Abstract
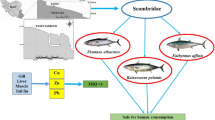
This paper aims to measure the metals (Fe, Cu, Cr, Co, As, Zn, Hg, and Pb) concentration and assesses human health risks posed by these eight metals in the five most available cultured fish species (Pangasiuspangasius, Oreochromisniloticus, Heteropneustesfossilis, Anabustestudineus, Clariasbatrachus) collected from the Mymenshing Sadar Upazila. Both fishes and three fish feeds were analyzed using energy dispersive X-ray fluorescence (EDXRF). In fish feeds, the concentrations of metals were detected in the following order Fe > Zn > Cu > Hg > Pb. Besides, Hg concentration in three feeds samples was found higher than the maximum residue limit. Fe, Cu, Cr, Co, As, Zn, Hg, and Pb concentration in fishes were 82.45 to 104.55, 24.47 to 32.88, 2.62 to 6.73, < 0.28, < 0.41, 70.01 to 96.56, 0.57 to 1.07, and 0.34 to 0.49 µg/g respectively as dry weight basis. Health Risk Index (HRI) was observed > 1 both for adults and children and maximum HRI values for Pb was found in both P. pangasius and C. batrachus fish species. Additionally, PCA explained 73% variation in data and CA comprised the similarity within sampling stations. Pearson correlation implied a strong association amongst the trace metals. Finally, the accumulation of metals tends to the massive health hazard that should be mitigated by adopting several measures on fish feeds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bangladesh has tremendous fishing and aquaculture potential because of its abundant inland waters and river systems. The country is recognized as one of the most attractive areas for fisheries in the world and third in inland fish production (Shamsuzzaman et al. 2017). Bangladesh’s fisheries sector is mainly divided into three sub-sectors: inland capture, inland culture, and marine fisheries. A significant contribution of 3.57% of the national Gross Domestic Product (GDP) and more than one-fourth (25.30%) to the agricultural of GDP is played by the fishing sector in the national economy (Department of Fisheries. 2018). In freshwater systems, fish is one of the aquatic foodstuffs that humans consume and also provide a good indicator of trace element pollution (Rashed. 2001). As fish are often at the top of aquatic food webs and may concentrate large amounts of metals from the water and sediments, through this process, heavy metals can easily enter the human body (Mansour and Sidkey. 2002). Fishes have been considered a significant source of proteins for human food consumption in the last several years. Consequently, the human body is likely to be enriched with a greater degree of heavy metal concentration (Ali and Khan 2018). In Bangladesh, fish farmers gradually use manufactured feeds rather than farm-made feeds (Mahmud et al. 2012). Fish feeds are commonly manufactured from rice bran, soya meal, flour, fish meal, shrimp meal, beef liver, silkworms, and earthworms (Asaduzzamanet al. 2006). Tannery solid wastes are also used as poultry and fish feeds in Bangladesh (Hossain et al. 2007). Due to certain anthropogenic activities and feeding fish feeds containing toxic metals, the closed water sources are polluted with high metals as various feedstuffs are generated with solid tannery waste as raw materials (Patwardhan 2013). Subsequently, intensive anthropogenic activities and natural sources can have an effect on the local aquatic environment, increasing the heavy metal contents in sediments and water and contributing to the associated ecological damage (Ogundele and Ayeku 2020; Alam et al. 2020). Some commercial fish feeds of Bangladesh have also been found to have heavy metals burden (Kundu et al. 2017). Heavy metals are ingested on the human body through the consumption of fish. In recent years, the science community has become profoundly responsive to human health's potential threats arising from the fishes exposed to toxic metals (Griboff et al. 2017). The ingestion of metal-contaminated fish and other foods polluted with chemical and organic contaminants from aquatic ecosystems can endanger human life (Aderinola et al. 2009). Some feeds contain substantial concentrations of contaminants, including heavy metals. Many of which can bio-accumulate and bio-concentrate in fish could harm humans (Maule et al. 2007; Indrajit et al. 2011). Trace elements (cobalt, copper, chromium, manganese, and nickel) are necessary for humans in minute amounts while heavy metal is carcinogenic or toxic, affecting the central nervous system (by mercury, lead, and arsenic), the kidneys or liver (by mercury, lead, cadmium, and copper) or skin, bones, or teeth (by nickel, cadmium, copper, and chromium) (Bhattacharya et al. 2007). Heavy metals exposure in human health causes dysfunctioning urinary organ and skeletal injury, medical speciality disturbances, endocrine disturbance, vessel disease, and cancer effects (Renjeri et al. 2019). Besides, an excessive amount of Hg, As, Pb, and Cd elements could be detrimental to the living cells, and prolonged exposure to the body can lead to illness or death. (Azaman et al. 2015).
Fish supplies comparatively cheap, and it is a readily available source of nutrients in several low and middle-income countries where diets are depending heavily on a narrow range of staple foods (Reksten et al. 2020). Commercial fish species are very cheap sources of protein, and the accumulation of metals in fish tissues act as a medium through which metals are ingested in the human body after consumption. Heavy metal contamination of the food chain is well documented in Bangladesh (Islam et al. 2014). Metals deposited into the aquatic environment may accumulate in the food chain and pose carcinogenic and other adverse effects on human health due to biomagnification over time (Malik et al. 2009). The excessive intake of heavy metal in small quantities contributes to the dysfunction of a variety of physiological and biochemical processes such as thalassemia, dermatitis, brain and kidney damage and cancer, which are known health risks linked to heavy metal exposure (Hu 2002; Tripathi et al. 2001). Heavy metals tend to accumulate in advanced organisms through bio-magnification effects in the food chain and accumulate in the human tissues to pose chronic toxicity (Qiao-qaio et al. 2007). The significant adverse effects of exposures to these heavy metals, even at low concentrations, are responsible for neurotoxicity and carcinogenicity (Jomova and Valko. 2010). This paper aims to determine the concentration of selected eight metals in five commercial fish species tissues (flesh), which are very available in Bangladesh and also assess the probable health risk due to the consumption of those fishes collected from the study area.
2 Materials and methods
2.1 Study area and sampling procedure
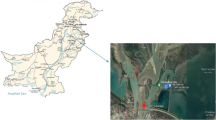
The study area (shown in Fig. 1) is located (latitude 24.38° N to 24.54° N and longitude 90.11° E to 90.31° E) in the Mymensingh Division of Bangladesh. Fish were sampled from this area because it is one of the concentrated aquaculture regions in Bangladesh. Fish were selected for sampling based on the species significance to local food habits and availability in the Bangladesh fish market. Five fish species (three of each) and fifteen fish samples (Table 1) were collected from five different fishing zones by the Multi-stage cluster method during the months of June to July 2019. Fishes were captured by using a seine net with the help of local fisherman and all the samples were in the mature stage. The samples were immediately placed in air-sealed plastic bags and kept in an icebox (Pinnacle TPX 60,091, Tokyo Plast Int., India) running at or below 40° F before further analysis laboratory of the Chemistry Division at Atomic Energy Center, Dhaka, Bangladesh. Sample collection time and fish growth stage are depicted in Table 1.
2.2 Sample preparation and analysis
All the samples were prepared in the laboratory of the Chemistry Division of Atomic Energy Center, Dhaka, Bangladesh. Collected fish were washed multiple times with deionized water. Fish collected from the study area were cut into small pieces (2–4 cm) with the stainless steel knife, separated into flesh, and all the samples, including fish feed, were dried in an oven at 800C until a constant weight was obtained (24–48 h in most cases). All dried samples were ground as powder by a carbide mortar and preserved in a plastic vial with an identification mark inside a desiccator. A 10-mm pellet with approximately 10 tons of hydraulic pressure was used to make by the pellet maker (Specac Ltd., UK) for 2–3 min for processing pellets for analyses using a sample of 0.1 g dried powdered materials. The pellets were then stored in desiccators for further experiments via X-ray Fluorescence Spectrometer (XRF) (Epsilon 5, PANalytical, The Netherlands). Pellets were loaded for 1000 s to complete the analysis in the X-ray excitement chamber. A system software package controlled the irradiation of the sample. The concentration of Iron (Fe), Chromium (Cr), Copper (Cu), Cobalt (Co), Arsenic (As), Zinc (Zn), Lead (Pb), and Mercury (Hg) in fifteen fishes and three feeds sample were measured.
Tuna fish Homogenate (IAEA-350) was used to construct the calibration curve for the elemental analysis in fish and feed samples, whereas CRM DORM-2 (National research council, Canada) was used to verify the accuracy of the calibration curve as well as to validate the analytical method. Moreover, it can be supplemented that EDXRF is a multi-elemental measurement technique and it can detect a range of elements at a time. In the present study, the model that is used can detect elements from K to Mo. So when a calibration curve has been constructed that can detect all the elements within this range (K to Mo) even though the standard reference material (SRM) may not contain those elements, thus the use of DORM-2 is justified. The results obtained for the elements and the standard values for their corresponding elements are shown in Table 2 where the values were found to be in good relation with the standard values.
2.3 Quality control and quality assurance measures
The samples were prepared and analyzed to ensure correct and reliable analytical data following a rigorous quality assurance/quality control (QA / QC) system. Prior to use, the disinfection equipment and sample containers were pre-cleaned with deionized water, and 100% Acetone (CH3)2CO was used for disinfection. Before being used for dissection, the stainless-steel scissor and knife were washed with 100% Acetone (CH3)2CO per flesh sample Clariasbatrachus e. Sterile laboratory gloves and nose masks were used throughout the experimental session to avoid hand contamination of the samples. All readings were taken in triplicates to minimize errors. All analytical values were exported from the Laboratory Information Management System (LIMS) to Microsoft Office 365 Excel 2013 version 15.0 to calculate means and standard deviations (SD). Statistical analyses were performed using GraphPad Prism 8.3.0 and Stata 13. After that, all the statistical analyses were rechecked by SPSS 16.
While constructing the calibration curve, Tuna homogenates were used as a commercially available fish standard. The following curves were constructed by plotting the sensitivities of the elements using the atomic number as a function. Subsequently, certified referenced materials were used to check the accuracy and precision of the constructed calibration curve (Hasan et al. 2020). The obtained result indicates that the proposed method is valid for determining the heavy metals in the fish and fish feed samples. The recoveries ranged from 87.66 to 108.27% and the resulted relative error and coefficient of variance were found less than 10% for most of the considered metals.
2.4 Health risk estimation
2.4.1 Metal pollution index (MPI)
The cumulative metal (Fe, Cu, Zn, Hg &Pb) concentrations contained on fish samples were determined by the Metal Pollution Index (MPI). The geometrical mean of the concentration of all metals on feeds and fishes was determined using the MPI index (Ureso et al. 1997). For computing, the MPI Eq. (1) is used
where Cfn = concentration of metal in n the sample.
2.4.2 Daily intake of metal (DIM)
DIM's daily intake was estimated to simulate the daily sorting of metals in the body system of established individual bodyweight. To calculate daily metal intake Eq. (2) (Islam et al. 2017) are used
where Cmetal is the metal concentration in fishes (µg/g), SDfish intake is the Daily intake of fishes (g/persons/day), Baverage body weight is the average body weight (65 kg used for this study for adult and 32.7 kg for children (Wang et al. 2005), Kconversion factor = 0.1455, based on the moisture content of the samples and their dry weight.
The mean current consumption of fishes in Bangladesh was 53 g/person/day as reported by the Department of Fisheries (DoF) in the Department of Fisheries Report (2015), though 60 g/person/day of fish intake by humans is considerable.
2.4.3 Health risk index (HRI)
HRI > 1 in any substance that is known as detrimental to human health. The daily metal intake (DIM) in any food and the oral reference dose (RfD) are used to estimate HRI values. The oral RfD is known to be an empirical approximation transmitted to humans by oral regular, which is not expected to be life-length threatening, including vulnerable subgroups(US-EPA IRIS. 2006). The health risk index for Fe, Cu, Zn, Hg, and Pb were calculated using Eq. (3) (Cui et al. 2004).
The value of oral RfD for Fe, Cu, Zn, Hg, and Pb are 0.7, 0.04, 0.3, 0.00016, and 0.004 mg/Kg/ day, respectively (USEPA 2006, 2010).
2.5 Non-carcinogenic health hazard
The target hazard quotient (THQ) estimation method indicates human health risk caused by exposure to pollutants. The risk-based concentration table of the USEPA Region III (USEPA 2011) is used to calculate the target hazard quotient (THQ) by Eq. (4).
where EF = the exposure frequency (365 days), ED = the exposure duration (30 years for noncancer risk as used USEPA 2011), FIR = the fish ingestion rate (62.58 g/person/day; DoF (Department of Fisheries) 2018), Cf = the conversion factor (0.208) to convert fresh weight (Fw)to dry weight (Dw) considering 79% of moisture content in fish, CM = the metal concentration in fish, WAB = the average body weight (70 kg), ATn = the average exposure time for non-carcinogens (EF \(\times\) ED) (ATn = 365 \(\times\) 30 = 10,950 days) as used in characterizing noncancer risk (USEPA. 2011), and RfD = the oral reference dose of metals.
2.6 Hazard index
Hazard Index (HI) is used to assess the risk of human health due to metal contamination (USEPA 1989), which is the sum of the Target Hazard Quotients (THQ) of metals (USEPA 2011). The Hazard Index is calculated for cultured fish samples by Eq. (5).
where HI is the Hazard Index, THQ (Fe) is the target hazard quotients for Fe intake and others.
2.7 Carcinogenic risk
For carcinogenic risk measurement, Target Cancer Risk (TR) is used. The method of estimating TR is also given in the USEPA Region III Risk-Based Concentration Table (USEPA 2011). The model for TR was followed by Eq. (6).
where CPSo is the Carcinogenic potency slope.
In this research work, the concentration of lead was found as a carcinogenic, and its effects were calculated by using the Oral Carcinogenic potency slope (i.e., CPSo), which has been reported by the Integrated Risk Information System (USEPA., 2010). The CPSo value for lead (Pb) (8.5 \(\times\) 10–3 mg/kg/day) and arsenic (As) (1.5 \(\times\) 10–3 mg/kg/day) were used respectively for the study.
3 Results
3.1 Metal concentration in feeds
Table 3 represents the metal concentration in fish feeds samples. The concentration of iron (Fe), copper (Cu), and lead (Pb) is 336.60, 62.48 and 0.48 µg/g, respectively, in fish feeds samples. Hg concentration exceeded the safe limit in all fish feeds samples (EU 2008). The chromium (Cr) concentration was found in the Paragon feed (9.02 µg/g), while the other two, Mega feed and Fresh feed, were not unrolled where values were < 0.41 µg/g. The concentration of Zn in Mega, paragon, and fresh feeds are 143.24 µg/gin, 94.90 µg/g, 163.47 µg/respectively. In opposition, As and Co were not detectable in any of the feed samples measured where the concentrations of them were < 0.41 µg/g and < 0.28 µg/g, respectively.
3.2 Metal concentration in fish species
Table 4 illustrates the eight metal concentrations of (Fe, Cu, Cr, Co, Zn, As, Hg & Pb) in fish species samples, which were analyzed in the laboratory. The mean concentration of Fe in all fish species was lower than the permissible limit 100 µg/g (FAO/WHO 1989), excepting the C.batrachus fish species (104.55 ± 6.13 µg/g). The mean concentrations of Zn were in the range of 70.01 ± 8.32 to 96.56 ± 9.23 µg/g. The lowest concentration of Hg, 0.57 ± 0.09 µg/g was measured in H. fossilis while the highest concentration, 1.07 ± 0.13 µg/g was found in P. pangasius. The concentration of Co was not detected in P. pangasius, and in O.niloticusfish species, Co concentration (< 0.28 µg/g) is the same. The mean concentrations of Co were found in other fish species but exceeded the permissible limit, which is 0.01 µg/g. The mean concentrations of Cr in all fish species ranged from 2.62 to 6.73 µg/g, with the highest concentration in A.testudineusfish species. The mean values of Pb were 0.48 ± 0.10 µg/g in P. pangasius, 0.34 ± 0.05 µg/g in O.niloticus, 0.45 ± 0.09 µg/g in H.fossilis, 0.35 ± 0.05 µg/g in A.testudineus, and 0.49 ± 0.11 µg/g in C.batrachus.
Safety limita: Allowable metal concentration in Fishes by FAO/ WHO (1989); Safety limitb: Maximum levels of metals in crustaceans by EC (European communities), EC regulation on. 1881/2006 (EC 2006).
3.3 Health risk estimation
3.3.1 Metal pollution index (MPI)
A higher value of MPI indicates more significant aggregate metal accumulation in the sample, which determines the health risks of human beings through metal toxicity. The value of MPI varied from 4.85 to 6.65 µg/g for fish samples and 12.09–18.21 µg/g for fish feeds. Figure 2 illustrates MPI values for fish and fish feed in which A.testudineusfish species showed the highest Metal Pollution index (6.657 µg/g) whereas O.niloticusfish species demonstrated the lowest (4.86 µg/g). The second highest value was in H.fossilis (6.18 µg/g)fish species. Otherwise, feed samples were ranked by MPI, the highest value was in Fresh feed (18.21 µg/g), and the values of MPI in Mega and Paragon feed were 12.09 and 13.88 µg/g respectively.
3.3.2 Daily intake of metal (DIM)
In this study, the DIM value was calculated both for adults and children. Table 5 reveals the Daily Intake of Metals (DIM) for adult and children consumers, in which daily Fe, Cu, Zn, Hg, Pb intake ranged are 9.78–12.40 µg/kg, 2.9–3.9 µg/kg, 9.17–11.45 µg/kg, 0.06–0.13 µg/kg, 0.04–0.06 µg/kg body weight respectively. For children consumers, all the daily intake of metal values were higher than the DIM value of adult consumers. This study revealed that the highest DIM value for Cu was found in C.batrachus, both adults and children (12.40 and 24.66 µg/kg, respectively). The maximum intake of Fe was found through the consumption of C.batrachus, both adult and children. The DIM value of Fe was the highest, and other parameter sequences order were Zn > Cu > Hg > Pb, respectively.
3.3.3 Health risk index (HRI)
For adults and children, the Health risk index (HRI) was calculated by dividing the Daily intake of metal and Reference Dose (RfD). Table 6 indicates that the concentration of metals for both adults and children had crossed the safe value of HRI for many folds. HRI for Fe exceeded the safe limit in adults; its value was 13–17 folds and 27–35 folds for children. HRI value for Hg was very high in all the fish species where it was higher than 1. The sequences of metals from higher to lower that exceeded the safe limit of HRI were Hg > Cu > Zn > Fe > Pb for adults and children. The HRI was maximum for Hg and minimum for Pb in all the measured samples for adults and children. However, the maximum HRI value for Pb was present in P. pangasius and C. batrachus species (14.41 for adults and28.65 for children).
3.3.4 Target hazard quotient (THQ)
Non-carcinogenic effects were determined to assume the consumption of the detected metals in contaminated fish species. Table 7 indicates the results of the target hazard quotient (THQ). The acceptable risk level for THQ is 1 (USEPA 2011). If THQ < 1 for all individual metals, which indicates having non-carcinogenic effects. In this research work, the values of THQ for all detected metals in five fish species are less than 1, expecting Hg. The highest THQ value for Hg (2.00) was in P. pangasius fish species, and the THQ sequences of Hg in other fish species were O.niloticus > C. batrachus > A.testudineus > H.fossilisrespectively.
3.3.5 Hazard index (HI)
The hazard index is the summed-up score of all detected metals in the specific samples. The higher magnitude of the HI indicates higher risks for consumers. Figure 3 reveals that P. pangasiussample had the highest HI value (2.23), whereas the lowest value (1.26) was calculated in H.fossilisfish samples. On the other hand, HI values for P. pangasiusm, A. testudineus, and O. niloticus were1.89,1.61 and 2.03, respectively. The sequences of HI in fishes from higher to lower order was:P. pangasius > O. niloticus > C. batrachus > A. testudineus > H. fossilis.
3.4 Target cancer risk
The target cancer risk was calculated for the intake of Pb and As. The acceptable level for cancer risk ranges from 10–4 to 10–6, where the risk of cancer is considered negligible below 10−6and above 10–4 is unacceptable (USEPA 1989,2011). Table 8 represents the target cancer risk, which points out that TR values for Pb ranged from 1.33 × 10–4 to 1.93 × 10–4 in the measured fish species, while the cancer risk for As was not detected due to low concentration (< 0.41 µg/kg). The values of TR for Pb in P. pangasius, O. niloticus, H. fossilis, A. testudineus, and C.batrachusfish species were 1.91 × 10–4, 1.33 × 10–4, 1.76 × 10–4, 1.37 × 10–4, and 1.93 × 10–4, respectively. However, The result of target cancer risk suggests that five commercial cultured fish species were capable of carcinogenic risk.
3.5 Principal component analysis of the metal parameters
PCA analysis was applied to determine the association between the parameters with the principal components. Eigenvalue was more significant than 1 were considered to demarcate the principal components. The scree plot of the PCA is shown in Fig. 4. Therefore, 5 principal components were derived from the analysis. These five components explained 73% of the variation in the data. Amongst the metal parameters, PC1 explains the highest 21% of the variation, whereas PC4 and PC5 explain 0.11% of the total variation. Cu and Co had the highest positive relation with PC1, while Pb and Fe had the highest negative association with PC1. Besides, Zn and Sr made the highest positive relation with PC2, whereas Cr and Rb had the highest negative association. The association between the parameters and PC2 follows the order Zn > Sr > Fe > Co > Cu > Se > Ca > Rb > Cr from strongly positive to strongly negative, respectively. Moreover, Se and Hg had the highest positive association with PC3, whereas Rb, Zn, and Cu had the highest negative relation. In PC4, most of the variables were found to have a positive association except for Zn, Se, and Rb. Furthermore, Se and Co showed a strong negative relation with PC5, respectively. On the contrary, Cr, Rb, and Sr illustrated strong positive associations, respectively. Table 9 depicts the PCA analysis of metal parameters.
3.6 Hierarchical cluster analysis over the sampling stations
Correlational agglomerated cluster analysis was applied to examine the similarity and uniformity within the sampling stations based on metal values. The dendrogram is shown in Fig. 5 was resulted from the average linkage. Sampling stations 5,15,13 and 14 showed uniformity amongst the sampling sites. Besides, 1,9 and 3 sampling stations made a cluster which was further made a sub-cluster with the 5,15,13 and 14 stations. After that, 6, 10, and 12 sampling stations were found to have a strong association within them. The notable heterogeneity was found from the 2, 11, and 7 sampling stations. Each of the stations individually revealed a weak association. Figure 5 shows the dendrogram of cluster analysis using average linkage.
3.7 Pearson correlation matrix within trace metals accumulated in fishes
Pearson correlation matrix determined the degree of association within fishes regarding metal accumulation. Ca had a strong positive relation with Co and Ni at 95% significant level while Fe and Zn had a strong negative association with Ca. Besides, Cr was found to have a moderate positive relation with Mn and Rb, whereas it shows a negative relation with Zn and Co at 0.05 significant level. Conversely, Mn did not have a notable positive association with these trace metals, while it had a moderate negative association with Co and Hg. After that, Fe had a significant positive relation with Pb. Also, Co seems to have a lower to moderate positive correlation with Ni, Co, and Hg. Ni had a positive association with As and Pb. On the contrary, Cu negatively correlated with Pb and Hg. Similarly, Zn positively associated with Sr. As was observed to have negatively associated with the maximum of the metals. Furthermore, As was positively associated with Ni and Ca. Then, Se was negatively correlated with Zn. Rb had negatively related with the maximum of the metals, while with Cr, it was positively correlated. Hg was positively associated with Ni. All the metals had remained at 95% significant level. Table 10 shows the correlation matrix within accumulated metals in fish species. Besides, comparatively toxic metals in fish species and fish feeds were taken into consideration. Table 11 shows the association of accumulated metals between fishes and fish feeds. Only one association was found to have a significant level. The table reveals no significant relation of one particular metal of fish feeds over the other metals in fish species. The specific metal from fish feeds might cause bioaccumulation of that metal in the fish species.
4 Discussion
The bioaccumulation of metal in cultured fish depends on numerous factors; among them, fish feeds act as relevant sources. In an aquatic environment, metal toxicity can be affected by the length and weight of fishes (Nsikak et al. 2007). Fish feeds are the primary sources of metal accumulation in the flesh of studied fish. Therefore, it is assumed that metals accumulated in the fish are mainly from fish feeds because there are no other anthropogenic sources of pollution in the study area of the cultured fishing zone. Besides, Ali and Khan (2018) also stated that, Possible factors have an associate influence on fish bioaccumulation, corresponding to fish feed groups. This is in agreement with the findings of Sabbir et al. 2018. They reported the Fish feeds could be a source of metal contamination of the fish body in the aquaculture system and the mean concentration of Pb, Cd, Mg, As and Cr for different fish feed collected from Khulna, Satkhira and Bagherhat districts of Bangladesh were 8.49 ± 3.66, 0.29 ± 0.8, < 0.02, < 0.05, and 8.57 ± 3.47 mg/kg respectively. Kundu et al., 2017 evaluated the metal concentration in four fish feeds and metal burden in cultured Oreochromis niloticus fish species in Bangladesh and the study found was reveal that the concentration of Cu, Pb, and Cd in fish feeds was 22.618 to 38.480 mg/kg, 7.671 to 12.232 mg/kg, and 8.082 to 9.771 mg/kg respectively. The observations from Anhwange et al., 2012 on metal contents on two synthetic fish feeds namely Multi, and Coppen feeds in Nigeria whereas the concentration of Cd, Cu, Fe, Ni, Pb and Zn in Multi feed are 30.00 μg/kg, 157.00 μg/kg, 2196.00 μg/kg, 92.00 μg/kg, 348.00 μg/kg and 1209.00 μg/kg respectively while the concentration of Cd, Cu, Fe, Ni, Pb and Zn in Coppen feed are Cd, Cu, Fe, Ni, Pb, and Zn are 20.00 μg/kg, 204.00 μg/kg, 2435.00 μg/kg, 8.00 μg/kg, 375.00 μg/kg and 3324.00 μg/kg respectively. Onsanti et al. 2012 measured mercury concentration in three types of fish feeds (dried pellet feed, forage fish, and fish viscera), about Mercury and stable isotope signatures in caged marine fish and fish feed in Fujian coastline, China. The recorded result of onsanti et al. showed that tuna viscera contained the highest level of mercury (0.20 μg/g THg and 0.13 μg/g MeHg), with pellet feed containing the lowest level (0.05 μg/g THg and 0.01 μg/g MeHg). Besides, Hu et al. (2016) measured that the mean value of Cd in marine fish, crustaceans and mollusks were 0.023 (0.002–0.223) mg/kg, 0.155 (0.002–1.90) mg/kg and 0.460 (0.002–3.06) mg/kg respectively and, the mean content of Pb in marine fish, crustaceans and mollusks were 0.08 (0.02–0.223) mg/kg, 0.08 (0.02–1.04) mg/kg and 0.30 (0.02–2.63) mg/kg respectively in the coastline China.
Fish feeds in the present study reveal that the concentration of iron (Fe), copper (Cu), and lead (Pb) in three types of feed samples containing lower than safety limits 500, 100, and 5.00 µg/g, respectively. Arsenic concentration in the present study was below the detection limit. Zn standards were not available as permitted standards. Hg concentrations in studied three types of feeds (mega, paragon and fresh) are higher than the acceptable standard limit which were 0.91 µg/g, 1.23 µg/g and 1.21 µg/g in mega, paragon and fresh feeds respectively whereas the concentration of Cr in paragon feed sample is 9.02 µg/g which are also very high from accepted safety limit. It was alarming to note that all feed samples collected for the present study contain metals close to the standard limit, and few are higher than the safety limit.
In Bangladesh, where malnutrition remains a significant development challenge, fish is an irreplaceable animal-source food in the diet of millions (Bogard et al. 2015). Fish is one of the most important sources of protein, but the ingestion of metal from anthropogenic and other sources, especially in the case of cultured fish species, can caused human health risk. Metal in fish flesh causes a severe health risk to humans (Onyia et al. 2010). Rahman et al. (2016) identify metal pollution of water and fishes in Balu and Brahmaputra rivers of Bangladesh where the Cr concentration of Balu river three fish species (Mastacem belusarmatus, Channapunctatus, and Mystusvittatus) are 0.72 mg/kg, 0.76 mg/kg and 0.51 mg/kg respectively and Cr concentration of Brahmaputra river three fish species (Mastacem belusarmatus, Channapunctatus, and Mystusvittatus) are 0.19 mg/kg, 0.17 mg/kg, 0.22 mg/kg respectively. Raknuzzaman et al. 2016 conduct a study about trace metal contamination in commercial fish in the coastal area of Bangladesh where Cr concentration range 0.15–2.2 mg/kg.
The present study finding showed that the concentration of Cr, Co, and Zn for all fish species was above the higher limit from the recommended value of FAO and WHO (1989). Cu concentration is high in Pangasius pangasius, Oreochromis niloticus, Hg concentration is high in Pangasius pangasius, Oreochromis niloticus, Anabustestudi neusand Clariasbatra chus. Copper high doses cause anemia, liver and kidney damage, and stomach and intestinal irritation and high levels of chromium, which can irritate skin and can produce ulcers; cause liver, kidney, circulatory and nerve tissue damage (Mandal and Ahmed 2014). Pb concentration is very close to the standard limit inPangasius pangasius and Heteropneustesf ossilisfish species. In humans, the exposure of Lead can inhabit the hemoglobin synthesis; distort the cardiovascular system (Ogwuebgu and Muhanga. 2005).
Given human health risk, MPI values for fish and fish feed in which A.testudineus fish species showed the highest Metal Pollution index.MPI values ranged from 2.38 to 4.89 µg/g in the freshwater prawn (Macrobrachium rosenbergii) of Bangladesh (Islam et al. 2017). The highest DIM value for Cu was found in C.batrachus, both adults and children. The DIM value of Fe was the highest, and other parameter sequences order were Zn > Cu > Hg > Pb, respectively. Zhong et al. (2018) identified the values of estimated daily intake for both adult and children in ten fish species in the central and eastern North China whereas the estimated daily intake of Cu, Pb, As, Cr and Zn for adult were 0.37, 0.13, 0.11, 2.24 and 6.36 respectively while the estimated daily intake of Cu, Pb, As, Cr and Zn for children were 0.70, 0.24, 0.21, 4.22 and 11.97 respectively.
Maximum HRI values for Pb was present in both P. pangasius and C. batrachus fish species. Furthermore, the highest HI value (2.23) recorded for this study in P. pangasius fishspecieswhereas the lowest value (1.26) was calculated in H.fossilis fish samples. Akoto et al. (2014) calculated HRIs of Fe, Cu, Pb and Zn in muscles of fish samples from the Fosu Lagoon, Ghana and the HRI value were 1.90 × 10–3, 7.25 × 10–4, 8.69 × 10–2 and 9.17 × 10−4respectively. Ahmed et al. (2015) estimated hazard index (HI) for different metals from the consumption of three fish species collected from Buriganga River, Bangladesh, and the HI values of Ailiacoila, Gagatayoussoufiand Mastacembeluspancaluswere 5.10 × 10–1, 4.49 × 10–1 and 1.03 × 10–1 respectively. The carcinogenic risk also showed that the examined fish species have carcinogenic effects through the consumption of the selected fish species.
5 Conclusions
The study demonstrated the concentration of metal in fish feeds and determined the metal concentration in the selected commercial fish flesh. However, the result of the present investigation indicates that the metal concentration in fish feeds may be responsible for bioaccumulation of metal in the fish flesh but future research work about fish feeds and fish metal accumulation considering other factors such as time, growth of fish and size of fish helps to understand about accumulation process and concentration. Health-related diseases may cause for consumption of the fishes with high degrees of metals. Amongst the measured fish species, Heteropneustesfossilis was found to have a lower accumulation of metals. According to the indices result, this fish species likely to be safer compared to other fish species. In contrast, Clariasbatrachus was observed to have a high contamination rate comparatively. As samples were collected from homogenous areas in different fishing zone, the degree of contamination might not vary significantly within the study area. The study provides useful tools for future ecotoxicological studies and the safety of consumers and helps the decision-maker implement rules and guidelines about the manufacture of commercial fish feed for fisheries. In developing countries, cultured fish are a regular part of the diet for most people, and from the consumer safety perspective, regular investigation and monitoring of related institutions and authorities are strictly required. A better aquaculture management system should be established for the conservation of the fish resources for the next generation, and more attention should be given on the manufacturing environment, and health-friendly fish feeds.
References
Abdel-Baki AS, Dkhil MA, Al-Quraishy S (2011) Bioaccumulation of some heavy metals in tilapia fish relevant to their concentration in water and sediment of Wadi Hanifah, Saudi Arabia. Afr J Biotechnol 10(13):2541–2547
Aderinola OJ, Clarke EO, Olarinmoye OM, Kusemiju V, Anatekhai MA (2009) Heavy metals in surface water, sediments, fish, and perwinklesof Lagos Lagoon. Am-Eurasian J Agric Environ Sci 5(5):609–617
Ahmed ATA, Mandal S, Chowdhury DA, Tareq ARM, Rahman MM (2012) Bioaccumulation of some heavy metals in ayre fish (Sperata Aor Hamilton, 1822), sediment, and water of dhaleshwari river in dry season. Bangladesh J Zool 40(1):147–153
Ahmed MK, Baki MA, Islam MS, Kundu GK, Habibullah-Al-Mamun M, Sarkar SK, Hossain MM (2015) Human health risk assessment of heavy metals in tropical fish and shellfish collected from the river Buriganga, Bangladesh. Environ Sci Pollut Res 22(20):15880–15890
Akoto O, Eshun F, Darko G, Adei E (2014) Concentrations and health risk assessments of heavy metals in fish from the Fosu Lagoon. Int J Environ Res 8:403–410
Alam R, Ahmed Z, Seefat SM, Nahin KTK (2020) Assessment of surface water quality around a landfill using multivariate statistical method, Sylhet. Bangladesh. Environ Nanotechnol Monit Manage 15(2021):100422
Ali H, Khan E (2018) Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fish. Risk to human health. Environ Chem Lett 16:903–917
Anhwang BA, Asemave K, Kim BC, Nyiaatagher DT (2012) Heavy metals contents of some synthetic fish feeds found within makurdi metropolis. Int J Food Nutr Saf 2(2):54–61
Asaduzzaman MY, Yang Y, Wahab MA, Diana JS, Ahmed ZF (2006) Farming system of giant freshwater prawn Macrobrachium rosenbergii in Bangladesh. In: Proceeding of the WAS Conference (AQUA 2006). A combination of tradition and technology: Florence, Italy
Azaman F, JuahiR H, Yunus K, Azid A, Dasuki A, Amran MA, Kamarudin MKA, Toriman MI, Hasnam CNC, Saudi ASM (2015) Heavy metal in fish: analysis and human health-a review. Jurnal Teknologi 77:61–69
Bhattacharya P, Welch AH, Stollenwerk KG, McLaughlin MJ, Bundschuh J, Panaullah G (2007) Arsenic in the environment: biology and chemistry. Sci Total Environ 379(2–3):109–120
Bogard JR, Thilsted SH, Marks GC, Wahab MA, Hossain MAR, Jakobsen J, Stangoulis J (2015) Nutrient composition of valuable fish species in Bangladesh and the potential contribution to recommended nutrient intakes. J Food Compos Anal 42:120–133
Chandra Sekhar K, Chary NS, Kamala CT, Suman Raj DS, Sreenivasa Rao A (2003) Fractionation studies and bioaccumulation of sediment-bound heavy metals in Kolleru lake by edible fish. Environ Int 29(7):1001–1008
Chi QQ, Zhu GW, Langdon A (2007) Bioaccumulation of heavy metals in fishes from Taihu Lake, China. J Environ Sci 19(12):1500–1504
Cui YJ, Zhu YG, Zhai RH, Chen DY, Huang YZ, Qiu Y, Liang JZ (2004) Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environ Int 30(6):785–791
DoF (Department of Fisheries) (2015) Department of Fisheries Report 2015. Department of Fisheries (DoF), Ministry of Fisheries and Livestock, Dhaka, Bangladesh
DoF (Department of Fisheries) (2018). Yearbook of Fisheries Statistics of Bangladesh 2017–18. Department of Fisheries (DoF), Ministry of Fisheries and Livestock, Dhaka, Bangladesh.
EC (2006). Setting maximum levels for certain contaminants in foodstuff European Commission, Regulation (EC) No. 1881/2006. Official J. Eur. Union L 364 (20), 5e24.
El-Moselhy KM, Othman AI, Abd El-Azem H, El-Metwally MEA (2014) Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egypt J Basic Appl Sci 1(2):97–105
EU, (2008) The opinion of the scientific committee on animal nutrition on undesirable substances in the feed. European Union, Brussels, Belgium
Griboff J, Wunderlin DA, Monferran MV (2017) Metals, As and Se determination by inductively coupled plasma-mass spectrometry (ICP-MS) in edible fish collected from three eutrophic reservoirs. Their consumption represents a risk for human health? Microchem J 130:236–244
Hossain AMMM, Monir T, Haque AMRU, Kazi ALM, Islam SM, Elahi SF (2007) Heavy metal concentration in tannery solid wastes used as poultry Teed and ecotoxicological consequences. Bangladesh J Sci Ind Res 42:397–426
Hu H (2002) Human health and heavy metals exposure. In: Michael M (ed) Life support. The environment and human health. MIT Press, Cambridge
Indrajit S, Ajay S, Shrivastava VS (2011) Study for determination of heavy metals in fish species of the River Yamuna (Delhi) by inductively coupled plasma-optical emission spectroscopy (ICP-OES). Adv Appl Sci Res 2(2):161–166
Islam GMR, Habib MR, Waid JL, Rahman MS, Kabir J, Akter S, Jolly YN (2017) Heavy metal contamination of freshwater prawn (Macrobrachium rosenbergii) and prawn feed in Bangladesh: a market-based study to highlight probable health risks. Chemosphere 170:282–289
Islam GMR, Khan FE, Hoque MM, Jolly YN (2014) Consumption of unsafe food in the adjacent area of Hazaribag tannery campus and Buriganga River embankments of Bangladesh: heavy metal contamination. Environ Monit Assess 186(11):7233–7244
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283(2–3):65–87
Hasan MK, Shahriar A, Hossain N, Shovon IK, Hossain A, Jolly YN, Begum BA (2020) Trace metals contamination in riverine captured fish and prawn of Bangladesh and Associated Health Risk. Expo Health. https://doi.org/10.1007/s12403-020-00378-1
Hu S, Su Z, Jiang J, Huang W, Liang X, Hu J, Chen M, Cai W, Wang J, Zhang X (2016) Lead, cadmium pollution of seafood and human health risk assessment in the coastline of the southern China. Stoch Env Res Risk Assess 30:1379–1386
Kundu GK, Alauddin M, Akter MS, Khan MS, Islam MM, Mondal G, Islam D, Mohanta LC, Huque A (2017) Metal contamination of commercial fish feed and quality aspects of farmed tilapia (Oreochromis niloticus) in Bangladesh. Biores Commun 3(1):345–353
Maule AG, Gannam AL, Davis JW (2007) Chemical contaminants in fish feeds used in federal Salmonid hatcheries in the USA. Chemosphere 67:1308–1315
Mahmud NA, Hasan MDR, Hossain MB, Minar MH (2012) Proximate composition of fish feed ingredients available in Lakshmipur region, Bangladesh. Am-Eurasian J Agric Environ Sci 12(5):556–560
Malik N, Biswas AK, Qureshi TA, Borana K, Virha R (2009) Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bhopal. Environ Monit Assess 160(1–4):267–276
Mandal S, Ahmed ATA (2014) Copper, Cadmium, Chromium and lead bioaccumulation in Stinging Catfish, Heteropneustes fossilis (Bloch) and freshwater mussel, Lamellidens corrianus Lia and to compare their concentration in sediments and water of Turag river. J Asiatic Soc Bangladesh Sci 39(2):231–238
Mansour SA, Sidky MM (2002) Ecotoxicological studies. 3. Heavy metals contaminating water and fish from Fayoum Governorate, Egypt. Food Chem 78(1):15–22
Nsikak UB, Joseph PE, Akan BW, David EB (2007) Mercury accumulation in fishes from tropical aquatic ecosystems in the Niger Delta, Nigeria. Curr Sci 92(6):781–785
Ogundele LT, Ayeku PO (2020) Source apportionment and associated potential ecological risk assessment of heavy metals in coastal marine sediments samples in Ondo, Southwest, Nigeria. Stoch Env Res Risk Assess 34:2013–2022
Ogwuegbu MOC, Muhanga W (2005) Investigation of lead concentration in the blood of people in the copperbelt province of Zambia. J Environ 1:66–75
Onyia LU, Milam C, Manu JM, Allison DS (2010) Proximate and mineral composition in some freshwater fishes in upper river benue, Yola, Nigeria. Continental J Food Sci Technol 4(2):1–6
Onsanit S, Chen M, Ke C, Wang WX (2012) Mercury and stable isotope signatures in caged marine fish and fish feeds. J Hazard Mater 203–204:13–21
Patwardhan AD (2013) Industrial solid wastes. TERI Press, New Delhi, India
Raknuzzaman M, Ahmed MK, Islam MS, Habibullah-Al-Mamun M, Tokumura M, Sekine M, Masunaga S (2016) Trace metal contamination in commercial fish and crustaceans collected from coastal area of Bangladesh and health risk assessment. Environ Sci Pollut Res 23(17):17298–17310
Rahman M, Islam M, Khan M (2016) Status of heavy metal pollution of water and fishes in Balu and Brahmaputra rivers. Progress Agric 27(4):444–452
Rashed MN (2001) Monitoring of environmental heavy metals in fish from Nasser lake. Environ Int 27:27–33
Rahman MS, Hossain MS, Ahmed MK, Akther S, Jolly YN, Akhter S, Jamiul Kabir M, Choudhury TR (2019) Assessment of heavy metals contamination in selected tropical marine fish species in Bangladesh and their impact on human health. Environ Nanotechnol Monitor Manag 11:100210
Rajeshkumar S, Li X (2018) Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol Rep 5:288–295
Reksten AM, Somasundaram T, Kjellevold M, Nordhagen A, Bøkevoll A, Pincus LM, Rizwan AAM, Mamun A, Thilsted SH, Htut T, Aakre I (2020) Nutrient composition of 19 fish species from Sri Lanka and potential contribution to food and nutrition security. J Food Compos Anal 91:103508
Renieri EA, Safenkova IV, Alegakis AK, Slutskaya ES, Kokaraki V, Kentouri M, Dzantiev BB, Tsatsakis AM (2019) Cadmium, lead and mercury in muscle tissue of gilthead seabream and seabass: risk evaluation for consumers. Food Chem Toxicol 124:439–449
Sabbir W, Rahman Z, Halder T (2018) Assessment of heavy metal contamination in fish feed available in three districts of South Western region of Bangladesh. Int J Fish Aquatic Stud 26(2):100–104
Shamsuzzaman MM, Islam MM, Tania NJ, Abdullah Al-Mamun M, Barman PP, Xu X (2017) Fisheries resources of Bangladesh: present status and future direction. Aquacult Fish 2(4):145–156
Tripathi MK, Agrawal IS, Sharma SD, Mishra DP (2001) The effect of untreated, HCl treated or copper and iodine supplemented high glucosinolate mustard (Brassica juncea) meals on nutrient utilization, liver enzymes, thyroid hormones, and calves’ growth. Anim Feed Sci Technol 92(1–2):73–85
Usero J, González-Regalado E, Gracia I (1997) Trace metals in the bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the Atlantic Coast of Southern Spain. Environ Int 23(3):291–298
USPEA (1989) Risk assessment guidance for superfund: human health evaluation manual (part A). United States Environment Protection Agency, Washington, DC, USA
USEPA (2010) Risk-based concentration table, Washington, DC, USA: United States Environment Protection Agency. Retrieved February 05, 2020 from: https://archive.epa.gov/region9/superfund/web/html/index-23.html
USEPA (2011) Regional screening level (RSL) summary table: November 2011. United States Environment Protection Agency, Washington, DC, USA
USEPA-IRIS (2006) United States Environmental Protection Agency. Integrated Risk Information System, Washington, DC, USA. Retrieved September 23, 2019, from: http://www.epa.gov/iris
Wang X, Sato T, Xing B, Tao S (2005) Health risk of heavy metals to the general publican Tianjin, China via consumption of vegetables and fish. Sci Total Environ 350:28–37
WHO/FAO (1989) National research council recommended dietary 626 allowances, 10th edn. National Academy Press, Washington
Zhong W, Zhang Y, Wu Z, Yang R, Chen X, Yang J, Zhu L (2018) Health risk assessment of heavy metals in freshwater fish in the central and eastern North China. Ecotoxicol Environ Saf 157:343–349
Acknowledgements
The authors would like to thank the authority of Bangladesh Atomic Energy Commission (BAEC) for providing their lab facilities and guidelines.
Funding
The study did not receive any funding.
Author information
Authors and Affiliations
Contributions
PG initiated and contributed to the preliminary concept of the study under the supervision of ZA and BB. Afterward, PG collected the primary data from the study area and completed the laboratory analysis according to the instruction of SA. Meanwhile, YNJ provided suggestions and guidelines. Statistical analyses were done by RA. Subsequently, RA and ZA completed the final draft and revised it critically. Finally, all the authors approved this final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghosh, P., Ahmed, Z., Alam, R. et al. Bioaccumulation of metals in selected cultured fish species and human health risk assessment: a study in Mymensingh Sadar Upazila, Bangladesh. Stoch Environ Res Risk Assess 35, 2287–2301 (2021). https://doi.org/10.1007/s00477-021-02026-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00477-021-02026-9