Abstract
Key Message
Neolithus fasciatus gall affected the host's efficiency to absorb and use the available light and functioned as a sink source for photoassimilates, water, and nutrients that would be allocated to plant development and reproduction of Sapium glandulatum.
Abstract
The knowledge on the effects of galls on the redistribution of resources and reproductive performance of the host is fragmentary. We used a combined approach of physiological and biochemical analyses to investigate the impact of galling on host ecophysiology and performance to aid in the bridging of this gap. We determined the frequency of galled hosts on 155 individuals of Sapium glandulatum in the field. The following ecophysiological parameters: oxidative stress, gas exchange, contents of chlorophyll, flavonoids, water, and total carbohydrates were recorded on galled and ungalled individuals. In addition, the impact of galling was recorded through the number of lateral shoots and fruit production. Approximately 75% of the studied host population had at least one galled shoot, and galls were most abundant on younger leaves. While galled and ungalled individuals did not differ in their oxidative stress, galled individuals showed higher stomatal conductance, internal carbon concentration, and flavonoid production, but lower net photosynthetic rate and effective quantum yield of PSII. Total carbohydrate and content of water were higher in galled tissues compared to healthy tissues of galled and ungalled host leaves. Galling induced attacked shoots to produce ca. four lateral shoots while ungalled shoots did not produce any lateral shoots. Last, while ungalled shoots produced an average of six fruits each, galled shoots did not bear any fruits. The presence of Neolithus fasciatus galls affected the host's efficiency to absorb and use the available light and functioned as a sink source for photoassimilates, water, and nutrients that would be allocated to plant development and reproduction. These data support the hypothesis that galling insects are important herbivores and their negative effects on the hosts can spread into several organs and functioning systems of the hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gall-inducing insects are sophisticated herbivores (e.g., Shorthouse et al. 2005) primarily because of their ability to control and drastically modify their host plant’s developmental trajectory to improve the insect’s fitness (Price et al. 1987; Harris and Pitzschke 2020). This parasitism often results in deep changes in the host physiology, anatomy, and metabolism. The impact begins when the gall-inducing female oviposits on the host and hatching larva first starts to manipulate the host cells. Initially, the galling larva suppresses the host’s immunity and influences its developmental mechanisms (e.g., Fernandes 1990; Favery et al. 2016; Giron et al. 2016; Schultz et al. 2019; Lemus et al. 2020). Since the success of the gall-inducing insect depends on the ability of the larvae to manipulate plant tissues (Fernandes 1990; Fernandes et al. 2003), females preferentially oviposit in tissues of rapid cell division or reactive sites (e.g., Rohfritsch 1992; Weis et al. 1988; Abrahamson and Weis 1997; Espírito-Santo et al. 2007). Some of these reactive sites are meristems, photoassimilates-sink points formed from partially or undifferentiated stem cells rich in nutrients and capable of cell division and tissue growth (Weis et al. 1988; Scofield and Murray 2006).
Among the initial changes triggered by the gall-inducing larva, there is a reduction in cell wall thickness, suppression of cuticle synthesis, and modification in the cytoplasmic density of cells inside the galls (e.g., Detoni et al. 2012; Tooker and Helms 2014). This process generates a cascade of oxidative reactions and genetic reprogramming (e.g., Detoni et al. 2011, 2012; Giron et al. 2016) that influence the host plant physiology and metabolism. The production of reactive oxygen species (ROS) is one of the first plant responses to gall induction (Oliveira et al. 2016; Lemus et al. 2020), which is also part of a redox regulatory network that allows plants to perceive and respond to fluctuating environmental conditions (Martin and Sies 2017). Reactive oxygen species (ROS) production above the neutralization capacity of the antioxidant system can damage the plant’s photosynthetic apparatus (Zhang et al. 2003; Roach and Krieger-Liszkay 2019). For example, a high concentration of hydrogen peroxide (H 2O2) affects enzyme activity in the Calvin cycle, particularly those containing Fe2+, D1/D2 proteins, and Mn clusters in the photosystem II (PSII) (Niyogi 1999; Zhang et al. 2003). An efficient mechanism of ROS dissipation in plant tissue is the production of secondary metabolites and enzymes that eliminate ROS (e.g., Aboul‐Enein et al. 2007). The synthesis of polyphenols, such as flavonoids, is a successful strategy to mitigate elevated cell ROS, because they eliminate free radicals and activate anti-oxidizing enzymes (Aboul‐Enein et al. 2007; Brunetti et al. 2018; Guedes et al. 2022). According to Czarnocka and Karpiński (2018), whether the production of ROS becomes harmful, protective, or a signaling factor depends on the balance between ROS production and its elimination by the host’s antioxidant system (e.g., Zhang et al. 2003).
The metabolic needs of gall-inducing insects are ensured by the supply of nutrients accumulated in the innermost cell layers of the galls or by solutes sucked directly from the phloem cells (Bronner 1992). Galls induced by Cecidomyiidae and Cynipidae are archetypes of insect galls and in these typical nutritive tissues accumulate proteins, lipids or sugar reducers (Bronner 1992; Rohfritsch 1992). Contrarily, phloem-sucking insects rarely develop true nutritive tissue in their galls, which makes their feeding sites restricted to the draw of solutes directly from the host’s phloem cells through their stylets (Bronner 1992). Some of the galls induced by these insects developed storage tissues with cells that accumulate starch close to the vascular bundles (e.g., Álvarez et al. 2009). Otherwise, more recent studies have shown the formation of nutritive tissues in a few galls induced by phloem-sucking insects of the neotropics (e.g., Oliveira et al. 2006; Oliveira and Isaias 2010; Carneiro and Isaias 2015).
The process of plant cell differentiation induced by the feeding behavior of the larva can also affect the contents of photosynthetic pigments and gas exchange rates of the host plant (Yang et al. 2003; Florentine et al. 2005; Patankar et al. 2011). When the gall tissue is unable to perform photosynthesis (e.g., galls on roots, stems, etc.) or does it at low intensity, the gall acts as a strong sink source for resources, draining photoassimilates, mineral nutrients, and water from the host plant (e.g., Kirst and Rapp 1974; Larson and Whitham 1991; Motta et al. 2005; Nabity et al. 2009; Oliveira et al. 2017). This is done by reversing regular carbohydrate transport patterns in the host and inducing mature leaves to import photoassimilates to meet the gall’s energy demands (e.g., Kirst and Rapp 1974; Wingler and Roitsch 2008; see also Miller and Raman 2019). In other words, as the gall develops, the host plant's resource distribution network is affected and, ultimately, precludes the host plant from simultaneously investing in growth, reproduction, and defense.
Although there have been good advances in the studies on gall development and impact on their hosts (e.g. Price and Louw 1996; Harris and Pitzschke 2020), speciose ecosystems, particularly those in the Neotropics, remain poorly investigated (Burckhardt and Queiroz 2021). One important gap is the lack of studies that evaluate the multiple effects of galling on the host plant, including that on fruit and/or seed production. Most studies generally focus solely on the ecophysiological aspects of galling without measuring the architectural or reproductive output of the host. We used a combination of physiological and biochemical analyses to investigate the effects of the galling insect Neolithus fasciatus (Psyllidae: Homoptera) on the resource allocation of its host plant Sapium glandulatum (L.) Morong (Euphorbiaceae), developmental, architectural, and reproductive output of the attacked host individuals. Although the first report on the interaction between these species is not new (see Manganaro 1916), to our knowledge there is no information on the impacts generated by the galls of N. fasciatus on S. glandulatum. Casual field observations indicated a high frequency of N. fasciatus galls on S. glandulatum in the islands of Atlantic rain forest in the summits of the Espinhaço Mountains in Brazil. This dense population of N. fasciatus galls in the region provided us with the ideal scenario to test some hypotheses on the ecophysiological impact of gall-inducing insects on their host plants. We tested the fllowing four hypotheses: (i) The "insect gall impact on host shoot development" hypothesis predicts that the development of shoots with galls is impaired or reduced. In addition, we expected that the more galls a shoot has the lower its development. (ii) The "insect gall impact on host ecophysiology" predicts that N. fasciatus galls trigger oxidative stress in the gall tissues and in the plant tissues adjacent to the galls, which leads to a decreased efficiency in the host’s light absorption and use. (iii) The hypothesis on "gall impact on host chemical defenses" predicts that N. fasciatus galls influence the production of defense secondary compounds such as flavonoids, in areas neighboring the galls. (iv) The hypothesis that "galls act as sinks'' by accumulating plant resources at a higher concentration than surrounding plant tissues. Carbon and water-based nutrients are translocated to the gall in a galling insect effort to preserve the natural development of the larval forms. (v) Last, the hypothesis that "galls impact the host architecture and performance". The presence of the N. fasciatus galls induce the formation of new lateral shoots but with reduced fruit production, resulting in the prevention of the host plant from reaching its reproductive potential.
Materials and methods
Study system
Sapium glandulatum is a perennial plant that occurs widely in the Lesser Antilles, Honduras, northeastern Argentina, Paraguay, Uruguay, and Brazil (Díaz 2020). It is a highly recommended species for the recovery of degraded areas due to its pioneer characteristics of providing initial soil cover, facilitating the entry and establishment of other plant species, among other reasons (Wuethrich 2007; Gagetti et al. 2016). Sapium glandulatum is the host plant of many herbivorous insects, among which is the psyllid N. fasciatus.
Study area
This study was carried out in Serra do Cipó (19°12′56.1″ S 43°32′02.5″ W), in the southern portion of the Espinhaço Mountains, southeastern Brazil. The region is classified as Cwb in the Köppen climate system with dry winters and rainy summers. The average annual precipitation is between 1250 and 1550 mm, and the average temperature ranges between 18 and 19 °C (Madeira and Fernandes 1999). Sapium glandulatum is common in sunny and open areas, being frequently found at the border of islands of tropical rainforests within a grassland matrix influenced by the rupestrian grassland vegetation (savannic campo rupestre: see Fernandes 2016) and the Atlantic rainforest biome (see Coelho et al. 2018).
Gall impact on shoot development
To test the first hypothesis that insect galls affect host shoot development, in August 2020, we randomly selected 155 individuals of S. glandulatum that were at least 10 m apart from each other to determine the frequency of galled individuals in the field. First, we counted the number of individuals with at least one gall and the number of ungalled individuals in the study population. Then, we randomly marked one shoot from each individual and determined the number of galls, diameter at the shoot’s base, and shoot length. These shoots were grouped into th following five categories according to the number of galls: 0 galls (n = 38, ungalled plants), 1–10 galls (n = 30), 11–30 galls (n = 32), 31–60 galls (n = 31), 61–312 galls (n = 24). To evaluate the effect of the number of galls on shoot’s biomass (weight: mg), we randomly selected 55 individuals among the sampled plants (galled and ungalled) and collected one shoot from each of them for measurements. In the laboratory, we recorded the number of galls and shoot’s weight. In this case, shoots were again grouped into five categories according to the number of galls as follows: 0 galls (n = 8), 1–10 galls (n = 7), 11–30 galls (n = 8), 31–60 galls (n = 11) and 61–312 galls (n = 21).
For identification of the gall-inducing insect and presence of parasitoids, pieces of galled shoots (n = 47 shoots) were cut at approximately 15 cm from the shoot apex and individually kept into glass pots until the emergence of adults of the gall-inducing insect or parasitoids. Pots were covered with a piece of tulle fabric and kept at room temperature (21–23 °C) for up to 5 days or when the shoots started to dry up. The humidity inside the pots was maintained through pieces of cotton soaked in potable water. Pots were monitored daily, and adults that emerged were collected and preserved in a 70% alcoholic solution. Insects that emerged were identified as the galling Neolithus fasciatus and its parasitoid Lochitoencyrtus gahani (Encyrtidade: Hymenoptera) (see Fernandes et al. 1988; Santis and Fernandes 1989). Finally, we determined the relative position of the galled leaves on the collected shoots of each of the 155 individuals to evaluate the spatial distribution of the galls on the host's shoots.
Ecophysiological responses of S. glandulatum
Twenty different S. glandulatum individuals in the population that were of similar size (ca. 130–150 cm height) were randomly selected to evaluate some ecophysiological aspects correlated with N. fasciatus galls. Firstly, we randomly selected and marked a galled individual, and then we located and marked its nearest neighbor ungalled individual for paired comparison of the ecophysiological measurements.
To test the hypothesis that N. fasciatus galls affect the host ecophysiology triggering oxidative stress in the gall tissues and the plant tissue adjacent to the galls, five galled leaves were collected from the galled individuals (n = 10). Neolithus fasciatus galls were carefully removed from the leaf lamina using razor blades. Analyses of oxidative stress were done for galled tissues, and ungalled leaf tissues of the galled leaf, separately. Likewise, five expanded leaves with no signs of herbivory or pathogens were collected from ten ungalled individuals (n = 10) for equivalent measurement of oxidative stress. Hence, analyses were made on gall tissue, leaf tissue from galled leaves, and leaf tissue from non-gall-bearing leaves. Reactive oxygen species (ROS) production was evaluated by quantifying H2O2, while the lipid peroxidation of the cell membrane was verified through the production of malondialdehyde (MDA). The H2O2 concentration was determined spectrophotometrically according to the method of Velikova et al. (2000) at a wavelength of 390 nm. The concentration of MDA reactive to the thiobarbituric acid was determined according to Hodges et al. (1999) by using a spectrophotometer (Shimadzu, UV-1800) at wavelengths of 440 nm, 532 nm and 600 nm.
We also evaluated gas exchange and chlorophyll a fluorescence in these galled and ungalled paired host individuals. Measurements were done on one galled leaf and one ungalled leaf per individual at the shoot apical region of these individuals (n = 10 each). On these leaves, we evaluated the net photosynthetic rate (A, μmol m−2 s−1), stomatal conductance (gs, mol m−2 s−1), transpiration rate (E, mmol m−2 s−1), internal CO2 concentration, instantaneous efficiency of water use (Wt = A/E, μmol CO2 mmol H2O m−2 s−1), electron transport rate (ETR), and ϕII from 0700 to 1100 h using an infrared gas analyzer (model 6400XT, Li-Cor Inc., Lincoln, NE, USA) attached to a fluorescence chamber (model 6400–40 leaf chamber fluorometer, Li-Cor Inc., Lincoln, NE, USA). The device was calibrated according to local characteristics to maintain the leaf temperature at 30 ºC and the vapor pressure deficit around 1.5 kPa. The flow rate was maintained at 300 μmol air min−1 and the CO2 concentration at 400 μmol mol−1 using a CO2 injection system. The leaf was positioned inside the chamber (2 cm2) and exposed to light until reaching a steady state for measurements (1500 μmol m−2 s−1).
To test the third hypothesis that insect galls affect secondary compounds’ production, we evaluated the content of flavonoids in the same leaves that we measured chlorophyll content. The chlorophyll content of galled (n = 10) and ungalled individuals (n = 10) was recorded in five leaves at the shoot apical region. Leaves of ungalled individuals had no signs of herbivory or pathogens. These measurements were performed on the left and right sides of each leaf and averaged by individual. The chlorophyll content was measured using specific wavelengths of light emitted by the Dualex (Dualex® 208 4.5 Scientific). This method was chosen due to its cost-effectiveness in addressing our research questions and practicality under field conditions (see Pihain et al. 2019; Perea et al. 2021). We used the proportion of chlorophyll content to flavonoids (which is associated with carbon/nitrogen ratio) in selected leaves to obtain the Nitrogen Balance Index, a proxy for leaf nitrogen content (Cartelat et al. 2005). These parameters are well-established indicators to evaluate the host plants’ ecophysiological conditions (Huang et al. 2014; Martini et al. 2020).
To test the fourth hypothesis that galls act as sinks by accumulating plant resources at a higher concentration than surrounding plant tissue, we evaluated the total carbohydrate and water content in galled individuals. For such analysis, five galled leaves were collected from the ten S. glandulatum individuals, and their galls were carefully removed with a razor blade. Each galling nymph was removed from the respective gall before the biochemical assay. Hence, we separately analyzed the tissue of the gall, and tissue of the leaf lamina of the galled leaves. Measurements from ungalled individuals (n = 10), were randomly done for five expanded leaves with no signs of herbivory or pathogens. The total carbohydrate content was quantified by the method of phenol sulfuric acid (Dubois et al. 1956). The leaf extract was prepared using 5 mg of plant biomass lyophilized in sulfuric acid (1 M). Glucose (Sigma-Aldrich, USA) was used as the standard, and the total carbohydrate content was expressed in milligrams of glucose equivalents per gram of dry weight. The water content was measured using the difference between the fresh weight (FW) and dry weight (DW) of the galls and the leaves (galled and ungalled) [\({\text{water content }}\left( \% \right)\, = \,{1}00 \, \left( {{\text{FW}} - {\text{DW}}} \right) \, /{\text{ DW}}\) ].
Lastly, water potential measurements were evaluated in one shoot per plant, totaling 10 measurements on galled and 10 measurements on ungalled hosts. The xylem water potential was assessed using a Scholander pressure chamber (3005 Series, Soilmoisture Equipment Corp., Santa Barbara, USA). These measurements were carried out at pre-dawn field conditions between 500 to 600 h.
Production of lateral shoots and fruits on host
To evaluate the hypothesis that galls impact the host’s lateral shoot growth and lead to lower fruit production, 10 galled and 10 ungalled individuals (same individuals used in the ecophysiological analysis) were selected in the field in August 2020. One shoot per S. glandulatum individual was marked to monitor the production of lateral shoots and fruits. We returned to these same individuals twice, the first time in October 2020 (60 days later), and the second in January 2021 (150 days later), for the final record of the number of lateral shoots and fruits produced on the galled and ungalled shoots.
Statistical analyses
We used Analysis of Variance (ANOVA) to evaluate the effect of gall abundance (categories: 0 galls, 1–10 galls, 11–30 galls, 31–60 galls and 61–312 galls) on the host plant’s shoot diameter, shoot length and shoot weight. Then, planned comparisons were performed using the general linear assumptions for multiple comparisons in the “multcomp” package in R (Hothorn et al. 2008). We use the generalized least squares (GLS) models to identify the leaf position on the shoot with the highest number of galled leaves and the differences in total contents of H2O2 and MDA between the galls and the leaves (galled and ungalled). We adjusted the models using maximum likelihood estimation using the “nlme” package (Bates et al. 2014) in R. Then, planned comparisons were performed using the general linear assumptions for multiple comparisons in the “multcomp” package in R (Hothorn et al. 2008). We used one-tailed t-tests to compare galled and ungalled leaves regarding the following parameters: A, gs, E, internal concentration of CO2 and chlorophyll, flavonoids, Nitrogen Balance Index, and water potential. We used the Wilcoxon sign rank tests to compare the Wt, ETR and ϕII since these parameters were not normally distributed. The differences in total carbohydrate and water content between the galls and the leaves (galled and ungalled) were verified using GLS models through the “nlme” package (Bates et al. 2014) in R. Subsequently, planned comparisons based on the study's hypotheses were performed using the “multcomp” package (Hothorn et al. 2008) in R. To test the effects of N. fasciatus gall on the production of lateral shoots and fruits, we used the one-tailed t-tests to compare the number of lateral shoots and the number of fruits produced on galled and ungalled shoots. We verified the residual graphs of all evaluated parameters through visual inspection for deviations of homoscedasticity or normality. All tests were performed in R (R Core Team 2020) and the graphs were constructed using the GraphPad Prism 5.0 software.
Results
Galls impact host shoot development
Galls induced by N. fasciatus (Fig. 1a) are glabrous projections clearly distinguishable from other organs of S. glandulatum (Fig. 1b). When at low numbers and isolated, galls are typically found as reddish spheroid structures in which only one larval chamber is found and in which only one nymph of N. fasciatus develops (Fig. 1c). At higher density, galls coalesce to form amorphous structures that can contain dozens of galls. In the study area, N. fasciatus galls were very abundant and hence mostly coalescent (Fig. 1d). Galls are associated with the central leaf’s vein or encompassing several young leaves and the apical meristem to form large structures with multiple galls of indistinct shape, often following the leaf’s lanceolate shape (Fig. 1e). Gall color varies from light green to deep red, turning reddish throughout their development. Adult N. fasciatus emerges from the gall after its opening from top to bottom (Fig. 1f–g). The gall’s walls split open to free the adult galling insect while in some cases we observed the last instar nymph leaving the split-open gall. After the emergence, galls dry and fall from the host plant (Fig. 1h–i). The N. fasciatus galls in this study differ from those reported by Fernandes et al. (1988: pg 21; Fig. 14) as no depressions were found at the top of the gall spheres. Besides, N. fasciatus galls are used by an abundant community of other insects such as parasitoids, thrips, acari, spiders, and often the adults of N. fasciatus. The parasitoid Lochitoencyrtus gahani (Encyrtidade) was rare (3 individuals) and emerged from small circular holes formed on the gall walls (see Santis and Fernandes 1989).
Neolithus fasciatus galls on Sapium glandulatum. a Newly emerged adult of N. fasciatus. b Simple N. fasciatus galls on S. glandulatum leaves. c Unilocular gall (one larval chamber) in which only one nymph develops. d–e Coalescent galls lead to amorphous galling structures covering the host’s leaf blades, petioles, and even shoots. f–g Recently emerged N. fasciatus galls. (h-i) Dried galls eventually dehisce due to their weight or persist attached to the host until the emergence of new shoots. Photos by G.W Fernandes
Among the S. glandulatum individuals of the studied population, 75.48% had at least one shoot galled. Shoots with more than 10 galls showed, on average, a 22.78% reduction in their diameter (p < 0.0001; Fig. 2a) and 29.90% in their length (p < 0.0001; Fig. 2b) in comparison with shoots with less than 10 galls or 0 galls (see Supplementary Data Table 1S). Besides, we found that shoots with more than 60 galls showed a weight increase of 44.40% compared to shoots with less than 60 galls (p < 0.0001; Fig. 2c; Supplementary Data Table 1S).
Regarding the phyllotaxis of galled individuals, we found that N. fasciatus galls were predominantly abundant on the three youngest leaves of the sampled shoots (p < 0.0001, F(15.493) = 3.119; Supplementary Data Fig. 1S).
Host ecophysiological and chemical responses
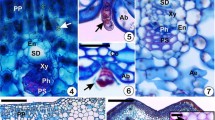
Gall’s presence negatively affected gas exchange in S. glandulatum individuals as well as their efficiency in absorbing and using light. Galled leaves showed higher gs (p = 0.042; Table 1) and internal carbon concentration compared to ungalled leaves (p < 0.0001; Table 1). However, carbon input was not associated with the A in galled individuals and was two times lower in the galled leaves than ungalled leaves (p = 0.0001; Table 1). Besides, galled leaves had a 17% reduction in their ETR (p = 0.001; Table 1) and 12% in their ϕII compared to ungalled leaves (p = 0.004; Table 1). There was no statistically significant change in the E of galled and ungalled leaves (p = 0.135; Table 1). However, galled leaves presented 52% lower Wt than ungalled leaves (p = 0.0003; Table 1). Also, the chlorophyll content in galled leaves was reduced by 45.44% compared to ungalled leaves (p < 0.0001; Table 1). Likewise, the nitrogen balance index was 31.92% lower in galled leaves than ungalled leaves (p = 0.004; Table 1).
The presence of N. fasciatus galls affected the production total carbohydrate content, and the water balance of their hosts. The total carbohydrate concentration was 72.75% higher in the gall than in ungalled leaves (P < 0.0001; Fig. 3a, Supplementary Data Table 2S). However, galled leaves had 46.8% less total carbohydrate content than ungalled leaves (P < 0.0001; Fig. 3a, Supplementary Data Table 2S). Also, the content of water in galled tissue was approximately four times higher than in tissues of galled and ungalled leaves (p < 0.0001; Fig. 3b, Supplementary Data Table 2S). Galled individuals also showed an increase of 62.7% in water potential (p = 0.002; Table 1).
Oxidative stress and nutritional content of galls and leaves (galled and ungalled) of Sapium gladulatum. a total carbohydrates, b water content, c Hydrogen peroxide (H2O2), d malondialdehyde (MDA) (x̅ ± SE). Different lowercase letters indicate a statistically significant difference between treatments (GLS and planned comparisons)
Hydrogen peroxide (H2O2) content was 55.5% higher in galls than galled and ungalled leaves (p = 0.025; Fig. 3c, Supplementary Data Table 2S). Consequently, the content of MDA was also twice as high in the gall tissue (p < 0.0001; Fig. 3d, Supplementary Data Table 2S). These results may be related to the increased production of secondary compounds. The production of flavonoids was 16.84% higher in galled leaves than ungalled leaves (p < 0.0001; Table 1).
Production of lateral shoots and fruits on host
The presence of N. fasciatus galls modified S. glandulatum production of lateral shoots and fruits. Galled shoots produced an average of 4 ± 0.262 new lateral shoots, while ungalled shoots did not present any new lateral shoots (Fig. 4a). Many of these lateral shoots grew just below the galled shoot’s apical region and did not bear any fruit. On the other hand, shoots of ungalled individuals had an average of 5.8 ± 0.611 fruits (Fig. 4b).
Discussion
Gall-inducing insects, unlike many of their free-living relatives, evolved the ability to discriminate among and select specific plants in a complex, natural environment. Physical and chemical characteristics of the plant (e.g., phyllotaxy and growth patterns, phenolics) are vital in host selection by gall-inducing insects (e.g., Fernandes and Price 1992; Fernandes et al. 2000; Miller and Raman 2019). Otherwise, a fascinating question yet to be answered is how gall-inducing insects have evolved to live and feed on plants with peculiar chemical characteristics (Miller and Raman 2019). The leaves of S. glandulatum are rich in defense compounds such as anthracene derivatives, monoterpenes, tannins and flavonoids (da Silva et al. 2012) and produces latex rich in proteolytic proteins (Sobottka et al. 2014). Nevertheless, even with a sophisticated anti-herbivore defense based on the production of potentially repellent and toxic substances, S. glandulatum is not free of herbivores. The frequency of S. glandulatum individuals with N. fasciatus galls was extremely high (Fig. 1) and influenced host plant development and performance.
Although N. fasciatus galls have been described on plant stems, inflorescences, and fruits (De Santis and Fernandes 1989), we only found them on the apical shoots and young leaves of the studied population of S. glandulatum (Fig. 1S). Apical portions of shoot tissues and young leaves have a high differentiation capacity, making them easier to manipulate during gall formation (Weis et al. 1988; Abrahamson and Weis 1997; Orcutt and Nilsen 2000). Besides, they present a strong sink capacity of photo-assimilated nutrients (Hodkinson 1984), which supports the formation of larger and more numerous galls (Price 2005; White et al. 2016).
The hypothesis that predicted that N. fasciatus galls would impact host development was supported. Galling, otherwise, varied from 1 to 312 galls per shoot (Fig. 2). Galled shoots were shorter and had a lower diameter compared to ungalled shoots. On the other hand, we highlight that the reductions in shoot length and diameter were influenced by the number of galls per shoot. Shoots bearing more than 11 galls were generally shorter and thinner compared to shoot categories with a lower number of galls. In terms of shoot weight, shoot categories with more than 60 galls were statistically heavier compared to other shoot categories with a lower number of galls. These results indicate the impact galls have on host development and architecture, that galls strongly influence host resource allocation patterns, and are powerful herbivores.
The influence of N. fasciatus galls on host ecophysiology was evaluated at different scales and processes and was generally supported. The high concentration of H2O2 and MDA in gall tissue corroborate the hypothesis that predicts that N. fasciatus galls trigger oxidative stress in the gall tissues (Fig. 3). The production of ROS is one of the first steps in the hypersensitivity response of plants to pathogens (Gill and Tuteja 2010; Jwa and Hwang 2017) and galling (Fernandes 1990). The production of ROS in the gall tissue is probably due to stress-induced by N. fasciatus activity on the inner tissues, along with the increased cellular metabolism rate that guarantees gall cellular machinery (Kmieć et al. 2018). Normally, ROS is generated at the feeding site of herbivores and spreads systemically all over the plant through the vascular bundles, mainly by the phloem (Kerchev et al. 2012). However, the production of ROS was restricted to the gall site/tissue. Curiously, the oxidative stress generated by the development of the gall was contained only to the galling tissue; indicating perhaps a strong capacity of manipulation of the host by this parasitic insect. The low efficiency of light absorption and utilization found in the galled leaves may be related to the translocation of plant nutrients to the galls (Table 1). Nitrogen participates in the composition of important molecules in plant metabolism; in fact, 75% of plant nitrogen is allocated to the production of chlorophyll and Rubisco (Evans 1989; Evans and Seemann 1989) (Table 1). Chlorophyll molecules are responsible for capturing light, resulting in the formation of reducing power and adenosine triphosphate (ATP) used in the Calvin-Benson cycle (Allen 2002; Croft et al. 2017). Rubisco catalyzes the combination of CO2 and ribulose 1.5-diphosphate (RuBP) to form 2 molecules of 3-phosphoglycerate, which are later converted into glucose (Benner 1989). Thus, chlorophyll and Rubisco contents are closely related to the plants’ photosynthetic capacity. Here, the low concentration of nitrogen negatively regulated the leaves’ chlorophyll content, causing the decline in A and ϕII. Besides, the high levels of gs and internal CO2 concentration also indicate that the reduction in A may be related to non-stomatal limitations, such as RuBP regeneration (von Caemmerer and Farquhar 1981; Medrano et al. 2002; Thompson et al. 2007). Future experimental studies on this system shall address photoassimilates’ origin and movement during gall formation under different host environmental conditions.
Galling insects are known to be able to control some chemical aspects of their host plant, which is not surprising given the intimate nature of the relationship (e.g., Fernandes 1990; Nyman and Julkunen-Tiitto 2000; Fernandes et al. 2019). Indeed, flavonoid content was two times higher in galled S. glandulatum individuals compared to ungalled individuals. The flavonoids are the main secondary compounds found in galled plants and may be related to defense against herbivory (Fernandes and Price 1991; Julião et al. 2014; Hall et al. 2017). Besides, chemicals such as tocopherol, carotenoids, and flavonoids also participate in eliminating ROS (Mittler 2002; Blokhina et al. 2003). Flavonoids may even be an important host trait in the selection of potential hosts by ovipositing galling female insects (Fernandes and Price 1991). Changes in cell redox potential affect MYB transcription factors (myeloblastosis) and activate the biosynthesis of flavonoids such as anthocyanins (Dubos et al. 2010; Agati et al. 2012). Indeed, a high concentration of ROS was found in the gall tissue induced by N. fasciatus. Agudelo et al. (2017) showed that gall induction by Neolithus spp. on S. haematospermum drastically increased the production of anthocyanins, especially in the final stages of gall maturation. Anthocyanins have been associated with the protection against light stress in galls (Solovchenko and Schmitz-Eiberger 2003; Karageorgou and Manetas 2006), signaling the presence of toxic substances (aposematic gall hypothesis) (Inbar et al. 2010), and antioxidant activity (Agati et al. 2012; Wiczkowski et al. 2013). In addition to advertising some toxicity to natural enemies, flavonoids could also play an essential role in reducing oxidative stress in the S. glandulatum galls. Furthermore, higher polyphenol content is also part of the plant stress hypothesis proposed by Fernandes and Price (1988, 1991) in which galling is facilitated by plant stress. Indeed, our host plant is mostly found at the sunny and warmer borders of the Atlantic rain forests studied.
Galls induced by sucking insects modify their host plant distribution of photoassimilates, and water, thus causing a deficit of these nutrients in galled leaves (Raman 1991; Mani and Raman 1994). As expected by the sink hypothesis, total carbohydrate concentration in galled tissue was ca. three-fold higher than in adjacent ungalled tissue of galled leaves, and about twice higher when compared to ungalled leaf tissue (Fig. 3). As homopterans mature, the bundle of stilettos in their oral apparatus becomes longer, enabling them to pass through the cells of superficial parenchyma and reach deeper vascular tissues (Raman 2003). Consequently, these cells become metaplastic and a source of nutrients within the gall (Raman 2003; Ferreira et al. 2019). However, the high concentration of total carbohydrates in galls may also be related to an increase in the cell wall’s pectic matrix (Castro et al. 2012). On the other hand, with few exceptions, the formation of nutritive tissues in psyllid galls is rare or not sufficiently reported (Meyer and Maresquelle 1983; Meyer 1987; Rohfritsch 1992). Future studies on the anatomy and ontogenesis of these galls as well as studies on the larval development and feeding mode should be developed to further explore this hypothesis.
The increase in the plant cell water content seems to be a necessary condition for the gall as galled individuals presented a greater water potential than ungalled individuals (Table 1). However, galls’ presence reduced the Wt; in other words, the assimilation of carbon with E among galled individuals (see Yoo et al. 2009). Thus, individuals of S. glandulatum with galls may be maintaining greater water potential by regulating their E (see Yoo et al. 2009; Li et al. 2017). Fay et al. (1993) argued that another strategy to keep high water potential is to invest in expanding the host’s root system, as observed in Silphium integrifolium. In our study, higher water content was recorded in host individuals with galls compared to individuals without galls. A similar pattern was also found directly on gall tissues, which had water content four times higher than in leaf tissue of ungalled plant individuals. Notably, the intense stress associated with the osmotic change during gall development attracts more water to the stressed cells than to the unaffected cells, thus creating intense hydrostatic pressure in the stressed cells (Zonia and Munnik 2007). This is certainly a plausible mechanism by which gall-inducing insects thrive on stressed hosts and habitats around the globe (e.g., Fernandes and Price 1991; Julião et al. 2014), albeit further studies are needed.
When apical shoots are damaged, the production of axillary shoots is activated, initiating a secondary axis growth in the host plant (Maschinski and Whitham 1989; Lortie and Aarssen 2000). Neolithus fasciatus galls impacted the host’s shoot production by inducing the formation of new lateral shoots; hence providing support for the impact of galls on host development and architecture (Fig. 4). This demonstrates the host plant’s plasticity to alter its architecture pattern as a response to an environmental disturbance (Bennett and Leyser 2006), as long as resources are available. As a result, galled plants can harness the available light and increase their net carbon gain (Aarssen and Irwin 1991; Fay and Throop 2005). Nevertheless, this is not a foolproof strategy. For instance, this secondary growth also represents a renewed opportunity for other N. fasciatus adults to oviposit and produce another generation or even establish a multivoltism pattern (Price et al. 1996; Price et al. 2005; Kurzfeld-Zexer et al. 2010). Indeed, Craig et al. (1986) reported that the galling sawfly Euura lasiolepis manipulates its host plant to induce new sprouts and immediately benefit subsequent generations, and called it the resource manipulation hypothesis. In addition, the constant production of lateral shoots could attract other herbivores looking for newly formed shoots/leaves (see Fernandes and Ribeiro 1990; Nakamura et al. 2003). In summary, hosts are not only manipulated to reallocate resources to galls, but also to the primary production of lateral shoots. On the other hand, no fruits were produced on these newly formed shoots, at least during the growing season. Contrarily, many fruits were produced on shoots of ungalled plant individuals. Therefore, N. fasciatus effects on S. glandulatum are not limited to the gall formation but also result in the modification of the shoot architecture and the reduction of the host’s reproductive performance.
Conclusions
This study demonstrates that insect galls affect shoot development and function as a sink source for photoassimilates, water, and nutrients that would be allocated throughout plant development and reproduction. The effects of N. fasciatus galls are felt at several scales and processes within its S. glandulatum host. Neolithus fasciatus also manipulates its host plant to induce new sprouts that benefit subsequent insect generations. These capillary effects ultimately reduce the host reproductive performance.
Author contribution statement
GWF and RAM: designed research and wrote the paper; RAM analyzed data; RAM and LAG: drew the figures; GWF, RAM, YO, LNPC collected data; and GWF, LAG, MB, EGP, MGCF revised the article.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Aarssen LW, Irwin DL (1991) What selection: herbivory or competition? Oikos 60:261–262
Aboul-Enein HY, Kruk I, Kładna A, Lichszteld K, MichalskaT, (2007) Scavenging effects of phenolic compounds on reactive oxygen species. Biopolymers 86:222–230. https://doi.org/10.1002/bip.20725
Abrahamson WG, Weis AR (1997) Evolutionary ecology across three trophic levels: goldenrods, gallmakers, and natural enemies. Princeton University Press, New Jersey
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76. https://doi.org/10.1016/j.plantsci.2012.07.014
Agudelo I, Wagner M, Ricco R (2017) Variación en el contenido de clorofilas, carotenos y antocianos en agallas inducidas por Neolithus spp. (Hemiptera: Psyllidae) sobre Sapium haematospermum (Euphorbiaceae). Lilloa 54:91–100
Allen J (2002) Photosynthesis of ATP-electrons, proton pumps, rotors, and poise. Cell 110:273–276. https://doi.org/10.1016/S0092-8674(02)00870-X
Álvarez R, Encina A, Hidalgo NP (2009) Histological aspects of three Pistacia terebinthus galls induced by three different aphids: Paracletus cimiciformis, Forda marginata and Forda formicaria. Plant Sci 176:303–314. https://doi.org/10.1016/j.plantsci.2008.11.006
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823
Benner AS (1989) Enzyme kinetics and molecular evolution. Chem Rev 89:789–806. https://doi.org/10.1021/cr00094a004
Bennett T, Leyser O (2006) Something on the side: axillary meristems and plant development. Plant Mol Biol 60:843–854. https://doi.org/10.1007/s11103-005-2763-4
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194. https://doi.org/10.1093/aob/mcf118
Bronner R (1992) The role of nutritive cells in the nutrition of Cynipids and Cecidomyiids. In: Shorthouse JD, Rohfritsch O (eds) Biology of insect-induced galls. Oxford University Press, New York, pp 11–140
Brunetti C, Fini A, Sebastiani F, Gori A, Tattini M (2018) Modulation of phytohormone signaling: a primary function of flavonoids in plant-environment interactions. Front Plant Sci 9:1042. https://doi.org/10.3389/fpls.2018.01042
Burckhardt D, Queiroz DL (2021) Psylloidea. Catálogo taxonômico da fauna do Brasil. PNUD. Available in: http://fauna.jbrj.gov.br/fauna/faunadobrasil/97523 [Accessed 10 Dec 2021]
Carneiro RGS, Isaias RMS (2015) Gradients of metabolite accumulation and redifferentiation of nutritive cells associated with vascular tissues in galls induced by sucking-insects. AoB Plants 7:plv086. https://doi.org/10.1093/aobpla/plv086
Cartelat A, Cerovic ZG, Goulas Y et al (2005) Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crops Res 91:35–49. https://doi.org/10.1016/j.fcr.2004.05.002
Castro AC, Oliveira DC, Moreira ASFP, Lemos-Filho JP, Isaias RMS (2012) Source-sink relationship and photosynthesis in the horn-shaped gall and its host plant Copaifera langsdorffii Desf. (Fabaceae). S Afr J Bot 83:121–126. https://doi.org/10.1016/j.sajb.2012.08.007
Coelho MS, de Siqueira NF, Perillo LN, Morellato LPC, Fernandes GW (2018) Forest archipelagos: a natural model of metacommunity under the threat of fire. Flora 238:244–249. https://doi.org/10.1016/j.flora.2017.03.013
Craig TP, Price PW, Itami JK (1986) Resource regulation by a stem-galling sawfly on the arroyo willow. Ecology 67:419–425
Croft H, Chen JM, Luo X, Bartlett P, Chen B, Staebler RM (2017) Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob Chang Biol 23:3513–3524. https://doi.org/10.1111/gcb.13599
Czarnocka W, Karpiński S (2018) Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Rad Biol Med 122:4–20. https://doi.org/10.1016/j.freeradbiomed.2018.01.011
Da Silva CHTP, Sobrinho TJSP, Saraiva AM, Pisciottano MNC, de Amorim ELC (2012) Phytochemical profile and antibacterial activity of bark and leaves of Caesalpinia pyramidalis Tul. and Sapium glandulosum (L.) Morong. J Med Plant Res 6:4766–4771. https://doi.org/10.5897/JMPR12.830
De Santis L, Fernandes GW (1989) Brazilian parasitoids of gall forming insects: two new chalcidoid species and host records. Entomol News 100:29–36
Detoni ML, Vasconcelos EG, Maia ACRG, Gusmão MAN, Isaias RMS, Soares GLG, Santos JC, Fernandes GW (2011) Protein content and electrophoretic profile of insect galls on susceptible and resistant host plants of Bauhinia brevipes Vogel (Fabaceae). Aust J Botany 59:509–514. https://doi.org/10.1071/BT11104
Detoni ML, Faria-Pinto P, Quellis LR, Rust NM, Tavares LS, Santos MO, Isaias RMS, Santos JC, Fernandes GW, Soares GLG, Vasconcelos EG (2012) Galls from Calliandra brevipes Benth (Fabaceae: Mimosoidae): evidence of apyrase activity contribution in a plant – insect interaction. Aust J Botany 60:559–567. https://doi.org/10.1071/BT12096
Díaz RF (2020) Revisión taxonómica del género Sapium Jacq (Euphorbiaceae) en Honduras. Intropica. https://doi.org/10.21676/23897864.3542
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 15:573–581. https://doi.org/10.1016/j.tplants.2010.06.005
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Evans JR, Seeman JR (1989) The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences, and control. In: Briggs W, Alan R (eds) Towards a broad understanding of photosynthesis. Alan R. Liss, New York, pp 183–205
Favery B, Quentin M, Jaubert-Possamai S, Abad P (2016) Gall-forming root-knot nematodes hijack key plant cellular functions to induce multinucleate and hypertrophied feeding cells. J Insect Physiol 84:60–69. https://doi.org/10.1016/j.jinsphys.2015.07.013
Fay PA, Throop HL (2005) Branching responses in Silphium integrifolium (Asteraceae) following mechanical or gall damage to apical meristems and neighbor removal. Am J Bot 92:954–959. https://doi.org/10.3732/ajb.92.6.954
Fay PA, Hartnett DC, Knapp AK (1993) Increased photosynthesis and water potentials in Silphium integrifolium galled by cynipid wasps. Oecologia 93:114–120. https://doi.org/10.1007/BF00321200
Fernandes GW (1990) Hypersensitivity: a neglected plant resistance mechanism against insect herbivores. Environ Entomol 19:1173–1182. https://doi.org/10.1093/ee/19.5.1173
Fernandes GW, Price PW (1992) The adaptive significance of insect gall distribution: survivorship of species in xeric and mesic habitats. Oecologia 90:14–20. https://doi.org/10.1007/BF00317803
Fernandes GW (2016) Ecology and conservation of mountain top grasslands in Brazil. Springer International Publishing, Switzerland
Fernandes GW, Price PW (1988) Biogeographical gradients in galling species richness: tests of hypotheses. Oecologia 76:161–167
Fernandes GW, Price PW (1991) Comparisons of tropical and temperate gal1ing species richness: the roles of environmental harshness and plant nutrient status. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW (eds) Plant-animal interactions: evolutionary ecology in tropical and temperate regions. Wiley, New York, pp 91–115
Fernandes GW, Ribeiro SP (1990) Plant response to herbivory: two examples from the neotropics. Ecotrópicos 3:77–86
Fernandes GW, Tameirão Neto E, Martins RP (1988) Ocorrência e caracterização de galhas entomógenas na vegetação do Campus Pampulha da Universidade Federal de Minas Gerais. Rev Bras Zool 5:11–29
Fernandes GW, Price PW, Gonçalves-Alvim SJ, Craig TP, Yanega D (2000) Response of the galling insect Aciurina trixa Curran (Diptera: Tephritidae) to host plant quality. An Soc Entomol Bras 29:423–431. https://doi.org/10.1590/S0301-80592000000300005
Fernandes GW, Duarte H, Lüttge U (2003) Hypersensitivity of Fagus sylvatica L. against leaf galling insects. Trees (berl West) 17:407–411. https://doi.org/10.1007/s00468-003-0252-4
Fernandes GW, Aguirre-Jaimes A, Araújo-Oliveira L (2019) Induction, engineering, and hijacking of defensive strategies of the host by a gall-inducing weevil. Ecology 100:e02693. https://doi.org/10.1002/ecy.2693
Ferreira BG, Álvarez R, Bragança GP, Alvarenga DR, Hidalgo-Pérez IRMS (2019) Feeding and other gall facets: patterns and determinants in gall structure. Bot Rev 85:78–106. https://doi.org/10.1007/s12229-019-09207-w
Florentine SK, Raman A, Dhileepan K (2005) Effects of gall induction by Epiblema strenuana on gas exchange, nutrients, and energetics in Parthenium hysterophorus. Biocontrol 50:787–801. https://doi.org/10.1007/s10526-004-5525-3
Gagetti BL, Piratelli AJ, Piña-Rodrigues FCM (2016) Fruit color preference by birds and applications to ecological restoration. Braz J Biol 76:955–966. https://doi.org/10.1590/1519-6984.05115
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Giron D, Huguet E, Stone GN, Body M (2016) Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. J Insect Physiol 84:70–89. https://doi.org/10.1016/j.jinsphys.2015.12.009
Guedes LM, Torres S, Sáez-Carillo K, Becerra J, Pérez CI, Aguilera N (2022) High antioxidant activity of phenolic compounds dampens oxidative stress in Espinosa nothofagi galls induced on Nothofagus obliqua buds. Plant Sci 312:111114. https://doi.org/10.1016/j.plantsci.2021.111114
Hall CR, Carroll AR, Kitching RL (2017) A meta-analysis of the effects of galling insects on host plant secondary metabolites. Arthropod Plant Interact 11:463–473. https://doi.org/10.1007/s11829-016-9486-0
Harris MO, Pitzschke A (2020) Plants make galls to accommodate foreigners: some are friends, most are foes. New Phytol 225:1852–1872. https://doi.org/10.1111/nph.16340
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611. https://doi.org/10.1007/s004250050524
Hodkinson ID (1984) The biology and ecology of the gall-forming Psylloidea. In: Ananthakrishnan TN (ed) The biology of gall forming insects. Edward Arnold, London, pp 59–77
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Huang MY, Huang WD, Chou HM, Chen CC, Chang YT, Yang CM (2014) Herbivorous insects alter the chlorophyll metabolism of galls on host plants. J Asia-Pac Entomol 17:431–434. https://doi.org/10.1016/j.aspen.2014.04.004
Inbar M, Izhaki I, Koplovich A, Lupo I, Silanikove GT, Gerchaman Y, Perevolotsky A, Lev-Yadun S (2010) Why do many galls have conspicuous colors? a new hypothesis. Arthropod Plant Interact 4:1-6. https://doi.org/10.1007/s11829-009-9082-7
Julião GR, Venticinque EM, Fernandes GW, Price PW (2014) Unexpected high diversity of galling insects in the Amazonian upper canopy: the savanna out there. PLoS ONE 9:e114986. https://doi.org/10.1371/journal.pone.0114986
Jwa NS, Hwang BK (2017) Convergent evolution of pathogen effectors toward reactive oxygen species signaling networks in plants. Front Plant Sci 8:1687. https://doi.org/10.3389/fpls.2017.01687
Karageorgou P, Manetas Y (2006) The importance of being red when young: anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light. Tree Physiol 26:613–621. https://doi.org/10.1093/treephys/26.5.613
Kerchev PI, Fenton B, Hancock FCH, RD, (2012) Plant responses to insect herbivory: interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant Cell Environ 35:441–453. https://doi.org/10.1111/j.1365-3040.2011.02399.x
Kirst GO, Rapp H (1974) Zur Physiologie der Galle von Mikiola fagi Htg. auf Blättern von Fagus sylvatica L. 2. Transport 14C markierter Assimilate aus dem befallenen Blatt und aus Nachbar-blättern in die Galle. Biochem Physiol Pflanz 165:445–455
Kmieć K, Rubinowska K, Golan K (2018) Tetraneura ulmi (Hemiptera: Eriosomatinae) induces oxidative stress and alters antioxidant enzyme activities in elm leaves. Environ Entomol 47:840–847. https://doi.org/10.1093/ee/nvy055
Kurzfeld-Zexer L, Wool D, Inbar M (2010) Modification of tree architecture by a gall-forming aphid. Trees (berl West) 24:13–18. https://doi.org/10.1007/s00468-009-0374-4
Larson KC, Whitham TG (1991) Manipulation of food resources by a gall-forming aphid: the physiology of sink-source interactions. Oecologia 88:15–21. https://doi.org/10.1007/BF00328398
Lemus PL, Tricard J, Duclercq J et al (2020) Salivary proteins of Phloeomyzus passerinii, a plant-manipulating aphid, and their impact on early gene responses of susceptible and resistant poplar genotypes. Plant Sci 294:110468. https://doi.org/10.1016/j.plantsci.2020.110468
Li Y, Li H, Li Y (2017) Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought resistant wheat. Crop J 5:231–239. https://doi.org/10.1016/j.cj.2017.01.001
Lortie CJ, Aarssen LW (2000) A test of the reserve meristem hypothesis using Verbascum thapsus (Scrophulariaceae). Am J Bot 87:1789–1792. https://doi.org/10.2307/2656830
Madeira JA, Fernandes GW (1999) Reproductive phenology of sympatric taxa of Chamaecrista (Leguminosae) in Serra do Cipó, Brazil. J Trop Ecol 15:463–479. https://doi.org/10.1017/S0266467499000954
Manganaro A (1916) Apuntes cecidiológicos. En: Actas de Primera Reunión Nacional de la Sociedad Argentina de Ciencias Naturales, Tucumán, pp 291–302
Mani T, Raman A (1994) Biochemical changes in relation to growth in two leaf gall systems induced by Trioza jambolanae and Microceropsylla longispiculata (Homoptera: Psylloidea). Phytophaga 6:59–64
Martin WF, Sies H (2017) Physiological evolution: genomic redox footprints. Nat Plants 3:17071. https://doi.org/10.1038/nplants.2017.71
Martini V, Moreira ASFP, Kuster VC, Oliveira DC (2020) Photochemical performance and source-sink relationships in galls induced by Pseudophacopteron longicaudatum (Hemiptera) on leaves of Aspidosperma tomentosum (Apocynaceae). Photosynthetica 58: 827–835https://doi.org/10.32615/ps.2020.033
Maschinski J, Whitham G (1989) The continuum of plant responses to herbivory: the influence of plant association, nutrient availability and timing. Am Nat 134:1–19
Medrano H, Escalona JM, Bota J, Gulías J, Flexas J (2002) Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Ann Bot 89:895–905. https://doi.org/10.1093/aob/mcf079
Meyer J (1987) Plant galls and gall inducers. Gebrüder Borntraeger, Berlin
Meyer J, Maresquelle HJ (1983) Anatomie des galles. Gebrüder Borntraeger, Berlin
Miller DG, Raman A (2019) Host–plant relations of gall-inducing insects. Ann Entomol Soc Am 112:1–19. https://doi.org/10.1093/aesa/say034
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. https://doi.org/10.1016/S1360-1385(02)02312-9
Motta LB, Kraus JE, Salatino A, Salatino ML (2005) Distribution of metabolites in galled and non-galled foliar tissues of Tibouchina pulchra. Biochem Syst Ecol 33:971–981. https://doi.org/10.1016/j.bse.2005.02.004
Nabity PD, Zavala JA, De Lucia EH (2009) Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Ann Bot 103:655–663. https://doi.org/10.1093/aob/mcn127
Nakamura M, Miyamoto Y, Ohgushi T (2003) Gall initiation enhances the availability of food resources for herbivorous insects. Funct Ecol 17:851–857. https://doi.org/10.1111/j.1365-2435.2003.00786.x
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50:333–359. https://doi.org/10.1146/annurev.arplant.50.1.333
Nyman T, Julkunen-Tiitto R (2000) Manipulation of the phenolic chemistry of willows by gall-inducing sawflies. Proc Natl Sci USA 97:13184–13187. https://doi.org/10.1073/pnas.230294097
Oliveira DC, Isaias RMS (2010) Cytological and histochemical gradients induced by a sucking insect in galls of Aspidosperma australe Arg. Muell (Apocynaceae). Plant Sci 178:350–358. https://doi.org/10.1016/j.plantsci.2010.02.002
Oliveira DC, Christiano JDCS, Soares GLG, Isaias RMS (2006) Reações de defesas químicas e estruturais de Lonchocarpus muehlbergianus Hassl. (Fabaceae) à ação do galhador Euphalerus ostreoides Crawf. (Hemiptera: Psyllidae). Rev Bras Bot 29:657–667. https://doi.org/10.1590/S0100-84042006000400015
Oliveira DC, Isaias RMS, Fernandes GW, Ferreira BG, Carneiro RGS, Fuzaro L (2016) Manipulation of host plant cells and tissues by gall-inducing insects and adaptive strategies used by different feeding guilds. J Insect Physiol 84:103–113. https://doi.org/10.1016/j.jinsphys.2015.11.012
Oliveira DC, Moreira ASFP, Isaias RMS, Martini V, Rezende UC (2017) Sink status and photosynthetic rate of the leaflet galls induced by Bystracoccus mataybae (Eriococcidae) on Matayba guianensis (Sapindaceae). Front Plant Sci 8:1249. https://doi.org/10.3389/fpls.2017.01249
Orcutt DM, Nilsen ET (2000) The physiology of plants under stress: soil and biotic factors. John Wiley and Sons, New York
Patankar R, Thomas SC, Smith SM (2011) A gall-inducing arthropod drives declines in canopy tree photosynthesis. Oecologia 167:701–709. https://doi.org/10.1007/s00442-011-2019-8
Perea R, Dirzo R, Bieler S, Fernandes GW (2021) Incidence of galls on sympatric California oaks: ecological and physiological perspectives. Divers 13:20. https://doi.org/10.3390/d13010020
Pihain M, Gerhold P, Ducousso A, Prinzing A (2019) Evolutionary response to coexistence with close relatives: increased resistance against specialist herbivores without cost for climatic-stress resistance. Ecol Lett 22:1285–1296. https://doi.org/10.1111/ele.13285
Price PW (2005) Adaptive radiation of gall-inducing insects. Basic Appl Ecol 6:413–421. https://doi.org/10.1111/j.1558-5646.2007.00069.x
Price PW, Fernandes GW, Waring GL (1987) Adaptive nature of insect galls. Environ Entomol 16:15–24. https://doi.org/10.1093/ee/16.1.15
Price PW, Louw S (1996) Resource manipulation through architectural modification of the host plant by a gall-forming weevil Urodontus scholtzi Louw (Coleoptera: Anthribidae). Afr Entomol 4: 103–110. https://hdl.handle.net/10520/AJA10213589_193
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Raman A (1991) Cecidogenesis of leaf galls on Syzygium cumini (L.) Skeels (Myrtaceae) induced by Trioza jambolanae Crawford (Homoptera: Psylloidea). J Nat Hist 25:653–663. https://doi.org/10.1080/00222939100770421
Raman A (2003) Cecidogenetic behavior of some gall-inducing thrips, psyllids, coccids, and gall midges, and morphogenesis of their galls. Orient Insects 37:359–413. https://doi.org/10.1080/00305316.2003.10417356
Roach T, Krieger-Liszkay A (2019) Photosynthetic regulatory mechanisms for efficiency and prevention of photo-oxidative stress. Ann Plant Rev Online 2:273–306
Rohfritsch O (1992) Patterns in gall development. In: Shorthouse JD, Rohfritsch O (eds) Biology of insect induced galls. Oxford University Press, New York, pp 60–86
Espírito Santo MM, Neves F, Andrade-Neto FR, Fernandes GW (2007) Plant architecture and meristem dynamics as the mechanisms determining the diversity of gall-inducing insects. Oecologia 153:353–364. https://doi.org/10.1007/s00442-007-0737-8
Schultz JC, Edger PP, Body MJA, Happel HM (2019) A galling insect activates plant reproductive programs during gall development. Sci Rep 9:1833. https://doi.org/10.1038/s41598-018-38475-6
Scofield S, Murray JA (2006) KNOX gene function in plant stem cell niches. Plant Mol Biol 60:929–946. https://doi.org/10.1007/s11103-005-4478-y
Shorthouse JD, Wool D, Raman A (2005) Gall-inducing insects - Nature’s most sophisticated herbivores. Basic Appl Ecol 6:407–411. https://doi.org/10.1016/j.baae.2005.07.001
Sobottka AM, Tonial F, Sytwala S, Melzig M (2014) Proteinase activity in latex of three plants of the family Euphorbiaceae. Braz J Pharm Sci 50:559–565. https://doi.org/10.1590/S1984-82502014000300015
Solovchenko A, Schmitz-Eiberger M (2003) Significance of skin flavonoids for UVB protection in apple fruits. J Exp Bot 54:1977–1984. https://doi.org/10.1093/jxb/erg199
Thompson AJ, Andrews J, Mulholland BJ, McKee JMT, Hilton HW, Horridge JS, Farquhar GD, Smeeton RC, Smillie IRS, Black CR, Taylor IB (2007) Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol 143:1905–1917. https://doi.org/10.1104/pp.106.093559
Tooker JF, Helms AM (2014) Phytohormone dynamics associated with gall insects, and their potential role in the evolution of the gall-inducing habit. J Chem Ecol 40:742–753. https://doi.org/10.1007/s10886-014-0457-6
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59-66. https://doi.org/10.1016/S0168-9452(99)00197-1
Von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387. https://doi.org/10.1007/BF00384257
Weis AE, Walton R, Crego CL (1988) Reactive plant tissue sites and the population biology of gall makers. Annu Rev Entomol 33:467–486
White AC, Rogers A, Rees M, Osborne CP (2016) How can we make plants grow faster? A source–sink perspective on growth rate. J Exp Bot 67:31–45. https://doi.org/10.1093/jxb/erv447
Wiczkowski W, Szawara-Nowak D, Topolska J (2013) Red cabbage anthocyanins: profile, isolation, identification, and antioxidant activity. Food Res Int 51:303–309. https://doi.org/10.1016/j.foodres.2012.12.015
Wingler A, Roitsch T (2008) Metabolic regulation of leaf senescence: interactions of sugar signaling with biotic and abiotic stress responses. Plant Biol 10:50–62. https://doi.org/10.1111/j.1438-8677.2008.00086.x
Wuethrich B (2007) Biodiversity: reconstructing Brazil’s Atlantic rainforest. Science 315:1070–1072. https://doi.org/10.1126/science.315.5815.1070
Yang CM, Yang MM, Huang MY, Hsu JM, Jane WN (2003) Herbivorous insect causes deficiency of pigment–protein complexes in an oval-pointed cecidomyiid gall of Machilus thunbergii leaf. Bot Bull Acad Sin 44:314–321
Yoo CY, Pence HE, Hasegawa PM, Mickelbart MV (2009) Regulation of transpiration to improve crop water use. Crit Rev Plant Sci 28:410–431. https://doi.org/10.1080/07352680903173175
Zhang S, Weng J, Pan J, Tu T, Yao S, Xu C (2003) Study on the photogeneration of superoxide radicals in Photosystem II with EPR spin trapping techniques. Photosynth Res 75:41–48. https://doi.org/10.1023/A:1022439009587
Zonia L, Munnik T (2007) Life under pressure: hydrostatic pressure in cell growth and function. Trends Plant Sci 12:90–97. https://doi.org/10.1016/j.tplants.2007.01.006
Funding
We thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Reserva Vellozia, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for their support to this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by T. Koike.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
468_2022_2280_MOESM1_ESM.ppt
Supplementary file1 (PPT 83 KB) Fig. 1S. Relationship between the number of Neolithus fasciatus galls per leaf and leaf position (phyllotaxy) on Sapium glandulatum shoots (x̅ ± SE) (GLS and planned comparisons)
468_2022_2280_MOESM2_ESM.doc
Supplementary file2 (DOC 35 KB) Table 1S. Morphometric parameters compared among shoots of Sapium glandulatum with different number of galls. Different lowercase letters indicate a statistically significant difference between treatments (ANOVA and planned comparisons)
468_2022_2280_MOESM3_ESM.doc
Supplementary file3 (DOC 36 KB) Table 2S. Oxidative stress and nutritional content of galls and leaves (galled and ungalled) of Sapium glandulatum. Different lowercase letters indicate a statistically significant difference between treatments (GLS and planned comparisons)
Rights and permissions
About this article
Cite this article
Fernandes, G.W., Maia, R.A., Arantes-Garcia, L. et al. Deep capillary impact of a psyllid gall on its host ecophysiology, architecture and performance. Trees 36, 1193–1206 (2022). https://doi.org/10.1007/s00468-022-02280-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-022-02280-6