Abstract
Galls are neoformed structures induced by specific animals, fungi, bacteria, virus or some parasitic plants on their host plant organs. Developmental processes are well known in Agrobacterium tumefasciens galls, but the animal-induced galls have a striking anatomical diversity, concerning several patterns, which were reunited herein. Anatomical traits observed in animal-induced galls involve manipulation of plant morphogenesis in convergent ways. Nematode, mite and insect galls usually contain homogeneous storage parenchyma and develop due to hyperplasia and cell hypertrophy. The development of typical nutritive tissues, giant cells, or hypertrophied vascular bundles may occur. Some other anatomical features may be usually restricted to galls induced by specific taxa, but they may eventually be related to the developmental potentialities of the host plants. The combination of distinct morphogenetic peculiarities in each gall system culminates in extant gall structural diversity. Convergent anatomical traits are observed according to the feeding mode of the gall inducers, representing potentiation or inhibition of similar events of host plant morphogenesis and cell redifferentiation, independent of gall-inducing taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The galls or cecidia are neoformed structures on plants, induced by very specific inducers, such as some species of animals, fungi, bacteria, virus and some parasitic plants (Mani, 1964; Meyer & Maresquelle, 1983; Meyer, 1987). The galls are usually species-specific structures, and their anatomy and metabolism are strongly related to the gall inducer species and to the host plants (Stone & Schonrögge, 2003; Moura et al., 2008; Carneiro et al., 2015; Ferreira et al., 2017b, 2018). The structural rearrangements in galls make them interesting models for the study of plant developmental processes, their causes and variations. The galls induced by the bacteria Agrobacterium tumefasciens on several plants, including crops, were studied in all plant development levels, since gall histogenesis to gene expression. These galls are induced by plasmidial DNA transference, from the bacterial genome to the host plant cells, which will induce several changes in the developmental features of the infested organ (Tooker & Helms, 2014; Taiz et al., 2015). However, in the case of galls induced by animals, no gene transference was observed. Until now, only some signaling molecules, but not all the reactions, are known to cause the initial modifications in plant cell development (see details in “Gall induction mechanisms” subsection, in this review).
Animal-induced galls, the zoocecidia, may develop on all plant organs, under the stimuli of gall-inducing organisms (the cecidozoa) (Mani, 1964; Meyer & Maresquelle, 1983; Roskam, 1992; Stone & Schonrögge, 2003; Oliveira et al., 2016). Gall anatomical structure may protect the gall inducers against natural enemies and unfavorable environmental conditions (Price et al., 1987; Fernandes & Price, 1992; Stone & Schonrögge, 2003). Moreover, gall tissue organization has been usually related to the taxa of insects and their feeding habit (Larew, 1981; Meyer & Maresquelle, 1983; Bronner, 1992; Rohfritsch, 1992; Ferreira et al., 2017a). Even though nematode and mite galls also have their own patterns and histological peculiarities (Mani, 1964; Larew, 1981), a thorough comparison among galls induced by Nematoda, Acarina and Insecta has been rarely performed (Larew, 1981; Meyer & Maresquelle, 1983; Ferreira et al., 2017a), and few of their systems has been well-studied (Table 1).
The histological profiles of the zoocecidia may be simple, i.e., similar to those of their host organs, or complex, with several neoformed tissues or structures, which provide a better microenvironment for the development of the gall inducers (Wells, 1920; Mani, 1964; Larew, 1981; Meyer & Maresquelle 1983; Ferreira et al., 2017a). Regarding the histological profiles, galls may be classified as organoids or histioids. The organoid galls do not histologically differ from their host organs, but macroscopic alterations may be observed (Sinnott, 1960). They are generally induced by fungi, viruses or bacteria, and may be simply shortened branched shoots, or sometimes with leaves with reduced surface area (Meyer, 1987). Witches’ brooms and fasciations are typical organoid galls. Fasciations modify host plant meristems, and originate ribbon-like, elongated, or crisped structures instead of polygonal or rounded axis (White, 1948). The histioid galls comprise alterations in histological structure and are usually induced by animals (Mani, 1964). They are divided into kataplasmatic galls (without a constant external shape and size), and prosoplasmatic galls (with a typical, constant and definitive shape and size) (Küster, 1911; Wells, 1920; Mani, 1964). Herein, we considered the number of events of structural modification (Ferreira et al., 2017a) to evaluate the level of gall complexity. Tissue reorganizing events, such as: (a) parenchyma homogenization; (b) occurrence of hyperplasia; (c) occurrence of cell hypertrophy or hypotrophy; (d) increment in the density of trichomes/emergences; (e) redifferentiation of a nutritive tissue and/or a storage tissue; (f) redifferentiation of a mechanical layer; (g) hypertrophy of vascular bundles; and (h) neo-formation (or redifferentiation) of specialized structures are discussed in the following sections. Simple and complex zoocecidia induced by Nematoda, Acarina and Insecta will be comparatively categorized.
In gall structural studies, plant developmental machinery is supposed to be similarly stimulated by the feeding habit of distinct gall-inducing taxa. In this case, the colonial habit is responsible only for potentiating the cecidogenetic factors, and therefore gall histological pattern do not alter according to the colonial or solitary habits of the cecidozoa. Recently discovered novelties on Neotropical galls, which have shown more diverse histological and cytological patterns from those studied in the Temperate regions, guided current discussion. Reuniting a vast literature data from 20th and 21th centuries, we expect to demonstrate that galls are results of a combination of distinct morphogenetical potentialities of the host plants, which results in the extant gall structural diversity. Therefore, the host plants should also be “protagonists” in the determination of gall structure, as the gall-inducing organisms are.
Glossary
The main terms used in this review are defined herein in order to make the reading easier:
-
Cecidium (plural:cecidia): gall (plural: galls).
-
Cecidozoa: animals capable of inducing galls.
-
Chamber: the locule or internal space inside the gall, where the inducer and/or its offspring lives and feeds. In some articles, it may be referred as crypt or “cell”.
-
Coalescent galls: polythalamous or multilocular galls. A group of galls fused during the morphogenetic process, containing several gall chambers, usually with one gall inducer per chamber.
-
Colonial galls: galls with several gall inducers per chamber.
-
Common storage tissue (CST): an outer storage parenchyma or sclerenchyma with intense accumulation of starch and other metabolites, which are not directly involved in the inducer’s feeding, but are important energy source for growth and maintenance of gall tissues.
-
Emergence: an appendicular structure of an organ, formed by epidermal and subepidermal tissues (ground system tissues and sometimes vascular tissues).
-
Filzgall: a mite-induced gall formed exclusively by increased density of trichomes and emergences, and sometimes parenchyma homogenization.
-
Gall: a structure originated by the stimuli of specific species of animals, fungi, bacteria, virus, or parasitic plants, causing simple or complex alterations in plant histogenesis and/or morphogenesis.
-
Inquilines: insects that cohabit galls induced by other insects, feeding on the gall tissues, but uncapable of inducing their own galls. Some inquilines may induce tissue alterations (a new gall) in the gall they cohabit.
-
Monothalamous galls: galls with one single gall chamber.
-
Nutritive-like tissue (NLT): tissue surrounding gall chambers, usually of hemipteran (phloem-sucking insect) galls, which is not a feeding source for the inducers. Its cells have prominent nucleus, dense cytoplasm, rich in proteins, lipids, reducing sugars, and/or starch.
-
Polythalamous galls: galls with two or more gall chambers.
-
Solitary galls: galls containing one chamber with one gall inducer.
-
Typical nutritive tissue (TNT): tissue surrounding gall chambers, directly used for the inducer’s feeding. Nutritive cells have dense cytoplasm, prominent nucleus, commonly rich in proteins, lipids and/or reducing sugars.
-
Zoocecidia: galls induced by animals (mites, insects or nematodes).
Gall Types
Some Simple Galls May Be Called Pseudogalls
Phylogenetic studies suggested that the galling habit, at least in some groups, evolved from leaf rolling and leaf folding galls (Inbar et al., 1995; Crespi & Worobey, 1998; Nyman et al., 2000). Therefore, the simplest galls should be the leaf rolling/folding galls, which may be induced by mites, thrips, aphids, psylloids, lepidopterans, hymenopterans, and cecidomyiids (Raman & Ananthakrishnan, 1983; Inbar et al., 1995; Nyman et al., 2000; Souza et al., 2000; Arduin et al., 2005; Rancic et al., 2006; Álvarez et al., 2009, 2016; Magalhães et al., 2014; Portugal-Santana & Isaias, 2014; Bedetti et al., 2013). These galls may be slight modifications of host tissues, as the eriophyid galls on Cirsium arvense (Rancic et al., 2006), or may also be formed by hyperplasia, cell hypertrophy and/or parenchyma homogenization, as demonstrated for thrips-induced galls on several host plants (Raman & Ananthakrishnan, 1983; Gopinathan & Ananthakrishnan, 1985; Jorge et al., 2016), aphid galls induced by Eriosoma ulmi on Ulmus minor (Sano & Akimoto, 2011; Álvarez et al., 2013; Ferreira et al., 2017a), psyllid galls on Baccharis reticularia (Formiga et al., 2015), cecidomyiid galls induced by Dasineura affinis on Viola odorata (Meyer, 1987), and sawfly (hymenopteran) galls on Salix spp. (Nyman et al., 2000).
Some authors consider leaf rolling galls as pseudogalls (Wool, 2004), since their development depends on the feeding activity and death of epidermal cells, which causes host leaf deformation. However, several leaf rolling galls are considered true galls, for they result from hypertrophy of vascular bundles, parenchyma and epidermal cells, overdifferentiation of trichomes, hyperplasia and parenchyma homogenization (Mani, 1964; Álvarez et al., 2013; Formiga et al., 2015; Jorge et al., 2016; Ferreira et al., 2017a). More complex leaf folding galls may have mechanical tissues (sclerenchyma layer surrounding gall chamber), multiseriate epidermis, and nutritive-like tissues or common storage tissues, as observed for some aphid galls (Álvarez et al., 2009, 2016; Kurzfeld-Zexer et al., 2015).
The concept of galls as new organs may be applied to galls containing redifferentiated tissues with new specialized functions (Oliveira & Isaias, 2010a, 2010b; Ferreira & Isaias, 2013, 2014; Ferreira et al., 2015; Richardson et al., 2017). Due to the variety of tissue reorganization, it is controversial to consider the leaf rolling/folding galls as new organs. Generally, the concept of galls as new organs will depend on the occurrence of tissue neo-formation and cell redifferentiation, and also on altered physiological parameters. Independent of the galls being new organs or not, Mani (1992, p. 3–4) amplified the concept of galls to “a bewildering variety of plant structures and growth forms, ranging from the nearly normal plant organs to extreme bizarre and highly complex growth abnormalities”, recognizing all types of tissue manipulation as galls.
Colonial, Solitary and Coalescent Galls
Herein, we consider colonial galls as those sheltering several individuals of the gall-inducing species in a single gall chamber. They are commonly induced by nematodes and mites, and sometimes by insects (such as Thysanoptera, Hemiptera: Aphididae and Hemiptera: Adelgidae) (Table 1). Solitary galls are those galls, which have one gall-inducing individual per gall chamber, and are mostly induced by insects. Several Hymenoptera may induce galls by ovipositing in very close sites of the host organs (Table 2). In such cases, the coalescent galls share common tissues, but each larva is solitary and occupies an exclusive chamber (Mani, 1964; Meyer, 1987) (Table 1). Coalescent galls are usually product of the morphogenetic fusion among solitary galls. Each coalescent gall has two or more gall chambers, and they can also be called polythalamous, multichambered or multilocular galls. The advantages of coalescent galls should be the relative protection of some individuals from predation or parasitism, considering that the individuals occupying superficial chambers are more susceptible to the attack of parasitoids (Stone & Schonrögge, 2003), while those inside the inner chambers are more difficult to access. It is important to stress that monothalamous galls are those galls with one single chamber, independent of the number of inducers inside the chamber.
Influence of Induction Modes on Solitary, Coalescent and Colonial Habits
The induction habit of the gall-inducing species is determinant for the colonial or solitary habit (Table 2). When a female induces galls by its feeding activity, and oviposits or generates its offspring parthenogenetically, these galls are usually colonial, containing the mother and its offspring. When the feeding activity (chewing or sucking plant tissues) of the larva or the nympha is the stimuli for gall induction, galls are usually solitary. In a few groups, the oviposition is responsible for the first steps of gall induction, and galls may be solitary or coalescent. Independent on the solitary or colonial feature, gall induction usually occurs in meristematic tissues (Mani, 1964; Álvarez, 2011; Dias et al., 2013a; Ferreira & Isaias, 2014; Fleury et al., 2015), but there are some cases in which parenchymatic or epidermal tissues are the oviposition sites (Oliveira & Isaias, 2010b; Ferreira & Isaias, 2013). These cases demonstrate that most living plant cells are truly totipotent cells, being capable of redifferentiating into any other plant cell type (see Lev-Yadun, 2003).
Both colonial and solitary galling habits are strategies that have arisen independently among Arthropoda. Colonial insect galls are induced by Thysanoptera, and Hemiptera such as Aphididae, Phylloxeridae and Adelgidae. The Thysanoptera females induce galls by feeding stimuli prior to oviposition, and the nymphs may intensify plant tissue responses. Similarly, aphid and phylloxerid galls are induced by a female, the fundatrix, and its offspring maintain the galling stimuli (see below). The solitary galls arose independently in distinct taxa of insects, and usually have one gall-inducing individual per chamber (Table 1, Table 2). The galls with chewing and rasping-sucking larvae are predominantly solitary (Table 2). It is important to notice that colonial galls are usually induced by hemimetabolous species (such as Thysanoptera and Hemiptera) (Kjer et al., 2006), cases in which the adults and their offspring have the same feeding habits. The solitary galling habit is of higher occurrence in taxa of Endopterygota (the holometabolous, such as Hymenoptera, Coleoptera, Lepidoptera and Diptera), with chewing or rasping larvae as inducing individuals.
Among the hemimetabolous, some Hemiptera (Psylloidea and Coccoidea) may induce solitary galls, where the nymphs, and not the adult females, are responsible for gall induction. The distinct stages of an individual, i.e., larva, pupa and adult of the holometabolous organisms usually inhabit solitary galls, while for the hemimetabolous, distinct generations may cohabit a colonial gall. Among the holometabolous, the Diptera: Cecidomyiidae and Hymenoptera: Cynipidae induce the most diverse galls (Espírito-Santo & Fernandes, 2007), which are histologically and histochemically peculiar (Wells, 1920; Rohfritsch, 1992; Harris, 1994; Stone & Schonrögge, 2003; Ferreira & Isaias, 2014). Such diversity may be consequence of the capability of these solitary gall-inducing organisms to both manipulate plant tissues and tolerate chemical constraints imposed by the host plants (Amorim et al., 2017).
A very interesting type of gall induction is performed by inquilines over the tissues of other growing galls. Inquilines are herbivores, which feed in the gall tissues. Some species of inquilines evolved from an ancestral gall-inducing species, and some of them may induce tissue alterations in the galls they cohabit (Roskam, 1992; Yang et al., 2001). The occurrence of morphological alterations induced by inquilines of the superfamily Psylloidea was firstly described by Yang et al. (2001). In this case, the inquiline Pachypsylla cohabitans (Psylloidea) induces alterations in the galls induced by several other species of Pachypsylla on Celtis spp. (Ulmaceae). The galls induced by the “true” Pachypsylla gall-inducers may be solitary or colonial. However, the inquilines (P. cohabitans) can only induce galls over a gall, producing a new lateral gall chamber in the original Pachypsylla galls. These galls, therefore, may be considered coalescent galls, specially induced by distinct species. Similar cases commonly occur in several Cecidomyiidae (Roskam, 1992) and Cynipidae galls (Yang et al., 2001; Brooks & Shorthouse, 1998). Brooks and Shorthouse (1998) described the histological alterations caused by the inquiline Periclistus pirata (Cynipidae) on the galls induced by Diplolepis nodulosa (Cynipidae) on stems of Rosa blanda (Rosaceae). One or more P. pirata females oviposit several eggs into the chamber of the young solitary galls of D. nodulosa. Several histological modifications were described, resulting in the formation of a bigger coalescent gall, with several chambers, over the original solitary gall. This multilocular gall is result of the redifferentiation of storage parenchyma cells surrounding nutritive tissues (Brooks & Shorthouse, 1998). Therefore, in both cases cited above, the galls modified by the inquilines are products of a rearrangement of the previous morphogenetic development. Considering the concepts used here, we must consider these inquilines as gall inducers, although they can only induce galls over the tissues of specific galls.
Influence of the Number of Individuals in Gall Growth and Development
The solitary and colonial habit may influence the size of the gall and the rates of hypertrophy and hyperplasia, but the tissue arrangement tends to be similar. In mite galls, the number of inducers and gall size are positively correlated, even though additional tissue layers and larger cells are not observed (Moura et al., 2009b). In galls induced by several species of thrips on Acacia spp., the higher the number of generations inside a gall, the greater is the gall inner surface area related to gall volume (Crespi & Worobey, 1998). The histological patterns of galls induced by colonial (aphids) (Fig. 2a, Fig. 3b) and solitary phloem-sucking insects (psylloids) are similar (Fig. 1). The size of aphid-induced galls is also directly related to the number of individuals inside the gall chamber (Zha et al., 2016). In such case, the lateral growth seems to be potentiated by the action of a plate meristem-like tissue (see Evert, 2006), with predominant anticlinal cell divisions in gall walls. Coalescent galls may also have potentiation of cell growth directly related to number of individuals inside their structure, as observed by Arduin et al. (1989) for hymenopteran galls induced on Struthanthus vulgaris leaves, and by Brooks and Shorthouse (1998) for galls induced by Diplolepis nodulosa (Cynipidae) on Rosa blanda stems. These data suggest that the number of inducers does not affect gall histological patterns, but only gall size, which seems to be merely a local response to increased concentrations of growth signals. Therefore, colonial and coalescent galls should be structurally similar to the solitary galls induced by closely-related gall-inducing species with the same feeding habit, reaching greater dimensions than those of solitary galls.
Network of feeding modes, gall-inducing groups, and gall anatomical traits. In the gall-inducing groups, clear boxes (yellow) indicate groups with colonial galls and darker boxes (purple) indicate groups with solitary galls. Uninterrupted lines indicate more frequent anatomical traits, and dashed lines indicate less frequent traits. The more spaced are the dashed lines, less frequent is the indicated feature. In anatomical traits, clear boxes (green) are related to feeding tissues, the box surrounded by a light grey board indicate the anatomical traits common to galls induced by all groups of animals, and the other boxes are features occurring in galls of a restricted gall-inducing taxon
Multiple Evolution of Galling Taxa
Taxonomical Distribution of Gall Inducers
Insecta is the richest group of independently-evolved families with gall-inducing species (Mani, 1964; Meyer, 1987; Roskam, 1992) (Table 1). Considering that only the most representative clades of gall-inducing species have been listed in Table 1, the galling habit appeared several independent times in metazoan evolution – representing more than 52 insect families (133,000 estimated species) (Fernandes et al., 2012), 4 mite families (3600 described species) (Raman et al., 2005), and at least 4 nematode families (without estimations on the number of galling species) (Meyer, 1987). The galling habit evolved in parallel in several distinct families of insects, from ancestral guilds with distinct life habits (Roskam, 1992). The gall-inducing Cecidomyiidae seems to have evolved directly from detritivorous/mycetophagous ancestors, while other insect groups have evolved from: spores/pollen feeders (such as Thysanoptera: Phloeothripidae); plant sap feeders (as Hemiptera: Adelgidae, Eriosomatidae, Psyllidae, and Coccidae); foliage feeders (as Coleoptera: Curculionidae and Hymenoptera: Tenthredinidae); stem borers (as Hymenoptera: Cephidae); leaf miners (as Lepidoptera: Gelechiidae, and Diptera: Tephritidae and Agromyzidae); and from zoophage parasitoids (as Hymenoptera: Cynipidae) (Roskam, 1992).
Gall Induction Mechanisms
Nematoda Galls
The most common nematode galls are induced in roots, where the adult females (Nacobbus, Rotylenchulus, Tylenchulus) or second-stage juveniles (Globodera, Heterodera, Meloidogyne) usually penetrate into plant tissues (Krusberg, 1963; Dropkin, 1969; Meyer, 1987; Wyss, 1997). The nematodes perforate cell walls and introduce salivary secretions, which immediately induce cytoplasm aggregation around the stylets (Krusberg, 1963; Dropkin, 1969; Wyss, 1997). Some proteins trigger cytoplasm changings, and influence the genetic expression of plant cells (Favery et al., 2016), activating the differentiation of meristematic cells into nutritive cells, or the redifferentiation of parenchymatic cells into new cell types (Goodey, 1948; Bird, 1961; Ferreira et al., 2017b).
Usually, the first feeding sites are meristematic cells, which respond to the galling stimuli of nematodes. The feeding of the nematodes causes the surrounding cells and nuclei to become larger, and the cytoplasm to be denser (Krusberg, 1963; Dropkin, 1969). There is an increase in ribosomes, polyribosomes and cell organelles in cortical parenchyma (as in Xiphinema galls) (Weischer & Wyss, 1976; Wyss, 1997, 2002) or in xylem parenchyma cells (as in Meloidogyne galls) (Bird, 1961; Yousif, 1979; Finley, 1981; Wyss, 1997, 2002; Di Vito et al., 2004) due to the nematodes feeding activity (Wyss, 1997). In general, the feeding activity of the nematodes induces the redifferentiation of meristematic or parenchymatic cells, and specialized nutritive tissues develop. The nutritive cells in root nematode galls may be multinucleate giant cells, non-hypertrophied uninucleate nutritive cells, or syncytia, depending on the gall-inducing species (Dropkin, 1969; Meyer, 1987; Wyss, 1997). The multinucleate cells are usually formed by nuclear divisions without cytokinesis, while syncytia are formed by the fusion of cell protoplasts after partial wall dissolution (Wyss, 1997). In fact, considering the distinct types of induced nutritive cells, each nematode species should be responsible for activating specific cell cycle genes of the host plant (Favery et al., 2016). Besides the formation of nutritive cells or tissues, the root parenchymatic tissues undergo hyperplasia, forming cysts or root-knots (Wyss, 1997).
In aerial parts, galling Nematoda usually penetrate young leaves or stems, inducing the redifferentiation of meristematic cells, and necrotic regions are rarely observed (Goodey, 1939, 1948; Dropkin, 1969; Watson & Shorthouse, 1979; Skinner et al., 1980). In 2 or few days, a typical nutritive tissue may be observed surrounding the colonies (Goodey, 1939, 1948; Skinner et al., 1980). The nutritive cells may be hypertrophied or not, and may have one or more nuclei and nucleoli (Watson & Shorthouse, 1979; Skinner et al., 1980). Even though most galling nematodes penetrate plant meristems, there are some exceptions. Ditylenchus gallaeformans remains external to plant tissues, and induces galls by feeding on the meristematic cell surfaces of Miconia spp. aerial parts (Ferreira et al., 2017a, 2017b).
Acarina Galls
Eriophyidae galls are also induced by adult females (Table 2) (Meyer, 1987), which feed on young leaf surfaces. After 15-20 min, it is possible to observe a cone-shaped callose thickening surrounding the perforation region of the outer periclinal wall of the epidermal cell. In one-hour time, the first nutritive cells differentiate next to the perforation, with dispersion of chromatin, nucleoli enlargement, vacuome formation, and cytoplasm enrichment (Westphal et al., 1981). Plant tissue invagination may occur after some hours to two days of motionless position of the female (Oldfield, 2005). The mites may feed only on epidermis, inducing the differentiation of superficial nutritive cells. Meyer and Maresquelle (1983) considered the direct differentiation of epidermal cells into nutritive cells in these galls as metaplasia, since these cells proliferate before redifferentiation, and do not dedifferentiate. In other cases, the feeding activity may cause cell degradation in feeding sites, and the mites may gradually penetrate plant tissues forming a gall chamber. Nutritive cells redifferentiate from common parenchyma cells, around the feeding route of the mites, which seems to be the main stimuli for cell redifferentiation (Kendall, 1930; Westphal et al., 1981; Ferreira et al., 2017a). After a variable time of feeding, the females oviposit (the eggs are often produced by parthenogenesis), and quit the gall (Oldfield, 2005).
Insecta Galls
The real causes of the insect galls were firstly unveiled by Marcello Malpighi in the seventeenth century, with his book “De Gallis” published in 1679 (Fagan, 1918). The mechanisms of gall induction were actually experimented and discussed along the late 19th and 20th centuries, by studies applying mechanical stimuli, insects’ salivary mixtures, and insects’ entire extracts to plant tissues (Felt, 1936; Hough, 1953). However, the physiological mechanisms involved in the induction of insect galls remain obscure (Wool, 2004; Oliveira et al., 2016). Some experiments may stimulate hyperplasia in host plant tissues, but the growth and development is not complete as they are in galls naturally induced by the insects (Hough, 1953; McCalla et al., 1962; Mapes & Davies, 2001). Accordingly, the role of the mechanical wounding (by perforation or chewing), and mainly the insect’s saliva or egg’s secretions are essential for gall induction (Felt, 1936; Hough, 1953; Raman et al., 2005). Additionally, plant growth regulators may be found in the gall-inducing insect extracts, reinforcing the hypothesis of gall induction by chemical manipulation (Byers et al., 1976; Meyer & Maresquelle, 1983; Hori, 1992; Mapes & Davies, 2001). It is also assumed that the constant feeding of the inducing animal on gall tissues is essential for gall development, maturation, and dehiscence (Felt, 1936; Hough, 1953; Oldfield, 2005). Moreover, the initial stimuli are not necessarily related to the feeding activity, as some insect galls may be induced by oviposition injury and associated secretions (Felt, 1936; Hough, 1953; Raman et al., 2005).
Feeding Activity
The inseminated female and offspring of the thrips (Thysanoptera) induce galls only by sucking on individual leaf epidermal cells (Mound & Morris, 2005; Raman, 2012), which end up dying, causing leaf distortion. The surrounding mesophyll cells are responsible for repairing this damage, originating outgrowths into the tissues often causing leaf folding (Mound, 1994; Mound & Morris, 2005; Raman, 2012). The process of leaf folding in thrips-induced galls may also be consequence of changes in the elongation axis of epidermal cells in Myrcia retorta (Jorge et al., 2018a) or by the development of hypersensitive areas in epidermis and mesophyll as in Myrcia splendens (Jorge et al., 2018b).
Some gall-inducing species of Hemiptera induce either solitary or colonial galls. Aphididae and Phylloxeridae induce colonial galls, by the feeding activity of a single female, usually the fundatrix, which reproduces parthenogenetically (Wool, 2004). Some genera of Aphididae: Fordini (Forda, Smynthurodes) may induce two distinct galls on a single plant species: the fundatrix (F1) induces a temporary solitary gall; in a few weeks, the fundatrix offspring, the fundatrigeniae (F2) induce a definitive gall. The parthenogenetic fundatrigenia oviposits inside the chamber, and therefore the definitive galls are colonial (Wool, 2004; Álvarez et al., 2014; Kurzfeld-Zexer et al., 2015). Solitary galls of Psylloidea (Hemiptera) are induced by the feeding activity of first-instar nymphs (1–3 individuals), and covering or pouch galls develop (Burckhardt, 2005), as is true for Nothotrioza spp. (Triozidae) on leaves of Psidium spp. (Myrtaceae) (Carneiro et al., 2014b; Carneiro & Isaias, 2015). Nevertheless, some species such as Baccharopelma dracunculifoliae (Psyllidae) nymphs may induce galls with one to 21 individuals inside the chamber. The sucking activity of B. dracunculifoliae nymphs is the stimulus responsible for the hyperplasia and cell hypertrophy, which culminate in the entire folding of Baccharis dracunculifolia (Asteraceae) leaves (Arduin et al., 2005). Bud galls may be induced by Psylloidea members, and, more rarely, stem, flower, rosette and root galls, as exemplified by Hodkinson (1984). Even though the similar feeding activity represents an important induction stimulus, the growth of these galls follows patterns other than those involved in pouch and leaf covering galls.
In Coccoidea (Hemiptera), the first nymphs (crawlers) were known to induce solitary galls by sucking on phloem or on parenchymatic tissues (Meyer, 1987). In the Neotropics, second-instar Eriococcidae nymphae are responsible for gall induction in three systems: Pseudotectococcus rolliniae – Rollinia laurifolia (Annonaceae), Eriogalococcus isaias – Pseudobombax grandiflorum (Malvaceae), and Bystracoccus mataybae – Matayba guianensis (Sapindaceae). The first-instar nymphs of P. rolliniae induce dormancy stem galls on R. laurifolia, and sexually dimorphic leaf galls are induced by the second-instar nymphs (Gonçalves et al., 2005, 2009). In the other two studied systems, the fist-instar nymphae use the bark of M. guianensis and P. grandiflorum as the sites for dormancy (diapause), but no tissue alteration is observed. After molting, they move to leaves and induce dimorphic galls, i.e., the female-induced galls are distinct from the male-induced galls (Hodgson et al., 2013; Magalhães et al., 2015).
In the case of Cecidomyiidae (Diptera), the main gall-inducing factor is cell disruption caused by the larva, in most cases using the prothoracic spatula for puncturing superficial cells (Rohfritsch, 1992; Harris, 1994). The released cellular fluids are extracorporeally digested and then sucked by the larvae. Probably the cell disruption and salivary fluids triggers the development of a pouch, covering growth, or distinct growth of buds (Rohfritsch, 1992; Harris, 1994; Ferreira & Isaias, 2014; Fleury et al., 2015). The larva may induce galls on the epidermal surface (and metaplasia may occur) or may penetrate into plant tissues, depending on the oviposition site and its behavior (Meyer, 1987).
The Lepidoptera induce galls through the voracious feeding of their larva, leading to the redifferentiation of typical nutritive cells. The oviposition occurs on superficial tissues, and when the eggs hatch (Meyer, 1987; Miller, 2005), the larvae chew plant cells, and enter the host plant organ. The nutritive cells accumulate lipids and are capable of self-regeneration (Meyer, 1987; Ferreira & Isaias, 2013; Vecchi et al., 2013; Ferreira et al., 2015). Even though Hymenoptera and Coleoptera also have chewing mouthparts, and their feeding activity is important to maintenance of gall development, their galls are initially induced by oviposition, and therefore they will be discussed below.
Oviposition
The oviposition is an important induction factor in Hymenoptera and Coleoptera galls (Table 2). Cynipidae (Hymenoptera) oviposition into plant tissues stimulates cell lysis and the formation of a gall chamber (Meyer, 1987; Brooks & Shorthouse, 1998; Csóka et al., 2005). The cells surrounding the oviposition site go through metaplasic reaction, and the cytoplasm becomes granulose. Gall development proceeds by cell hypertrophy and hyperplasia of surrounding tissues. Several Cynipidae galls, however, are induced only after the eclosion of the larvae (McCalla et al., 1962), which feeds, and induces the redifferentiation of additional nutritive cells in the outer layers (Meyer, 1987; Bronner, 1992; Ferreira et al., 2017a).
The oviposition is also the determinant factor for tissue modification in Coleoptera galls (Korotyaev et al., 2005). The adult females of Curculionidae excavate the host stems, oviposit and immediately leave plant tissues. Controlled experiments have shown that the females digging without oviposition is followed by fast reintegration of stem tissues. Accordingly, the oviposition fluids and the egg secretions were essential for the formation of hyperplasic and nutritive tissues in these galls (McCalla et al., 1962; Barnewall & De Clerck-Floate, 2012). However, the complete development of Coleoptera- and Hymenoptera-induced galls requires larval feeding by chewing the typical nutritive cells (Meyer, 1987; Rohfritsch, 1992; Barnewall & De Clerck-Floate, 2012).
Histological and Histochemical Convergences in Galls
During gall development, the more morphogenetic changes there are, the more complex galls are (Formiga et al., 2015; Ferreira et al., 2017a). Therefore, histochemical, histological, and histometric evaluations must be considered in order to determine the comparative complexity levels of galls (Ferreira et al., 2017a), considering distinct types of developmental alterations (Table 3).
Comparing galls induced by mites, nematodes and insects, not only the feeding habit, but also the gall-inducing taxa, determine patterns in gall development. For example, nematodes and mites have similar feeding habits, and induce galls with several cytohistological distinctions, mainly their nutritive tissue types (Fig. 1). Mite-induced galls are usually simple, and a typical nutritive tissue is not always essential, while nematode-induced galls may have different levels of complexity. Nematode galls may have diverse nutritive tissues, whose patterns are related to the inducing species. Sucking animals, as eriophyids, nematodes, hemipterans, and thrips induce distinct galls, according to their feeding sites on plant organs (Fig. 2a-e, Fig. 3a-b). Phloem-sucking insects, with long stylets, induce phloem hypertrophy (Figs. 2a and 3b), while superficial sucking animals, as thrips, mites, nematodes, and rasping-sucking and chewing insects may induce galls with typical nutritive tissues around the larval chamber (Figs. 2c–e and 3a). Usually, the gall-inducing individuals induce the growth and enrichment of the cells,which are sources of their feeding. Simple galls of distinct groups result at least from one of these three basic processes: parenchyma homogenization, cell hypertrophy or hyperplasia (Fig. 1). Complex galls result from these processes and additional ontogenetic alterations (Table 3).
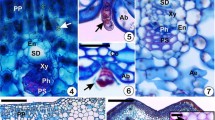
Schematic drawings of cross-sectioned galls induced by insects, mites and nematodes. a Leaf-rolling gall induced by Eriosoma ulmi (Hemiptera: Aphididae) on Ulmus minor (Ulmaceae). Hypertrophied vascular bundles (arrows) in hypertrophic regions of this gall. bFilzgall induced by an unidentified Eriophyidae on leaves of Miconia ibaguensis (Melastomataceae). The overdifferentiated indumentum is formed by emergences. c Leaf gall induced by Ditylenchus gallaeformans (Nematoda) on Miconia albicans (Melastomataceae). The trichomes are longer and denser when covering galls, and occur also in adaxial surface of epidermis, which does not occur in non-galled portions of leaves. d Fusiform stem gall induced by unidentified Lepidoptera on Marcetia taxifolia (Melastomataceae). e Lenticular leaf gall induced by Anguina balsamophila (Nematoda) on Wyethia amplexicaulis (Asteraceae). Abbreviations: CST, common storage tissue; Em: emergence; GC: gall chamber; HT: healing tissue; NGP, non-galled portion of leaf; Ph, phloem; TNT, typical nutritive tissue; Tr: trichomes; Xy, xylem. Scale bars: (a), (c), (d) 500 μm; (b) 200 μm; (e) 3 mm
Schematic drawings of galls showing histological patterns of galls and of a typical nutritive tissue. a Cross-sectioned lenticular gall induced by Aceria tristriata (Eriophyidae) on Juglans regia (Juglandaceae). b Globoid gall induced by Tetraneura ulmi (Aphididae) on Ulmus minor (Ulmaceae), longitudinal section. c Detail of nutritive cells of fusiform galls induced on the midribs of Eucalyptus camaldulensis (Myrtaceae) leaves by Leptocybe invasa (Hymenoptera: Eulophidae). The typical nutritive tissue shows a gradient of density of the cytoplasm (shown by darker shades of purple) towards the gall chamber; and smaller vacuoles (white) near gall chamber. Typical nutritive cells in contact with the inducer have more prominent nuclei and nucleoli. More information about anatomy and cytology of these galls may be encountered in the article of Isaias et al. (2018). Abbreviations: CST, common storage tissue; Cy: cytoplasm; GC, gall chamber; NGP, non-galled portion of leaf; NLT, nutritive-like tissue; Nu: nucleus; Ph: phloem; TNT, typical nutritive tissue; Va: vacuole; Xy: xylem. Scale bars: (a) 200 μm; (b) 500 μm; (c) 20 μm
Hyperplasia
The increasing cell divisions, hyperplasia, is observed in almost all galls (Fig. 1), and must be diagnosed by the increment of cell layers or number of cells per tissue area (Goodey, 1948; Mani, 1964; Meyer, 1987; Moura et al., 2008; Ferreira & Isaias, 2013; Ferreira et al., 2017a). In some cases, the intumescence of host organs results only from parenchyma hyperplasia (Goodey, 1948; Ferreira et al., 2017a).
Hyperplasia occurs mainly in the beginning of gall development, being persistent in most galls until the end of gall growth and development phase (Rohfritsch, 1992; Arduin & Kraus, 1995). The increment of cell layers occurs mainly before cell hypertrophy and differentiation, and contributes to the final shape of the galls, depending on its topographical concentration. The intumescence of a stem gall is due to the hyperplasia in the cortical cells, where periclinal divisions occur during growth and development phase (Goodey, 1948; Ferreira & Isaias, 2013). Similarly, the formation of pouch galls (Moura et al., 2009b; Oliveira & Isaias, 2010a; Álvarez, 2011; Dias et al., 2013a; Carneiro & Isaias, 2015), leaf rolling and folding galls (Souza et al., 2000; Arduin et al., 2005; Álvarez et al., 2009; Ferreira et al., 2017b) occur by unequal hyperplasia and hypertrophy rates between the adaxial and abaxial ground meristems. In the formation of a covering gall (Arduin & Kraus, 1995), periclinal and oblique divisions give rise to emergences, which cover the gall-inducing egg, larva or nymph. In some galls, the hyperplasia rates are high until the end of the maturation phase, due to the constant feeding activity of the larva (Ferreira & Isaias, 2013).
Cell Hypertrophy
Cell hypertrophy may occur either in outer or inner gall tissue compartments (sensu Bragança et al., 2017). In the most complex galls induced by nematodes, cell hypertrophy is limited to the inner compartment, with redifferentiation of giant nutritive cells (Bird, 1961; Watson & Shorthouse, 1979). Such cells are polynucleated, which may explain their great hypertrophy rates, when compared to other galls (Jones & Northcote, 1972; Rodiuc et al., 2014). In mite-induced galls, the hypertrophy may occur in the outer parenchyma cells, even though it is not a strict pattern (Moura et al., 2009b; Ferreira et al., 2017a). Nevertheless, insect-induced galls usually have outer tissue compartments with a large rate of cell hypertrophy (Kostoff & Kendall, 1929; Mani, 1964; Kraus et al., 2002; Álvarez et al., 2009; Isaias et al., 2011; Ferreira & Isaias, 2013, 2014; Suzuki et al., 2015), which develop from the end of growth and development toward the maturation phase. Outer parenchyma cells are usually vacuolated and store starch, being named the ‘common storage tissue’ (see next subsection), for they are not directly involved in the nutrition of the galling insect (Oliveira et al., 2011; Bragança et al., 2017; Ferreira et al., 2017a). Outer parenchyma cells may also accumulate secondary metabolites, being related to UV protection, defense against natural enemies, or to conferring distinct colors to the galls (Dias et al., 2013b; Bragança et al., 2017; Ferreira et al., 2017c). Phenolics and auxins have been co-localized in these tissue compartments, which have been associated with cell hypertrophy (Hori, 1992; Bedetti et al., 2014; Suzuki et al., 2015).
Hypertrophy of Internal Glands
Internal glands are common on some host plant families and the activity of the gall inducing agents causes their hypertrophy. Hypertrophied internal glands immersed on phloem commonly occur in galls induced by phloem-sucking hemipterans on Anacardiaceae (Álvarez, 2011; Dias et al., 2013a; Álvarez et al., 2014, 2016; Muñoz-Viveros et al., 2014). In this case, the hypertrophy of the internal glands accompanies the hypertrophy of the phloem, the primary feeding source of the inducing aphids, and therefore the responsive tissue in their associated galls. Other phloem-sucking insects induce the hypertrophy of secretory cavities not associated to vascular bundles in host leaves (Arduin et al., 2005). The hypertrophy of secretory structures seems to accompany the common processes of hypertrophy and hyperplasia of the outer parenchyma cells (Arduin et al., 2005; Magalhães et al., 2014), which is not necessarily associated to the direct stimuli of the feeding activity. Instead, it may be an indirect response to hormonal (e.g., auxins and cytokinins) translocation and synergism, and consequent alteration of cell expansion and proliferation.
Parenchyma Homogenization
Independent on gall ontogenesis, the occurrence of parenchyma homogenization in galls induced by nematodes, mites, and insects is common (Fig. 1). It is observed in simple leaf rolling galls (Álvarez et al., 2013; Ferreira et al., 2017a), filzgalls (Ferreira et al., 2017a), leaf folding galls (Souza et al., 2000; Álvarez et al., 2009; Bedetti et al., 2013; Magalhães et al., 2014; Formiga et al., 2015), pouch galls (Moura et al., 2009b; Carneiro et al., 2014b; Carneiro & Isaias, 2015), covering galls (Ferreira et al., 2017a), and stem/root swelling galls (Kraus & Tanoe, 1999; Ferreira & Isaias, 2013). Parenchyma homogenization may be related to some developmental factors: (1) the overwhelming of gall-inducing stimuli upon the environmental and endogenous signals for cell elongation in the host plant organ; (2) the maintenance of some meristematic sites in gall tissues; and (3) the combination of high hypertrophic and hyperplasic rates all over gall structure. These developmental factors isolated or grouped are commonly diagnosed in gall developmental sites.
Regarding gall development, some histological features reflect cytological aspects, such as the rearrangement of cellulose microfibrils, which favors the homogenization of gall tissues, at least in the beginning of gall development (Magalhães et al., 2014; Suzuki et al., 2015). Homogeneous parenchyma may persist until gall maturity and senescence (Moura et al., 2008, 2009b; Isaias et al., 2011; Ferreira & Isaias, 2014; Ferreira et al., 2017a), or change during the final phases of growth and development depending on the final gall morphotype (Ferreira & Isaias, 2013; Magalhães et al., 2014; Suzuki et al., 2015).
Changes in Indumentum Density
A dense indumentum is usually related to the increment of a boundary layer in plants, which ends up decreasing the transpiration rates and reflecting the excessive light, as well as increasing the defenses against herbivores (Schreuder et al., 2001; Carmona et al., 2011). The increment of indumentum may occur in several gall systems (Ferreira et al., 2017a), and is usually related to the protection of the gall-inducing agent against abiotic stresses and natural enemies, even though these effects must be tested (Stone & Schonrögge, 2003).
The overdifferentiation of trichomes or emergences (Fig. 2b) may be the unique process involved in the formation of filzgalls (Fig. 1), which are considered the simplest mite galls for they are characterized by the formation of dense indumentum and no or few other modifications (Mani, 1964). These galls are open, and the trichomes or emergences are usually the feeding sites of eriophyids (Mani, 1964; Ferreira et al., 2017a). The cells of these emergences or hypertrophied trichomes usually have scanty protoplasm (Mani, 1964), and no other cytological features of typical nutritive cells, such as granulose cytoplasm and hypertrophied nucleus, occur. In filzgalls induced on Miconia ibaguensis, the cells of the emergences accumulate reducing sugars (Ferreira et al., 2017a).
Feeding and Storage Tissues
There are three types of storage tissues in galls: common storage tissues, typical nutritive tissues, and nutritive-like tissues (sensu Ferreira et al., 2017a), which are herein grouped as storage tissues lato sensu.
Important developmental alterations in histological profiles of complex galls usually involve the increment and enrichment of the gall-inducer feeding tissues (Ferreira et al., 2017a). Most complex zoocecidia present differentiation of specialized nutritive or reserve cells (Fig. 1). Typical nutritive tissues of complex nematode, mite and insect galls have cells with conspicuous nucleus, dense or granulose cytoplasm, which store proteins, reducing sugars and/or lipids, and are sources for the nutrition of the inducers (Kendall, 1930; Bird, 1961; Dropkin, 1969; Westphal et al., 1981; Brooks & Shorthouse, 1998; Ferreira et al., 2015, 2017a, 2017b; Bragança et al., 2017). These cells are in direct contact with the inducers (Fig. 2c-e, Fig. 3a), and their redifferentiation may involve the process of metaplasia (Meyer & Maresquelle, 1983; Meyer, 1987), when the cells redifferentiate into cells with higher metabolic and cytological activities, without previous dedifferentiation and proliferation. The cytoplasm granulation (Fig. 3c), observed in these nutritive cells, may be consequence of an accumulation of ribosomes and enzymes, increment of Golgi apparatus and endoplasmic reticulum, and in the number of mitochondria, as observed in some Neotropical galls (Oliveira et al., 2011; Vecchi et al., 2013; Ferreira et al., 2015). A nutritive cell may have one or several nuclei, depending on the gall-inducing or host plant species (Mani, 1964). In nematode galls, independent on the host plant or organ, the nutritive tissues may have promeristematic and totipotent cells or giant polynucleated cells (Fig. 1). Polynucleated nutritive cells are usually very hypertrophied, as those giant cells of root-knot nematode galls (Bird, 1961; Jones & Northcote, 1972; Rodiuc et al., 2014). Cyst nematode galls have syncytial nutritive tissues, formed by the induced digestion of parenchyma cell walls (Rodiuc et al., 2014). Promeristematic nutritive tissues, i.e., with totipotent cells, are observed in galls induced by the nematode Ditylenchus gallaeformans on Miconia spp. (Fig. 2c) (Ferreira et al., 2017a, 2017b). Distinctly from most cases, the nucleus may be absent in nutritive cells of some galls, a feature reported for the lepidopteran galls on Tibouchina pulchra (Vecchi et al., 2013), and for the cecidomyiid horn-shaped galls on Copaifera langsdorfii (Fabaceae) (Oliveira et al., 2011). These cells are supposedly controlled by adjacent cells, similarly to the role of companion cells over phloem sieve elements.
But how have the nutritive cells been stimulated to granulate? Nutritive cells with their conspicuous nuclei, accumulation of high-quality reserves (Fig. 3c), and few unpalatable secondary metabolites, are similar to meristematic cells, such as the cambium initials (Vecchi et al., 2013), or the cells in axillary buds (Ferreira et al., 2017b). Vascular cambium cells may store starch and lipids, and respond to several plant signals to begin or to cease cell divisions for a while (Evert, 2006; Begum et al., 2010). Maybe the gall inducers’ stimuli are responsible for the maintenance of the meristematic features of these cells, representing a convergence among galls induced by phylogenetically distant organisms. Distinct taxa of galling organisms may induce the differentiation of granulose cells, even though their galls may be histologically very distinct. Contrary to the groups cited above, the phloem-sucking insects (as Psylloidea, Coccoidea and Aphididae) induce galls with hypertrophied phloem bundles (Fig. 3b). These galls do not have typical nutritive tissues (Larew, 1981; Bronner, 1992; Ferreira et al., 2017a), reflecting the direct influence of the feeding habit on the differentiation of nutritive cells. The increment in area of phloem in phloem-sucking galls should reflect the increment of sieve elements, companion cells, and/or transfer cells, which are nutrient-rich cells, and therefore are comparable to nutritive tissues in the galls induced by other zoocecidia.
As the inner tissue compartments of galls are usually related to the nutrition of the gall-inducing agents (Bragança et al., 2017), secondary metabolites may be suppressed or at least less accumulated in such gall tissues. For instance, an impairment of accumulation of terpenes and of phenolics occurs in the nutritive tissues of the Cecidomyiidae galls on Piper arboreum (Bragança et al., 2017), and of Lepidopteran galls on Marcetia taxifolia (Ferreira & Isaias, 2013), as well as in the inner storage tissues of Psylloidea galls on Psidium spp. (Carneiro et al., 2014a).
In general, “common storage tissues” (Fig. 1, Fig. 2c-e, Fig. 3a), with vacuolated starch-rich cells, and peripheral cytoplasm (Ferreira et al., 2017a) largely occur in galls induced by distinct taxa (Brooks & Shorthouse, 1998; Álvarez et al., 2009, 2013; Oliveira & Isaias, 2010a; Isaias et al., 2011; Carneiro & Isaias, 2014, 2015; Ferreira et al., 2017a; Richardson et al., 2017). When the storage cells are cytologically similar to the typical nutritive cells of galls, but are not directly involved in the inducer’s feeding, they are called “nutritive-like tissues” (Ferreira et al., 2017a; Richardson et al., 2017). The reserves accumulated in these cells are probably used for the energetic maintenance of gall structure (Oliveira & Isaias, 2010a). Some exceptions of this general pattern may occur, for example in galls induced by Pachypsylla spp., a piercing-sucking Psyllidae, on Celtis occidentalis. This insect feeds directly on nutritive polynucleated cells developed by the dissolution of cell walls, and not in phloem cells, as usually occur in hemipteran galls (Meyer, 1987).
Ambrosia Galls
Some Cecidomyiidae galls do not have a typical nutritive tissue (Arduin & Kraus, 2001; Dorchin et al., 2002; Rohfritsch, 2008; Sá et al., 2009), instead, their larva feed on a mycelium inoculated in plant tissues by oviposition time (Arduin & Kraus, 2001; Stone & Schonrögge, 2003; Rohfritsch, 2008; Sá et al., 2009; Chao & Liao, 2013). The nutritive resources are provided by the hyphae, which are intimately associated with the surrounding plant cells (Fig. 1), forming a nutritive ‘pseudoparenchyma’ (Rohfritsch, 2008). Similar to the ectomycorrhizae associated to some plant roots, the hyphae do not penetrate the cells at gall developmental site, but surround cell walls, increasing the absorption surface (Nylund & Unestam, 1982; Münzenberger et al., 2012). In ambrosia galls, pseudoparenchyma plant cells with dense cytoplasm may be observed adjacent to the inoculated mycelium (Meyer, 1987; Arduin & Kraus, 2001; Chao & Liao, 2013). Similar to the ectomycorrhizae, these enriched plant cells may provide water and nutrients to the fungi, which are consumed by the gall-inducing cecidomyiid. Similarities between the ectomycorrhizae and the fungi of ambrosia galls need further studies toward elucidating the physiological and ecological functions of the fungi in the associated gall systems.
The habit of inducing ambrosia galls is considered plesiomorphic in gall-inducing Cecidomyiidae, and the derived clades of gall inducers specialize in herbivory (Roskam, 1992; Arduin & Kraus, 2001; Rohfritsch, 2008). The complexity of ambrosia galls may vary according to their associated inducing species, and they may be histologically simple and parenchymatic, or may be complex structures consisting of a mechanical layer and outer neoformed tissues (Chao & Liao, 2013).
Outer Tissue Compartments
In general, the galls may be divided into outer and inner tissue compartments (Bragança et al., 2017). The inner compartments of several galls comprise the typical nutritive tissues, while the outer compartment is of general occurrence, and may constitute or not a common storage tissue (Figs. 1, 2 and 3).
The outer compartment usually accumulates secondary metabolites, such as phenolic derivatives (hydrolysable tannins, flavonoids, anthocyanins), alkaloids, and terpenoids (Ferreira & Isaias, 2013; Carneiro et al., 2014a; Bragança et al., 2017). These secondary metabolites are supposed to function as protective barriers against excessive sun radiation and UV light (Dias et al., 2013a, 2013b; Bragança et al., 2017), as well as against the natural enemies of the galling organism (Stone & Schonrögge, 2003; Bragança et al., 2017). Another possibility is the attractiveness of the gall inducer. The type of monoterpenes and sesquiterpenes accumulated in galls, such as those induced on Lantana camara (Verbenaceae), for instance, determines the choice of the galling Aceria lantanae (Eriophyidae) for plants with red flowers instead of plants with white or rose flowers (Moura et al., 2008, 2009a, 2009b). The volatile compounds are important for the recognition of host plants and for gall induction. Once in contact with host plant tissues, the differential content of terpenes of the host plants requires an adaptive development of the inducers, which must be able to detoxify for their successful gall induction and establishment.
The cells of the gall outer compartments may have more rigid walls than those of the inner compartments due to their distinct lignin and pectin composition (Formiga et al., 2013; Oliveira et al., 2014; Carneiro et al., 2015). As the cells of the gall outer tissues are usually hypertrophied, it is also possible that the pectin composition influences water uptake to these cells. The rigidity of gall walls may also be guaranteed by cell wall lignification of sclereids redifferentiated from parenchyma cells (Álvarez et al., 2009, 2016; Carneiro et al., 2014a, 2014b, 2015; Kurzfeld-Zexer et al., 2015). The sclerenchymatic cells may also provide mechanical protection for the gall inducers against natural enemies in maturation phase (Stone & Schonrögge, 2003; Carneiro et al., 2014b).
A sclerenchyma layer with sclereids or fibers may occur between the typical nutritive tissue and the outer tissue compartments in insect galls (Fig. 1), mainly Diptera and Hymenoptera galls (Arduin et al., 2005; Oliveira et al., 2011, 2016; Castro et al., 2012; Fleury et al., 2015; Amorim et al., 2017). The differentiation of sclerenchymatic cells may rely on the morphogenetic pattern of the host plant (Ferreira & Isaias, 2014). Mechanical layers may differentiate from the vascular cambium or pericyclic cells, similarly to the non-galled host organs (Fig. 2d) (Bedetti et al., 2013; Ferreira & Isaias, 2013). The differentiation of sclerenchyma layers surrounding the gall nutritive or nutritive-like tissues seems to be consequence of the accumulation of reactive oxygen species (ROS) due to the feeding activity of the gall inducers (Isaias et al., 2015; Oliveira et al., 2016). The ROS are captured by cinnamyl alcohols (precursors of lignins) and immobilized when deposited in cell walls (Barceló, 1997; Apel & Hirt, 2004). Outside the sclerenchymatic cells, specialized types of parenchyma, as an aerenchyma, for instance, may differentiate in the gall outer tissue compartment, and favor gas exchanges among the cells in galls deprived of stomata (Amorim et al., 2017). Collenchyma cell layers may also differentiate as a conservative feature of the host organs (Amorim et al., 2017), or as a neoformed feature (Goodey, 1948).
Changes in Dermal System
Some galls outer epidermal pavement cells may be suberized (Kraus et al., 2002) or metacutinized (Oliveira & Isaias, 2010b; Formiga et al., 2011), protecting the gall from desiccation (Isaias et al., 2013). Moreover, distinct epidermal appendages, such as trichomes, may be related to mechanical protection against natural enemies and stabilization of the microclimate inside the gall (Moura et al., 2008; Dias et al., 2013a). Stomata can also be deformed or suppressed in galls induced by nematodes, mites and insects (Goodey, 1939; Moura et al., 2008; Álvarez et al., 2009, 2016; Dias et al., 2013a; Amorim et al., 2017), which affect gas exchanges in gall outer tissue compartments. Inner epidermal cells, limiting the chamber, in phloem-sucking insect galls may have reduced cell surface area and thin cuticle (Dias et al., 2013a; Carneiro & Isaias, 2014), facilitating the feeding of the galling organism (Carneiro & Isaias, 2014).
As commented in other sections, metaplasia may occur during induction and development of the galls under the feeding stimuli of gall inducers. The epidermal cells of Eriophyidae and Cecidomyiidae galls may directly redifferentiate into nutritive cells, or nutritive hairs (Westphal et al., 1981; Oliveira & Isaias, 2010b), without previous cell dedifferentiation and proliferation (Meyer & Maresquelle, 1983).
Promeristems
The cell layers lining the larval chamber may keep their meristematic feature. Moreover, some galls maintain promeristems or totipotent regions capable of redifferentiating the three plant tissue systems (Ferreira et al., 2017b). The maintenance of a unique promeristematic nutritive tissue impacts the longevity of D. gallaeformans galls on Miconia spp. (Fig. 2c), once it allows the replication of several generations of nematodes in the same gall (Ferreira et al., 2017a, 2017b). Accordingly, lateral buds differentiated in rosette galls of Pisphondylia brasiliensis (Cecidomyiidae) on Guapira opposita (Nyctaginaceae) produce true chlorophyllous leaf primordia (Fleury et al., 2015). The activity of these lateral buds is distinct from that of the non-galled buds, for instead of ordinary leaves, they produce modified leaves responsible for the rosette shape of the galls.
Conclusions
Plant development follows the morphogenetic patterns determined in plant meristems, which however can be manipulated by galling organisms, leading to the overdifferentiation or inhibition of some plant features, as well as the differentiation of distinct cell types. Gall development obeys the inducer’s feeding chemical and mechanical stimuli toward convergent anatomical traits observed in galls induced by at least 50 independently evolved animal groups. Even though galls may be considered extended phenotypes of their inducers (Carneiro et al., 2015), their host plant genome and cell machinery may be stimulated in similar ways related both to the mode of feeding and feeding sites (Fig. 1). This concept is aligned with galls as new plant organs, as stated by Shorthouse et al. (2005, p. 407): “they [the insect galls] are in a sense new plant organs because it is the plant that produces the gall in response to a specific stimulus provided by the invading insect. Each species of inducer produces galls that are anatomically and physiologically different from those induced by other related species”. The combination of anatomical convergent traits in each gall system culminates in the extant gall structural diversity.
We propose galls as elegant models for developmental studies due to their constant and repetitive cycles in nature, and hope such studies may help elucidate the roles of signaling molecules in plant developmental processes. The studies focusing on the developmental patterns of galls induced by unrelated animal taxa on related host plant species should help elucidating the pathways on plant cell redifferentiation. We also propose that structurally simple galls, i.e., with few anatomical alterations, should be the starting point to studying gall development using transcriptomes.
References
Álvarez, R. 2011. Initial stages in the formation of galls induced by Geoica utricularia in Pistacia terebinthus leaflets: Origin of the two vascular bundles which characterize the wall of the galls. American Journal of Plant Sciences 2: 175–179.
Álvarez, R., Encina, A. & Pérez-Hidalgo, N. 2009. Histological aspects of three Pistacia terebinthus galls induced by three different aphids: Paracletus cimiciformis, Forda marginata and Forda formicaria. Plant Science 176: 303–314.
Álvarez, R., González-Sierra, S., Candelas, A. & Martinez, J.J.I.. 2013. Histological study of galls induced by aphids on leaves of Ulmus minor: Tetraneura ulmi induces globose galls and Eriosoma ulmi induces pseudogalls. Arthropod-Plant Interactions 7: 643–650.
Álvarez, R., Martinez, J.J.I., Muñoz-Viveros, A.L., Molist, P., Abad-González, J. & Nieto-Nafría, J.M. 2016. Contribution of gall microscopic structure to taxonomy of gallicolous aphids. Plant Biology 8: 868–875.
Álvarez, R., Molist, P., González-Sierra, S., Martinez, J.J.I. & Nieto-Nafría, J.M. 2014. The histo structure of galls induced by aphids as a useful taxonomic character: The case of Rectinasus (Hemiptera, Aphididae, Eriosomatinae). Zootaxa 3861: 487–492.
Amorim, D.O., Ferreira, B.G. & Fleury, G. 2017. Plant potentialities determine anatomical and histochemical diversity in Mikania glomerata Spreng. Galls. Brazilian Journal of Botany 40: 517–527.
Apel, K. & Hirt, H. 2004. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55: 373–399.
Arduin, M. & Kraus, J.E. 1995 Anatomia e ontogenia das galhas foliares de Piptadenia gonoacantha (Fabales, Mimosaceae). Boletim de Botânica da Universidade de São Paulo 14: 109–130.
Arduin, M., Kraus, J.E. 2001. Anatomia de galhas de ambrosia em folhas de Baccharis concinna e Baccharis dracunculifolia (Asteraceae). Brazilian Journal of Botany 24: 63–72.
Arduin, M., Kraus, J.E., Otto, P.A. & Venturelli, M. 1989. Caracterização morfológica e biométrica de galhas foliares em Struthanthus vulgaris Mart. (Loranthaceae). Revista Brasileira de Biologia 49: 817–823.
Arduin, M., Kraus, J.E., Fernandes, G.W. & Kraus, J.E. 2005. Morphogenesis of galls induced by Baccharopelma dracunculifoliae (Hemiptera: Psyllidae) on Baccharis dracunculifolia (Asteraceae) leaves. Brazilian Journal of Biology 65: 559–571.
Barceló, R. 1997. Lignification in plant cell walls. International Review of Cytology 176: 87–132.
Barnewall, E. & De Clerck-Floate, R.A. 2012. A preliminary histological investigation of gall induction in an unconventional galling system. Arthropod-Plant Interactions 6: 449–459.
Bedetti, C.S., Ferreira, B.G., Castro, N.M. & Isaias, R.M.S. 2013. The influence of parasitoidism on the anatomical and histochemical profiles of the host leaves in a galling Lepidoptera–Bauhinia ungulata system. Revista Brasileira de Biociências 11: 242–249.
Bedetti, C.S., Modolo, L.V. & Isaias, R.M.S. 2014. The role of phenolics in the control of auxin in galls of Piptadenia gonoacantha (Mart.) MacBr (Fabaceae: Mimosoideae). Biochemical Systematics and Ecology 55: 53–59.
Begum, S., Nakaba, S., Oribe, Y., Kubo, T. & Funada, R. 2010. Changes in the localization and levels of starch and lipids in cambium and phloem during cambial reactivation by artificial heating of main stems of Cryptomeria japonica trees. Annals of Botany 106: 885–895.
Bird, A.F. 1961. The ultrastructure and histochemistry of a nematode-induced giant cell. Journal of Biophysical and Biochemical Cytology 11: 701–715.
Bragança, G.P., Oliveira, D.C. & Isaias, R.M.S. 2017. Compartmentalization of metabolites and enzymatic mediation in nutritive cells of Cecidomyiidae galls on Piper arboretum Aubl. (Piperaceae). Journal of Plant Studies 6: 11–22.
Bronner, R. 1992. The role of nutritive cells in the nutrition of cynipids and cecidomyiids. Pp. 118–140. In: J.D. Shorthouse & O. Rohfritsch, (eds.), Biology of insect-induced galls. Oxford University Press, New York, USA.
Brooks, S.E. & Shorthouse, J.D. 1998. Developmental morphology of stem galls of Diplolepis nodulosa (Hymenoptera: Cynipidae) and those modified by the inquiline Periclistus pirata (Hymenoptera: Cynipidae) on Rosa blanda (Rosaceae). Canadian Journal of Botany 76: 365–381.
Burckhardt, D. 2005. Biology, ecology and evolution of gall-inducing psyllids. Pp. 143–157. In: A. Raman, C.W. Schaefer & T.M. Withers, (eds.), Biology, ecology and evolution of gall-inducing arthropods. Science Publishers Inc., Enfield, USA.
Burckhardt, D., Queiroz, D.L. 2012. Checklist and comments on the jumping plant-lice (Hemiptera: Psylloidea) from Brazil. Zootaxa 3571: 26–48.
Byers, J.A., Brewer, J.W. & Denna, D.W. 1976. Plant growth hormones in pinyon insect galls. Marcellia 39: 125–134.
Carmona, D., Lajeunesse, M.J. & Johnson, M.T.J. 2011. Plant traits that predict resistance to herbivores. Functional Ecology 25: 305–432.
Carneiro, R.G.S. & Isaias, R.M.S. 2014. Cytological cycles and fates in Psidium myrtoides are altered towards new cell metabolism and functionalities by the galling activity of Nothotrioza myrtoidis. Protoplasma 252: 637–646.
Carneiro, R.G.S., & Isaias, R.M.S. 2015. Gradients of metabolite accumulation and redifferentiation of nutritive cells associated with vascular tissues in galls induced by sucking insects. AoB Plants 7: plv086.
Carneiro, R.G.S., Castro, A.C. & Isaias, R.M.S. 2014a. Unique histochemical gradients in a photosynthesis-deficient plant gall. South African Journal of Botany 92: 97–104.
Carneiro, R.G.S., Oliveira, D.C. & Isaias, R.M.S. 2014b. Developmental anatomy and immunocytochemistry reveal the neo-ontogenesis of the leaf tissues of Psidium myrtoides (Myrtaceae) towards the globoid galls of Nothotrioza myrtoidis (Triozidae). Plant Cell Reports 33: 2093–2106.
Carneiro, R.G.S., Pacheco, P. & Isaias, R.M.S. 2015. Could the extended phenotype extend to the cellular and subcellular levels in insect-induced galls? PLoS One 10(6): e0129331.
Castro, A.C.R., Oliveira, D.C. & Isaias, R.M.S. 2012. Morphological patterns of a hymenopteran gall on the leaflets of Caryocar brasiliense Camb. (Caryocaraceae). American Journal of Plant Sciences 3: 921–929.
Chao, J.F. & Liao, G.I. 2013. Histocytological aspects of four types of ambrosia galls on Machilus zuihoensis Hayata (Lauraceae). Flora 208: 157–164.
Crespi, B. & Worobey, M. 1998. Comparative analysis of gall morphology in Australian gall Thrips: The evolution of extended phenotypes. Evolution 52: 1686–1696.
Csóka, G., Stone, G.N. & Melika, G. 2005. Biology, ecology and evolution of gall-inducing Cynipidae. Pp. 573–642. In: A. Raman, C.W. Schaefer & T.M. Withers, (eds.), Biology, ecology and evolution of gall-inducing arthropods. Science Publishers Inc., Enfield, USA.
Di Vito, M., Vovlas, N. & Castillo, P. 2004. Host–parasite relationships of Meloidogyne incognita on spinach. Plant Pathology 53: 508–514.
Dias, G.G., Ferreira, B.G., Moreira, G.R.P. & Isaias, R.M.S. 2013a. Developmental pathway from leaves to galls induced by a sap-feeding insect on Schinus polygamus (Cav.) Cabrera (Anacardiaceae). Anais da Academia Brasileira de Ciências 85: 187–200.
Dias, G.G., Moreira, G.R.P., Ferreira, B.G. & Isaias, R.M.S. 2013b. Why do the galls induced by Calophya duvauae Scott on Schinus polygamus (Cav.) Cabrera (Anacardiaceae) change color? Biochemical Systematics and Ecology 48: 111–122.
Dorchin, N., Freidberg, A. & Aloni, R. 2002. Morphogenesis of stem gall tissues induced by larvae of two cecidomyiid species (Diptera: Cecidomyiidae) on Suaeda monoica (Chenopodiaceae). Candaian Journal of Botany 80: 1141–1150.
Dropkin, V.H. 1969. Cellular responses of plants to nematode infections. Annual Review of Phytopathology 7: 101–122.
Espírito-Santo, M.M. & Fernandes, G.W. 2007. How many species of gall-inducing insects are there on earth, and where are they? Annals of the Entomological Society of America 100: 95–99.
Evert, R.F. 2006. Esau's plant anatomy: Meristems, cells, and tissues of the plant body: Their structure, function, and development. John Wiley & Sons, New Jersey, USA.
Fagan, M.M. 1918. The uses of insect galls. The American Naturalist 52: 155–176.
Favery, B., Quentin, M., Jaubert-Possamai, S. & Abad, P. 2016. Gall-forming root-knot nematodes hijack key plant cellular functions to induce multinucleate and hypertrophied feeding cells. Journal of Insect Physiology 84: 60–69.
Felt, E.P. 1936. The relations of insects and plants in gall production. Annals of the Entomological Society of America 29: 694–700.
Fernandes, G.W. & Price, P.W. 1992. The adaptive significance of insect gall distribution: Survivorship of species in xeric and Mesic habitats. Oecologia 90: 14–20.
Fernandes, G.W., Carneiro, M.A.A. & Isaias, R.M.S. 2012. Gall-inducing insects: From anatomy to biodiversity. Pp. 369–395. In: A.R. Panizzi & J.R.P. Parra, (eds.), Insect bioecology and nutrition for integrated pest management. CRC Press, Boca Raton, USA.
Ferreira, B.G. & Isaias, R.M.S. 2013. Developmental stem anatomy and tissue redifferentiation induced by a galling Lepidoptera on Marcetia taxifolia (Melastomataceae). Botany 91: 752–760.
Ferreira, B.G., & Isaias, R.M.S. 2014. Floral-like destiny induced by a galling Cecidomyiidae on the axillary buds of Marcetia taxifolia (Melastomataceae). Flora 209: 391–400.
Ferreira, B.G., Álvarez, R., Avritzer, S.C. & Isaias, R.M.S. 2017a. Revisiting the histological patterns of storage tissues: Beyond the limits of gall-inducing taxa. Botany 2017a;95: 173–184.
Ferreira, B.G., Avritzer, S.C. & Isaias, R.M.S. 2017b. Totipotent nutritive cells and indeterminate growth in galls of Ditylenchus gallaeformans (Nematoda) on reproductive apices of Miconia. Flora 227: 36–45.
Ferreira, B.G., Carneiro, R.G.S. & Isaias, R.M.S. 2015. Multivesicular bodies differentiate exclusively in nutritive fast-dividing cells in Marcetia taxifolia galls. Protoplasma 252: 1275–1283.
Ferreira, B.S., Falcioni, R., Guedes, L.M., Avritzer, S.C., Antunes, W.C., Souza, L.A. & Isaias, R.M.S. 2017c. Preventing false negatives for histochemical detection of phenolics and lignins in PEG-embedded plant tissues. Journal of Histochemistry and Cytochemistry 65: 105–116.
Ferreira, B.S., Oliveira, D.C., Moreira, A.S.F.P., Faria, A.P., Guedes, L.M., França, M.G.C., Álvarez, R. & Isaias, R.M.S. 2018. Antioxidant metabolism in galls due to the extended phenotypes of the associated organisms. PLoS ONE 13: e0205364.
Finley, A.M. 1981. Histopathology of Meloidogyne chitwoodi (Golden et al.) on russet Burbank potato. Journal of Nematology 13: 486–491.
Fleury, G., Ferreira, B.G., Oliveira, D.C., Soares, G.L.G. & Isaias, R.M.S. 2015. Elucidating the determination of the rosette galls induced by Pisphondylia brasiliensis Couri & Maia 1992 (Cecidomyiidae) on Guapira opposita (Vell.) Reitz (Nyctaginaceae). Australian Journal of Botany 63: 608–617.
Formiga, A.T., Soares, G.L.G. & Isaias, R.M.S. 2011. Responses of the host plant tissues to gall induction in Aspidosperma spruceanum Müell. Arg. (Apocynaceae). American Journal of Plant Sciences 2: 823–834.
Formiga, A.T., Oliveira, D.C., Ferreira, B.G., Magalhães, T.A., Castro, A.C., Fernandes, G.W. & Isaias, R.M.S. 2013. The role of pectic composition of cell walls in the determination of the new shape-functional design in galls of Baccharis reticularia (Asteraceae). Protoplasma 250: 899–908.
Formiga, A.T., Silveira, F.A.O., Fernandes, G.W. & Isaias, R.M.S. 2015. Phenotypic plasticity and similarity among gall morphotypes on a superhost, Baccharis reticularia (Asteraceae). Plant Biology 17: 512–521.
Gonçalves, S.J.M.R., Isaias, R.M.S., Vale, F.H.A. & Fernandes, G.W. 2005. Sexual dimorphism of Pseudotectococcus rolliniae Hodgson & Gonçalves 2004 (Hemiptera Coccoidea Eriococcidae) influences gall morphology on Rollinia laurifolia Schltdl. (Annonaceae). Tropical Zoology 18: 161–169.
Gonçalves, S.J.M.R., Moreira, G.R.P., Isaias, R.M.S. 2009. A unique seasonal cycle in a leaf gall-inducing insect: The formation of stem galls for dormancy. Journal of Natural History 43: 843–854.
Goodey, J.B. 1939. The structure of the leaf galls of Plantago lanceolata L. induced by Anguillulina dipsaci (Kühn) Gerv. & v. Ben. Journal of Helminthology 17: 183–190.
Goodey, J.B. 1948. The galls caused by Anguillulina balsamophila (Thorne) Goodey on the leaves of Wyethia amplexicaulis Nutt. And Balsamorhiza sagittata Nutt. Journal of Helminthology 22: 109–116.
Gopinathan, K. & Ananthakrishnan, T.N. 1985. Morphogenesis and histochemistry of some thrips (Thysanoptera: Insecta) induced galls. PNAS B51: 413–456.
Harris, K.M. 1994. Gall midges (Cecidomyiidae): Classification and biology. In: M.A.J. Williams, (ed.), Plant galls: Organisms, interactions, populations. The Systematics Association Special Volume. 49: 201–211.
Hodgson, C., Oliveira, D.C. & Isaias, R.M.S. 2013. A new gall-inducing genus and species of Eriococcidae (Hemiptera: Sternorrhyncha: Coccoidea) on Sapindaceae from Brazil. Zootaxa 3734: 317–330.
Hodkinson, I.P. 1984. The biology and ecology of the gall-forming Psylloidea (Homoptera). Pp. 59–77. In: T.N. Ananthakrishnan, (ed.), Biology of gall insects. Oxford & IBH Publishing Co., New Delhi, India.
Hough, J.S. 1953. Studies on the common spangle gall of oak. II. A general consideration of past work on gall induction. New Phytologist 52: 218–228.
Hori, K. 1992. Insect secretions and their effect on plant growth, with special reference to hemipterans. Pp. 157–170. In: J.D. Shorthouse & O. Rohfritsch, (eds.), Biology of insect-induced galls. Oxford University Press, New York, USA.
Inbar, M., Eshel, A. & Wool, D. 1995. Interspecific competition among phloem-feeding insects mediated by induced host–plant sinks. Ecology 76: 1506–1515.
Isaias, R.M.S., Oliveira, D.C. & Carneiro, R.G.S. 2011. Role of Euphalerus ostreoides (Hemiptera: Psylloidea) in manipulating leaflet ontogenesis of Lonchocarpus muehlbergianus (Fabaceae). Botany 89: 581–592.
Isaias, R.M.S., Carneiro, R.G.S., Oliveira, D.C. & Santos, J.C. 2013. Illustrated and annotated checklist of Brazilian gall morphotypes. Neotropical Entomology 42:230–239.
Isaias, R.M.S., Oliveira, D.C., Moreira, A.S.F.P., Soares, G.L.G. & Carneiro, R.G.S. 2015. The imbalance of redox homeostasis in arthropod-induced plant galls: Mechanisms of stress generation and dissipation. Biochimica et Biophysica Acta 1850: 1509–1517.
Isaias, R.M.S., Ferreira, B.G., Alvarenga, D.R., Barbosa, L.R., Salminen, J.-P. & Steinbauer, M.J. 2018. Functional compartmentalisation of nutrients and phenolics in the tissues of galls induced by Leptocybe invasa (Hymenoptera: Eulophidae) on Eucalyptus camaldulensis (Myrtaceae). Austral Entomology 57: 238–246.
Jones, M.G.K. & Northcote, D.H. 1972. Nematode-induced syncytium – A multinucleate transfer cell. Journal of Cell Science 10: 789–809.
Jorge, N.C., Cavalleri, A., Bedetti, C. & Isaias, R.M.S. 2016. A new leaf-galling Holopothrips (Thysanoptera: Phlaeothripidae) and the structural alterations on Myrcia retorta (Myrtaceae). Zootaxa 4200: 174–180.
Jorge, N.C., Alvarenga, D.R., Cavalleri, A. & Isaias, R.M.S. 2018a. Anatomical and histometric explanations for leaf folding on Holopothrips striatus galls in Myrcia retorta. Flora 244–245: 24–28.
Jorge, N.C., Souza-Silva, E.S., Alvarenga, D.R., Saboia, G., Soares, G.L.G., Zini, C.A., Cavalleri, A. & Isaias, R.M.S. 2018b. Structural and chemical profiles of Myrcia splendens (Myrtaceae) leaves under the influence of the galling Nexothrips sp. (Thysanoptera). Frontiers in Plant Science 9: 1521.
Kendall, J. 1930. The structure and development of certain eriophyid galls. Zeitschrift Fur Parasitenkunde 2: 477–501.
Kjer, K.M., Carle, F.M., Litman, J. & Ware, J. 2006. A molecular phylogeny of Hexapoda. Arthropod Systematics & Phylogeny 64: 35–44.
Korotyaev, B.A., Konstantinov, A.S., Lingafelter, S.W., Mandelshtam, M.Y. & Volkovitsh, M.G. 2005. Gall-inducing Coleoptera. Pp. 239–271. In: A. Raman, C.W. Schaefer & T.M. Withers, (eds.), Biology, ecology and evolution of gall-inducing arthropods. Science Publishers Inc., Enfield, USA.
Kostoff, D. & Kendall, J. 1929. Studies on the structure and development of certain Cynipid galls. Biological Bulletin 56: 402–458.
Kraus, J.E. & Tanoe, M. 1999. Morpho-ontogenetic aspects of entomogenous galls in roots of Cattleya guttata (Orchidaceae). Lindleyana 14: 204–213.
Kraus, J.E., Arduin, M. & Venturelli, M. 2002. Anatomy and ontogenesis of hymenopteran leaf galls of Struthanthus vulgaris Mart. (Loranthaceae). Revista Brasileira de Botânica 25: 449–458.
Krusberg, L.R. 1963. Host response to nematode infection. Annual Review of Phytopathology 1: 219–240.
Kurzfeld-Zexer, L., Lev-Yadun, S. & Inbar, M. 2015. One aphid species induces three gall types on a single plant: Comparative histology of one genotype and multiple extended phenotypes. Flora 210: 19–30.
Küster, E. 1911. Dier Gallen der Pflanzen. S. Hirzel, Leipzig, Germany.
Larew, H.G. 1981. A comparative anatomical study of galls caused by the major cecidogenetic groups, with special emphasis on the nutritive tissue. PhD Thesis, Oregon State University, USA. Available from: http://ir.library.oregonstate.edu/xmlui/handle/1957/9413
Lev-Yadun, S. 2003. Stem cell in plants are differentiated too. Current Topics in Plant Bioogy 4: 93–100.
Magalhães, T.A., Oliveira, D.C., Isaias, R.M.S. 2015. Population dynamics of the gall inducer Eriogallococcus isaias (Hemiptera: Coccoidea: Eriococcidae) on Pseudobombax grandiflorum (Malvaceae). Journal of Natural History 49: 789–801.
Magalhães, T.A., Oliveira, D.C., Suzuki, A.Y.M. & Isaias, R.M.S. 2014. Patterns of cell elongation in the determination of the final shape in galls of Baccharopelma dracunculifoliae (Psyllidae) on Baccharis dracunculifolia DC (Asteraceae). Protoplasma 251: 747–753.
Mani, M.S. 1964. Ecology of plant galls. Dr. W. Junk Publishers, The Hague, Netherlands.
Mani, M.S. 1992. Introduction to Cecidology. Pp. 3–7. In: J.D. Shorthouse & O. Rohfritsch, (eds.), Biology of insect induced galls. Oxford University Press, New York, USA.
Mapes, C.C. & Davies, P.J. 2001. Indole-3-acetic acid and ball gall development on Solidago altissima. New Phytologist 151: 195–202.
McCalla, D.R., Genthe, M. & Hovanitz, W. 1962. Chemical nature of an insect gall growth-factor. Plant Physiology 37: 98–103.
Meyer, J. & Maresquelle, H.J. 1983. Anatomie des galles. Gebrüder Borntraeger, Berlin, Germany.
Meyer, J. 1987. Plant galls and gall inducers. Gebrüder Borntraeger, Berlin, Germany.
Miller, W.E. 2005. Gall-inducing Lepidoptera. Pp. 431–465. In: A. Raman, C.W. Schaefer & T.M. Withers, (eds.), Biology, ecology and evolution of gall-inducing arthropods. Science Publishers Inc., Enfield, USA.
Mound, L.A. 1994. Thrips and gall induction: A search for patterns. Pp. 131–149. In: M.A.J. Williams, (ed.), Plant galls: Organisms, interactions, populations. The Systematics Association Special Volume 49: 131–149.
Mound, L.A., & Morris, D.C. 2005. Gall-inducing Thrips: An evolutionary perspective. Pp. 59–72. In: Raman, C.W. Schaefer & T.M. Withers, (eds.), Biology, ecology and evolution of gall-inducing arthropods. Science Publishers Inc., Enfield, USA.
Moura, M.Z.D., Soares, G.L.G. & Isaias, R.M.S. 2008. Species-specific changes in tissue morphogenesis induced by two arthropod leaf gallers in Lantana camara L. (Verbenaceae). Australian Journal of Botany 56: 153–160.
Moura, M.Z.D., Soares, G.L.G., Alves, T.M.A. & Isaias, R.M.S. 2009a. Intra-specific phenotypic variations in Lantana camara leaves affect host selection by the gall maker Aceria lantanae. Biochemical Systematics and Ecology 37: 541–548.
Moura, M.Z.D., Soares, G.L.G., & Isaias, R.M.S. 2009b. Ontogênese da folha e das galhas induzidas por Aceria lantanae Cook (Acarina: Eriophyidae) em Lantana camara L. (Verbenaceae). Revista Brasileira de Botânica 32: 271–282.
Muñoz-Viveros, A.L., Martinez, J.J.I., Molist, P., González-Sierra, S., González, P. & Álvarez, R. 2014. Microscopic study of galls induced by three species of Geopemphigus (Hemiptera: Aphididae: Eriosomatinae) on Pistacia mexicana. Arthropod-Plant Interactions 8: 531–538.
Münzenberger, B., Schneider, B., Nilsson, R.H., Bubner, B., Larsson, K.H. & Hüttl, R.F. 2012. Morphology, anatomy, and molecular studies of the ectomycorrhizal formed axenically by the fungus Sistotrema sp. (Basidiomycota). Mycological Progress 11: 817–826.
Nylund, J.E. & Unestam, T. 1982. Structure and physiology of ectomycorrhizae. New Phytologist 91: 63–79.
Nyman, T., Widmer, A. & Roiniken, H. 2000. Evolution of gall morphology and host-plant relationships in willow-feeding sawflies (Hymenoptera: Tenthredinidae). Evolution 54: 526–533.
Oldfield, G.N. 2005. Biology of gall-inducing Acari. Pp. 35–58. In: A. Raman, C.W. Schaefer & T.M. Withers, (eds.), Biology, ecology and evolution of gall-inducing arthropods. Science Publishers Inc., Enfield, USA.
Oliveira, D.C. & Isaias, R.M.S. 2010a. Cytological and histochemical gradients induced by a sucking insect in galls of Aspidosperma australe Arg. Muell (Apocynaceae). Plant Science 178: 350–358.
Oliveira, D.C., Isaias, R.M.S. 2010b. Redifferentiation of leaflet tissues during midrib gall development in Copaifera langsdorffii (Fabaceae). South African Journal of Botany 76: 239–248.
Oliveira, D.C., Carneiro, R.G.S., Magalhães, T.A. & Isaias, R.M.S. 2011. Cytological and histochemical gradients on two Copaifera langsdorffii Desf. (Fabaceae) – Cecidomyiidae gall systems. Protoplasma 248: 829–837.
Oliveira, D.C., Isaias, R.M.S., Fernandes, G.W., Ferreira, B.G., Carneiro, R.G.S. & Fuzaro, L. 2016. Manipulation of host plant cells and tissues by gall-inducing insects and adaptive strategies used by different feeding guilds. Journal of Insect Physiology 84: 103–113.
Oliveira, D.C., Magalhães, T.A., Ferreira, B.G., Teixeira, C.T., Formiga, A.T., Fernandes, G.W. & Isaias, R.M.S. 2014. Variation in the degree of pectin methylesterification during the development of Baccharis dracunculifolia kidney-shaped gall. PLoS One 9: e94588.
Portugal-Santana, A. & Isaias, R.M.S. 2014. Galling insects are bioindicators of environmental quality in a conservation unit. Acta Botanica Brasilica 28(4): 594–608.
Price, P.W., Fernandes, G.W. & Waring, G.L. 1987. Adaptive nature of insect galls. Environmental Entomology 16: 15–24.
Raman, A. 2012. Gall induction by hemipteroid insects. Journal of Plant Interactions 7: 29–44.
Raman, A., & Ananthakrishnan, T.N. 1983. Studies on some thrips (Thysanoptera: Insecta) induced galls. 2. Fine-structure of the nutritive zone. Proceedings of the Indian National Science Academy. Part B, Biological Sciences 49: 525–561.
Raman, A., Schaefer, C.W. & Withers, T.M. 2005. Biology, ecology and evolution of gall-inducing arthropods. Science Publishers Inc., Enfield, USA.
Rancic, D., Stevanovic, B., Petanović, R., Magud, B., Tosevski, I. & Gassmann, A. 2006. Anatomical injury induced by the eriophyid mite Aceria anthocoptes on the leaves of Cirsium arvense. Experimental and Applied Acarology 38: 243–253.
Richardson, R.A., Body, M., Warmund, M.R., Schultz, J.C. & Appel, H.M. 2017. Morphometric analysis of young petiole galls on the narrow-leaf cottonwood, Populus angustifolia, by the sugarbeet root aphid, Pemphigus betae. Protoplasma 254: 203–216.
Rodiuc, N., Vieira, P., Banora, M.Y. & Engler, J.A. 2014. On the track of transfer cell formation by specialized plant-parasitic nematodes. Frontiers in Plant Sciences 5: 1–14.
Rohfritsch, O. 1992. Patterns in gall development. Pp. 60–86. In: J.D. Shorthouse & O. Rohfritsch, (eds.), Biology of insect induced galls. Oxford University Press, New York, USA.
Rohfritsch, O. 2008. Plants, gall midges, and fungi: A three-component system. Entomologia Experimentalis et Applicata 128: 208–216.
Roskam, J.C. 1992. Evolution of the gall-inducing guild. Pp. 34–49. In: J.D. Shorthouse & O. Rohfritsch, (eds.), Biology of insect induced galls. Oxford University Press, New York, USA.
Sá, C.E.M., Silveira, F.A.O., Santos, J.C., Isaias, R.M.S. & Fernandes, G.W. 2009. Anatomical and developmental aspects of leaf galls induced by Schizomyia macrocapillata Maia (Diptera: Cecidomyiidae) on Bauhinia brevipes Vogel (Fabaceae). Brazilian Journal of Botany 32: 319–327.
Sano, M. & Akimoto, S.-Y. 2011. Morphological phylogeny of gall-forming aphids of the tribe Eriosomatini (Aphididae: Eriosomatinae). Systematic Entomology 36: 607–627.
Schreuder, M.D.J., Brewer, C.A. & Heine, C. 2001. Modelled influences of non-exchanging trichomes on leaf boundary layers and gas exchange. Journal of Theoretical Biology 210: 23–32.
Shorthouse, J.D., Wool, D. & Raman, A. 2005. Gall-inducing insects—nature’s most sophisticated herbivores. Basic and Applied Ecology 6: 407–411.
Sinnott, E.W. 1960. Plant morphogenesis. McGraw-HillBook Co. Inc., New York, USA.
Skinner, J.A., Orr, C.C. & Robinson, A.F. 1980. Histopathogenesis of the galls induced by Nothanguina phyllobia in Solanum elaeagnifolium. Journal of Nematology 12: 141–150.
Souza, S.C.P.M., Kraus, J.E., Isaias, R.M.S. & Neves, L.J. 2000. Anatomical and ultrastructural aspects of leaf galls in Ficus microcarpa L. F. (Moraceae) induced by Gynaikothrips ficorum Marchal (Thysanoptera). Acta Botanica Brasilica 14: 57–69.
Stone, G.N. & Schonrögge, K. 2003. The adaptive significance of insect gall morphology. Trends Ecology and Evolution 18: 512–522.
Suzuki, A.Y.M., Bedetti, C.S., Isaias, R.M.S. 2015. Detection and distribution of cell growth regulators and cellulose microfibrils during the development of Lopesia sp. galls on Lonchocarpus cultratus (Fabaceae). Botany 93: 435–444.
Taiz, L., Zeiger, E., Moller, I.M. & Murphy, A. 2015. Plant physiology and development. 6th. ed. Sinauer Associates, Sunderland, UK.
Tooker, J.F. & Helms, A.M. 2014. Phytohormone dynamics associated with gall insects, and their potential role in the evolution of the gall-inducing habit. Journal of Chemical Ecology 40: 742–753.
Vecchi, C., Menezes, N.L., Oliveira, D.C., Ferreira, B.G. & Isaias, R.M.S. 2013. The redifferentiation of nutritive cells in galls induced by Lepidoptera on Tibouchina pulchra (Cham.) Cogn. Reveals predefined patterns of plant development. Protoplasma 250: 1363–1368.
Watson, A.K. & Shorthouse, J.D. 1979. Gall formation on Cirsium arvense by Ditylenchus dipsaci. Journal of Nematology 11: 16–22.
Weischer, B. & Wyss, U. 1976. Feeding behaviour and pathogenicity of Xiphinema index on grapevine roots. Nematologica 22: 319–325.
Wells, B.W. 1920. Early stages in the development of certain Pachypsylla galls on Celtis. American Journal of Botany 7: 275–285.
Westphal, E., Bronner, R. & Le Ret, M. 1981. Changes in leaves of susceptible and resistant Solanum dulcamara infested by the gall mite Eriophyes cladophthirus (Acarina, Eriophyoidea). Canadian Journal of Botany 59: 875–882.
White, O.E. 1948. Fasciation. The Botanical Review 14: 319–358.
Williams, M.A.J. 1994. Plant galls: Organisms, interactions, populations. The systematics association special volume 49. The Systematics Association, Oxford, UK.
Wool, D. 2004. Galling aphids: Specialization, biological complexity, and variation. Annual Review of Entomology 49: 175–192.
Wyss, U. 1997. Root parasitic nematodes: An overview. Pp. 5–22. In: C. Fenoll, F.M.W. Grundler & S.A. Ohl, (eds.), Cellular and molecular aspects of plant-nematode interactions. Kluwer Academic Publishers, Dordrecht, Netherlands.
Wyss, U. 2002. Feeding behavior of plant-parasitic nematodes. Pp. 462–513. In: D. Lee, (ed.), The biology of nematodes. CRC Press, London, UK.
Yang, M.M., Mitter, C. & Miller, D.R. 2001. First incidence of inquilinism in gall-forming psyllids, with a description of the new inquiline species (Insecta, Hemiptera, Psylloidea, Psyllidae, Spondyliaspidinae). Zoologica Scripta 30: 97–113.
Yousif, G.M. 1979. Histological responses of four leguminous crops infected with Meloidogyne incognita. Journal of Nematology 11: 395–401.
Zha, Y.-P., Yang, Z.-X., Chen, J.-Y. & Cai, S.-S. 2016. Relationship between population dynamics and gall development of the gall aphid Kaburagia rhusicola (Hemiptera: Aphididae). Acta Entomologica Sinica 59: 791–796.
Acknowledgments
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) provided the scholarship for BGF doctoral internship in Universidad de León, Spain (99999.003956/2015-06); and doctoral scholarship for GPB and BGF. Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) provided a doctoral scholarship for BGF (140226/2017-6), a postdoctoral grant for BGF (171182/2017-0), and research grants for RMSI (307011/2015-1). Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil) provided financial resources for project execution (APQ-02617-15), and a scholarship for DRA.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ferreira, B.G., Álvarez, R., Bragança, G.P. et al. Feeding and Other Gall Facets: Patterns and Determinants in Gall Structure. Bot. Rev. 85, 78–106 (2019). https://doi.org/10.1007/s12229-019-09207-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-019-09207-w