymphocytes, germ cells, cerebellum and hippocampus, and spermatozoa. The results of these studies showed that the effect of thermal stress on DNA damage had the greatest impact. Most of the studies have also emphasized the impacts of thermal stress including the spread of negative impacts on oxidative DNA damage in the future, how high temperatures and humidity creates hyperthermic cell killing risks and inhibit the repair of DNA damage challenges; how thermal stress will affect the integrity of the genome, and how these are often intertwined with heat shock responses. Four publications came from Russia, three from the USA, two from Japan, two from Canada, one from Australia, one from India, one from Taiwan, one from Italy, one from Ukraine, and one from Scotland. To measure DNA damage, five studies used comet assay, two used FISH, three used ELISA kit, two used surviving fraction, one used MN assay, one used gene chip system and bioinformatics tools, one used gel electrophoresis (EMSA), one used PFGE, and one used immunofluorescence. Concerning the biomarker and the techniques used to quantify DNA damage, 2 studies used 8-OHdG (Houston et al. 2018; Liu et al. 2015) with either comet assay or ELISA kit. A total of 4 studies used tail moment, tail length, and tail DNA (%), with the comet assay technique (Roti Roti et al. 2010; Ryabchenko et al. 2013; Velichko et al. 2012, 2015), one used HSF with FISH or surviving fraction (Bettaieb and Averill-Bates 2015), and 3 studies used γ-H2AX with immunofluorescence (molecular probes), PFGE, and comet assay (Hunt et al. 2007; Laszlo and Fleischer 2009; Velichko et al. 2012). Of the studies using different climate conditions, all of the studies were conducted in a range of (33–50) mild to hot temperatures.
Discussion
In our review, we explored the effects of heat stress on DNA damage in various cells. Heat stress triggers active and passive cellular reactions that depend on environmental conditions as an important factor and the dominant response. High temperatures can induce the denaturation of thermal proteins and impose adverse effects on local proteins, disrupting protein synthesis and inducing DNA damage via affecting intracellular metabolic pathways and components (Lepock and Borrelli 2005). On the other hand, DNA repair suppression and complications such as cellular apoptosis are triggered by exposure to physical stressors such as radiation, thermal stress, and cytotoxic agents. Heat stress can lead to a variety of DNA damages, depending on the cell cycle phase and the ambient temperature. Regarding the global warming trend, it is necessary to understand the genetic damages caused by thermal stress, the pathogenic mechanisms of heat-related illnesses (HRIs), and related physiological and perceptual responses at molecular levels to develop preventive and therapeutic strategies in the future.
Sensitive molecular markers of DNA damage
HSPs belong to a family of the proteins produced by cells in response to stress. These proteins were first recognized in relation to heat shock, but now, they are known to be expressed in response to other stresses such as exposure to cold and UV radiation, during wound healing and tissue regeneration, and in diseases that are directly related to inflammation. The upregulation of these proteins under these conditions is regulated at the transcriptional level. The tight regulation of the expression of heat shock proteins is an important part of the heat shock reaction and is primarily induced by the heat shock factor. These proteins are responsible for preserving cellular integrity and regulating the signaling pathways that are essential for cell survival. HSPs include a combination of groups known as HSP27 (also named HSPB), HSP60 (HSPD), HSP70 (HSPA), HSP90 (HSPC), and HSP110 (also called HSPH), which have been recognized as highly preserved guardians of molecules and have remained highly protected throughout evolution (Dubrez et al. 2020). In a study conducted by Yang et al., it was suggested to use HSP70 as a biomarker for heat-induced DNA damage (Yang et al. 2008). Wu et al. also reported that HSPs could provide useful biomarkers for assessing cellular damages among those working in hot environments (Wu et al. 2001). The quantification of γ-H2AX was recommended as a primary marker to assess cellular responses and a sensitive tool for detection of DNA DSBs, resolution, and recognizing DNA damage initiation (Mah et al. 2010). Lazlo et al., however, revealed no role for heat stress-induced γ-H2AX in assessing DSBs and cell death (Laszlo and Fleischer 2009). In this regard, the mechanisms by which γ-H2AX is induced following thermal stress should be further investigated.
Heat stress and gene response
There are a few studies on the effects of delayed heat stress on gene expression regulation and cellular function. Heat stress has been noted to trigger p21-dependent cell aging only in the early S phase, which is similar to cell cycle arrest that induces DNA SSBs (Velichko et al. 2015). Harrouk et al. showed that the exposure of sperms to heat during fertilization could alter the expression of DNA repair genes in the early stages of embryonic development (Harrouk et al. 2000). In an animal model, exposure to thermal stress could reduce the expression of polyADP ribose polymerase (PARP) through two pathways, including BER and NER, which contribute to the detection of SSBs (Tramontano et al. 2000; Van’t Veer et al. 2002). Also, exposure to heat stress (the range of 39–42 °C) increased DNA damage in Sertoli cells and significantly boosted oxidative stress-induced damages in the exposed cells compared to the control group (Nezhad et al. 2013). Cryptorchidism can lead to thermal stress, in which the expression of the repair genes acting in the final stages of DNA repair is reduced, such as DNA polymerase beta, which promotes the recruit of DNA ligase III (Tramontano et al. 2000). An in vitro study showed that cigarette smoking could enhance the formation of micronuclei in the human lymphocytes exposed to heat stress (Feng et al. 1998). The formation of heat-induced γ-H2AX foci is dependent on ataxia-telangiectasia mutated (ATM) protein, which is known as a DNA damage sensor. Moreover, thermal stress, by activating a subset of ATM proteins, can interfere with IR-induced signaling pathways involved in the repair of chromosomal DNA DSBs. Hunt et al. showed that high temperatures could enhance cells’ sensitivity to radiation (Hunt et al. 2007). Heat stress was also shown to induce partial DNA replication, boost cellular DNA content, and cause excessive centrosome growth in the early S phase, triggering an aging-like phenotype in human HeLa cells (Petrova et al. 2016). Further investigations are warranted to divulge the molecular mechanisms underlying the exit of tumor cells from the aging stage under various thermal stressors. Heat stress can stimulate different DNA damage responses (DDRs) in the S phase, leading to the suppression of DNA replication (the S phase) and the formation of DNA DSBs (in the interphase at the stages of G1 and G2), which depend on H2AX phosphorylation and γ-H2AX foci formation, respectively (Velichko et al. 2015). Heat stress-induced DSBs and DNA damage depend on the cell type, cell cycle phase, and ambient temperature (Kantidze et al. 2016). Nevertheless, more studies are needed to confirm the role of hyperthermia in inducing DSBs. The combination of heat stress and infrared radiation was shown to exaggerate genotoxic effects and DNA damage in cells (Ryabchenko et al. 2013). A study revealed a link between heat stress-induced tRNA depletion and motility in HeLa cells. Research has suggested that heat stress-induced tRNA granules in the nucleus may be applicable as important sensors for detecting DNA damage (Miyagawa et al. 2012). Tabuchi et al. mentioned that mild heat stress (41 °C for 30 min) could induce the differential expression of common genes in normal human fibroblasts (Tabuchi et al. 2013). Some studies have negated a direct role for heat stress in creating DSBs (Hunt et al. 2007; Laszlo and Fleischer 2009); however, others have reported that heat stress can provoke DSBs via inducing γ-H2AX (Nam et al. 2013; Velichko et al. 2012).
Heat stress and HSPs
In the cells exposed to various climatic conditions, especially hot-dry and hot-humid, HSPs (HSP27, HSP70, and HSP90) can promote cell death and inflammatory responses (Maghsudlu and Yazd 2017; Nam et al. 2013). Yan et al. asserted the main role of HSPs in cellular responses to heat stress and protecting cells against heat stress by regulating the body’s temperature and facilitating the intracellular trafficking of repair proteins, as well as inducing either the refolding of denatured proteins or their degradation following stress or injury. Therefore, HSPs can prevent the adverse metabolic effects of incorrect protein folding and subsequently proteotoxic-induced cell death (Yan et al. 2006). Stocker et al. noted that heat shock could affect cellular proliferation by blocking the entry of precursors into the cell cycle; however, further studies are needed to clarify this issue (Stocker et al. 2006). After exposure to heat stress, HSP70 levels alter in lymphocytes and plasma, suggesting these proteins as biomarkers to investigate protective responses. Nevertheless, other factors regulating the production of intracellular and extracellular stress proteins remain completely unknown (Yang et al. 2008). A close relationship has been noted between HSP70 expression and DNA damage in peripheral blood lymphocytes (Venugopal et al. 2018). Heat stress can also increase HSP72 plasma level, a biomarker of cardiovascular disease (Tang-Chun et al. 1995). In the lymphocytes exposed to acute heat stress, there was a good association between positive autoantibodies (respective to negative antibodies) and increased DNA damage (Yili et al. 1997). The expression of HSP70 was reported as a sensitive biomarker for a wide range of detrimental physical and chemical stressors in cultured cells (Bierkens 2000). Thermal stress induces a wide range of complex cellular responses, the most important of which is the induction of HSPs, ROS production, disruption of proteins, DNA and RNA damage, abnormal protein homeostasis, imbalanced cell cycle progression, and, finally, cell death (Tabuchi et al. 2013). Nasr et al. indicated that mitochondrial proteins, especially HSP70 proteins, could protect DNA-binding proteins and oxidative stress-scavenging systems following exposure to thermal stress up to 52 °C (Nasr et al. 2019).
Heat stress exposure and effect on male fertility and pregnancy outcome
Normal testicular function depends on the temperature of the body and the ambient environment. A rise in testes temperature may occur in the men residing in tropical and subtropical countries, especially during summer and in those working in hot outdoor environments. Exposure to occupational heat stress happens in various professions requiring working in hot environments, such as bakery, construction, municipal services, farming, mining, and welding. Also, heavy workload, prolonged exposure to heat, reduced air movement around the skin, and wearing personal protective equipment (PPE) can lead to an increase in the core body temperature and alteration in other physiological parameters in hot and humid environments (Habibi et al. 2021b; Paul et al. 2008). Thermal stress significantly affects spermatogenesis in mammals, inflicting damage to DNA damage in germ cells and increasing apoptosis in these cells, culminating in infertility due to processes such as hypoxia, oxidative stress, and apoptosis (Paul et al. 2009). Heat stress can increase the temperature in testes, increasing the production of abnormal and immature sperms and leading to infertility and ejaculation. In the mice kept in hot environments, there were reports of alterations in DNA integrity, reduced sperm quality, and loss of germ cells with normal chromatin packaging. Exposure to thermal stress (up to 40 °C) led to the premature loss of the fetus, spermatocytes, and spermatids. In the thermal range of 40 to 42 °C, there was a report of testicular dysfunction, as well as changes in testicular weight, increased apoptotic biomarkers, and increased rate of death in germ cells (MacLachlan et al. 1995; Paul et al. 2008). Rockett et al. described an increased rate of apoptosis and the upregulation of heat-induced proteins, Hsp70-1 and Hsp70-3, in spermatocytes after exposure to 43 °C for 20 min. Also, heat can increase DNA SB in Pachytene stage spermatocytes via inducing primary cellular responses leading to the overexpression of γ-H2AX, a highly specific and sensitive molecular marker (Rockett et al. 2001). The ability to reproduce is affected by exposure to extreme heat, as evidenced by the abnormal growth of germ cells and reduced sperm quality. Exposure to heat stress increases mitochondrial ROS levels in sperms and induces oxidative DNA damage and DNA SSBs in germ cells in men (Houston et al. 2018). Heat stress also upregulates the mRNA expression of the hypoxia-inducible factor 1 alpha (Hif1a) gene and promotes mild testicular hyperthermia and the translocation of the HIF1A protein to the nucleus in germ cells (Paul et al. 2009). As well, Pena et al., in a study on boars, showed that heat stress increased DNA damage in sperms and decreased sperm quantity in a hot summer. It has been suggested that DNA integrity assessment in the sperm nucleus can be a good indicator of sperm quality in exposure to hot weather (Peña et al. 2019), especially in tropical and subtropical areas where changes in temperature are beyond the thermal comfort zone of animals and humans (Penã et al. 2017). In a female animal model exposed to heat stress (36 °C for 24 h), a decrease in the number of embryos and an increase in heat stress responses were observed (Zhu and Setchell 2004). In male mice, the whole-body temperature was shown to affect sperm count, with a decrease in fertility rate within 10 to 14 days after exposure to thermal stress (36 °C for 24 h) (Yaeram et al. 2006). In an investigation on the effects of thermal stress on mice sperm count, the results showed a reduction in fertility rate, altered fetal weight, and changes in the expression of the repairing genes involved in embryonic growth before implantation and in the single-cell stage (Harrouk et al. 2000). Alekseenko et al. showed that heat stress could induce apoptosis via different mechanisms in human embryonic stem cells (ESC) and their differentiating daughter cells (Alekseenko et al. 2012).
Limitations and current research gaps and future directions
Due to gaps in our knowledge about the effects of exposure to heat stress on DNA damage, there is a need for further investigations. As mentioned before, two studies have addressed these effects in animal models, and one study has been conducted on human workers. Other studies have examined these effects in vitro (Table 1). In addition, there are no epidemiological studies on the epidemiological aspects of diseases and the effects of heat stress on DNA alterations in men and women exposed to heat in different work environments, which should be addressed in upcoming studies. In addition, the effects of heat stress on fertility (spermatozoa) observed in male animal models should also be evaluated in humans (Houston et al. 2018). We also need to scrutinize the effects of heat stress at the cellular level on the reproductive organs of both females and males. Animal and human studies are needed to discover all the mechanisms behind heat stress-induced formation of DNA DSBs.
This is important considering that the outcomes of studies can be variable depending on parameters such as study design, the animal used, ambient temperature, relative humidity, the number of animals, and the duration of exposure to heat. So, these determinants should be taken into mind when generalizing results to humans (Kampinga et al. 2005). Such new approaches can help characterize the concepts related to thermal stress biology and its molecular indicators and investigate their relationships with epidemiological parameters, such as the incidence and prevalence of diseases. In addition, some studies have identified the adverse effects of heat stress (e.g., inducing cell damage, altering cellular function, and triggering apoptosis) on immune cells such as monocyte-derived dendritic cells (DCs) in vitro; however, these effects have not been validated in vivo that should be considered in future studies (Beachy and Repasky 2011). Climate change and global warming have potentially exposed millions of humans and animals to hot-humid and hot-dry environments. Therefore, it is essential to characterize the cellular damages caused by this phenomenon and its relationship with HSPs’ levels in tissues and organs so that we can implement preventive interventions, management strategies, and protective instructions in different societies and countries according to climate conditions (Habibi et al. 2021b; Venugopal et al. 2018). Finally, if a link between heat stress and DNA damage is established, it will be necessary to review and adjust the regulations of working in hot-humid and hot-dry environments so that the health of employees is warranted.
Conclusion
Heat stress induces DNA DSBs in human and animal models, which is one of the deadliest types of DNA damage. Nonetheless, the effects of heat stress on the molecular mechanisms involved in DNA damage are not well-understood. Although cellular and molecular responses to heat stress have been extensively studied in recent decades, in this systematic review of the literature, we found that a few studies have been conducted to scrutinize the effects of heat stress on DNA damage, DNA replication, and nucleic acid repair mechanisms. Studying the physiological responses to heat stress and their molecular mechanisms can help understand the biological effects of heat stress, especially in tropical and subtropical countries. As well, identifying the main biomarkers of molecular responses to heat stress, including HSPs and other related biomarkers, can help early detect heat-induced cellular damages and destructive effects, especially in people working in hot environments. Finally, it is required to implement appropriate interventions, such as technical-engineering, managerial, and therapeutic measures, and to design and develop standard preventive guidelines to avoid the rise of heat-induced diseases.
References
Alekseenko LL, Zemelko VI, Zenin VV, Pugovkina NA, Kozhukharova IV, Kovaleva ZV, ..., Nikolsky NN (2012a) Heat shock induces apoptosis in human embryonic stem cells but a premature senescence phenotype in their differentiated progeny. Cell Cycle 11(17):3260-3269.https://doi.org/10.4161/cc.21595
Beachy SH, Repasky EA (2011) Toward establishment of temperature thresholds for immunological impact of heat exposure in humans. Int J Hyperth 27(4):344–352
Bettaieb A, Averill-Bates DA (2015) Thermotolerance induced at a mild temperature of 40°C alleviates heat shock-induced ER stress and apoptosis in HeLa cells. Biochimica Et Biophysica Acta - Molecular Cell Research 1853(1):52–62. https://doi.org/10.1016/j.bbamcr.2014.09.016
Bierkens JG (2000) Applications and pitfalls of stress-proteins in biomonitoring. Toxicology 153(1–3):61–72
Dubrez L, Causse S, Borges Bonan N, Dumetier B, Garrido C (2020) Heat-shock proteins: chaperoning DNA repair. Oncogene 39(3):516–529. https://doi.org/10.1038/s41388-019-1016-y
Evans MD, Dizdaroglu M, Cooke MS (2004) Oxidative DNA damage and disease: induction, repair and significance. Mutation Research/reviews in Mutation Research 567(1):1–61
Feng H, Sun L, Li Y, Yang B (1998) Study on induced mutagenesis interaction of the high temperature and/or cigarette smoke. Wei sheng yan jiu= Journal of hygiene research, 27(6):379–381
Golbabaei F, Heydari A, Moradi G, Dehghan H, Moradi A, Habibi P (2020) The effect of cooling vests on physiological and perceptual responses: a systematic review. International J Occup Saf Ergon(just-accepted) 1–36.
Habibi P, Momeni R, Dehghan H (2015) Relationship of environmental, physiological, and perceptual heat stress indices in Iranian Men. International Journal of Preventive Medicine 6
Habibi, P., Moradi, G., Moradi, A., & Golbabaei, F. (2021a). A review on advanced functional photonic fabric for enhanced thermoregulating performance. Environmental Nanotechnology, Monitoring & Management, 100504.
Habibi P, Moradi G, Moradi A, Heydari A (2021b) The impacts of climate change on occupational heat strain in outdoor workers: a systematic review. Urban Climate 36:100770
Harrouk W, Codrington A, Vinson R, Robaire B, Hales BF (2000) Paternal exposure to cyclophosphamide induces DNA damage and alters the expression of DNA repair genes in the rat preimplantation embryo. Mutation Research/DNA Repair 461(3):229–241
Houston BJ, Nixon B, Martin JH, De Iuliis GN, Trigg NA, Bromfield EG, ..., Aitken RJ (2018) Heat exposure induces oxidative stress and DNA damage in the male germ line. Biol Reprod 98(4):593-606
Hunt CR, Pandita RK, Laszlo A, Higashikubo R, Agarwal M, Kitamura T, ..., Pandita TK (2007) Hyperthermia activates a subset of ataxia-telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res 67(7):3010-3017. https://doi.org/10.1158/0008-5472.can-06-4328
Kampinga HH, Laszlo A, Takahashi A, Mori E, Ohnishi T (2005) DNA double strand breaks do not play a role in heat-induced cell killing [1] (multiple letters). Can Res 65(22):10632–10633. https://doi.org/10.1158/0008-5472.CAN-05-0006
Kantidze OL, Velichko AK, Luzhin AV, Razin SV (2016) Heat Stress-Induced DNA Damage Acta Naturae 8(2):75–78. https://doi.org/10.32607/20758251-2016-8-2-75-78
Kjellstrom T, Lemke B, Hyatt O, Otto M (2014) Climate change and occupational health: a South African perspective. Samj South Afr Med J 104(8), 586-+. https://doi.org/10.7196/samj.8646
Laszlo A, Fleischer I (2009) The heat-induced-H2AX response does not play a role in hyperthermic cell killing. Int J Hyperth 25(3):199–209. https://doi.org/10.1080/02656730802631775
Lepock JR, Borrelli MJ (2005) How do cells respond to their thermal environment? Int J Hyperth 21(8):681–687. https://doi.org/10.1080/02656730500307298
Liu FW, Liu FC, Wang YR, Tsai HI, Yu HP (2015) Aloin protects skin fibroblasts from heat stress-induced oxidative stress damage by regulating the oxidative defense system. PloS one 10(12). https://doi.org/10.1371/journal.pone.0143528
MacLachlan TK, Sang N, Giordano A (1995) Cyclins, cyclin-dependent kinases and cdk inhibitors: implications in cell cycle control and cancer. Critical Reviews™ in Eukaryotic Gene Expression 5(2)
Maghsudlu M, Yazd EF (2017) Heat-induced inflammation and its role in esophageal cancer. J Dig Dis 18(8):431–444. https://doi.org/10.1111/1751-2980.12511
Mah L, El-Osta A, Karagiannis T (2010) γH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia 24(4):679–686
Maroni P, Bendinelli P, Tiberio L, Rovetta F, Piccoletti R, Schiaffonati L (2003) In vivo heat-shock response in the brain: signalling pathway and transcription factor activation. Brain Res Mol Brain Res 119(1):90–99. https://doi.org/10.1016/j.molbrainres.2003.08.018
Milani V, Horsman M (2008) Cellular and vascular effects of hyperthermia. Int J Hyperth 24(1):1–2. https://doi.org/10.1080/02656730701858313
Miyagawa R, Mizuno R, Watanabe K, Ijiri K (2012) Formation of tRNA granules in the nucleus of heat-induced human cells. Biochem Biophys Res Commun 418(1):149–155. https://doi.org/10.1016/j.bbrc.2011.12.150
Mohammadi B, Safaiyan A, Habibi P, Moradi G (2021) Evaluation of the acoustic performance of polyurethane foams embedded with rock wool fibers at low-frequency range; design and construction. Appl Acoust 182:108223
Nam JW, Kim SY, Yoon T, Lee YJ, Kil YS, Lee YS, Seo EK (2013) Heat shock factor 1 inducers from the bark of Eucommia ulmoides as cytoprotective agents. Chem Biodivers 10(7):1322–1327. https://doi.org/10.1002/cbdv.201200401
Nasr MA, Dovbeshko GI, Bearne SL, El-Badri N, Matta CF (2019) Heat shock proteins in the “hot” mitochondrion: identity and putative roles. Bioessays 41(9). https://doi.org/10.1002/bies.201900055
Nezhad FS, Lavvaf A, Karimi S (2013) Influence of heat stress on DNA damage on sheep’s Sertoli cells. International Research Journal of Applied and Basic Sciences 6(10):1396–1400
Paul C, Murray AA, Spears N, Saunders PT (2008) A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction 136(1):73–84. https://doi.org/10.1530/rep-08-0036
Paul C, Teng S, Saunders PT (2009) A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol Reprod 80(5):913–919. https://doi.org/10.1095/biolreprod.108.071779
Penã ST, Gummow B, Parker AJ, Paris DBBP (2017) Revisiting summer infertility in the pig: could heat stress-induced sperm DNA damage negatively affect early embryo development? Animal Production Science 57(10):1975–1983. https://doi.org/10.1071/AN16079
Peña ST, Stone F, Gummow B, Parker AJ, Paris DBBP (2019) Tropical summer induces DNA fragmentation in boar spermatozoa: implications for evaluating seasonal infertility. Reprod Fertil Dev 31(3):590–601. https://doi.org/10.1071/RD18159
Petrova NV, Velichko AK, Razin SV, Kantidze OL (2016) Early S-phase cell hypersensitivity to heat stress. Cell Cycle 15(3):337–344. https://doi.org/10.1080/15384101.2015.1127477
Prandini MN, Neves A, Lapa AJ, Stavale JN (2005) Mild hypothermia reduces polymorphonuclear leukocytes infiltration in induced brain inflammation. Arq Neuropsiquiatr 63(3B):779–784. https://doi.org/10.1590/s0004-282x2005000500012
Raaphorst GP, LeBlanc JM, Li LF, Yang DP (2005) Hyperthermia responses in cell lines with normal and deficient DNA repairs systems. J Therm Biol 30(6):478–484. https://doi.org/10.1016/j.jtherbio.2005.05.010
Richter K, Haslbeck M, Buchner J (2010) The heat shock response: life on the verge of death. Mol Cell 40(2):253–266
Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ (2001) Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod 65(1):229–239
Roti Roti JL, Pandita RK, Mueller JD, Novak P, Moros EG, Laszlo A (2010) Severe, short-duration (0–3 min) heat shocks (50–52°C) inhibit the repair of DNA damage. Int J Hyperth 26(1):67–78. https://doi.org/10.3109/02656730903417947
Ryabchenko NM, Rodionova NK, Sychevska IS, Muzalev II, Mykhailenko VM, Druzhina MO (2013) Genotoxic effects of radiation and hyperthermia in linear mice with different radiation sensitivity. Cytol Genet 47(1):39–43. https://doi.org/10.3103/s0095452713010088
Shamseer LMD, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj 2:7647
Sirriyeh R, Lawton R, Gardner P, Armitage G (2012) Reviewing studies with diverse designs: the development and evaluation of a new tool. J Eval Clin Pract 18(4):746–752
Stocker AJ, Madalena CR, Gorab E (2006) The effects of temperature shock on transcription and replication in Rhynchosciara americana (Diptera: Sciaridae). Genetica 126(3):277–290. https://doi.org/10.1007/s10709-005-7407-8
Tabuchi Y, Furusawa Y, Kariya A, Wada S, Ohtsuka K, Kondo T (2013) Common gene expression patterns responsive to mild temperature hyperthermia in normal human fibroblastic cells. Int J Hyperth 29(1):38–50. https://doi.org/10.3109/02656736.2012.753163
Tang-Chun W, Han-Zhen H, Tanguay RM, Yang W, Dai-Gen X, Currie RW, ..., Guo-gao Z (1995) The combined effects of high temperature and carbon monoxide on heat stress response. J Tongji Med Univ 15(3):178-183
Tramontano F, Malanga M, Farina B, Jones R, Quesada P (2000) Heat stress reduces poly (ADPR) polymerase expression in rat testis. Mol Hum Reprod 6(7):575–581
Van’t Veer LJ, Dai H, Van De Vijver MJ, He YD, Hart AA, Mao M, . . ., Witteveen AT (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415(6871): 530–536.
Vanos J, Vecellio DJ, Kjellstrom T (2019) Workplace heat exposure, health protection, and economic impacts: a case study in Canada. Am J Ind Med 62(12):1024–1037. https://doi.org/10.1002/ajim.22966
Velichko AK, Petrova NV, Kantidze OL, Razin SV (2012) Dual effect of heat shock on DNA replication and genome integrity. Mol Biol Cell 23(17):3450–3460. https://doi.org/10.1091/mbc.E11-12-1009
Velichko AK, Petrova NV, Razin SV, Kantidze OL (2015) Mechanism of heat stress-induced cellular senescence elucidates the exclusive vulnerability of early S-phase cells to mild genotoxic stress. Nucleic Acids Res 43(13):6309–6320. https://doi.org/10.1093/nar/gkv573
Venugopal V, Krishnamoorthy M, Venkatesan V, Jaganathan V, Paul S (2018) Occupational heat stress, DNA damage and heat shock protein—a review. Medical Research Archives 6(1)
Venugopal V, Krishnamoorthy M, Venkatesan V, Jaganathan V, Shanmugam R, Kanagaraj K, Paul SF (2019) Association between occupational heat stress and DNA damage in lymphocytes of workers exposed to hot working environments in a steel industry in Southern India. Temperature 6(4):346–359
Wu T, Chen S, Xiao C, Wang C, Pan Q, Wang Z, ..., Tanguay RM (2001) Presence of antibody against the inducible Hsp71 in patients with acute heat-induced illness. Cell Stress Chaperones 6(2):113
Xiang JJ, Bi P, Pisaniello D, Hansen A (2014) Health impacts of workplace heat exposure: an epidemiological review. Ind Health 52(2):91–101. https://doi.org/10.2486/indhealth.2012-0145
Yaeram J, Setchell B, Maddocks S (2006) Effect of heat stress on the fertility of male mice in vivo and in vitro. Reprod Fertil Dev 18(6):647–653
Yan Y-E, Zhao Y-Q, Wang H, Fan M (2006) Pathophysiological factors underlying heatstroke. Med Hypotheses 67(3):609–617
Yang X, Yuan J, Sun J, Wang H, Liang H, Bai Y, ..., Wang J (2008) Association between heat-shock protein 70 gene polymorphisms and DNA damage in peripheral blood lymphocytes among coke-oven workers. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 649(1-2):221-229
Yili X, Tangchun W, Yongxing Z, Tanguay R, Nicole L, Ye Y, Guogao Z (1997) Preliminary studies on the relationship between autoantibodies to heat stress proteins and heat injury of pilots during acute heat stress. J Tongji Med Univ 17(2):83–85
Zhu B-K, Setchell BP (2004) Effects of paternal heat stress on the in vivo development of preimplantation embryos in the mouse. Reprod Nutr Dev 44(6):617–629
Acknowledgements
The researchers express their gratitude to the School of Public Health Research Council of Tehran University of Medical Sciences, Tehran, Iran, for their advices and support.
Author information
Authors and Affiliations
Contributions
PH and AH participated in the study design and data collection. SNO, SA, and ARF participated in data analysis, manuscript writing, revising, and editing. Study was done under the supervision of FG and MRM. All authors read, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Habibi, P., Ostad, S.N., Heydari, A. et al. Effect of heat stress on DNA damage: a systematic literature review. Int J Biometeorol 66, 2147–2158 (2022). https://doi.org/10.1007/s00484-022-02351-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-022-02351-w