Abstract
Key message
The Mexican beech undergoes masting events, on average, every 5.5 years. These events depend directly on precipitation.
Abstract
Climate change has considerably impacted the protective functions of tropical montane cloud forests, possibly influencing the synchronicity of phenological processes and the distribution and physiology of plants. In particular, climatic fluctuations cause changes in the distribution of tree species. Mexican beech (Fagus grandifolia subsp. mexicana) is considered an endangered species, due to its restricted distribution and its being a Miocene relict, limited to tropical montane cloud forests in the mountains of the Sierra Madre Oriental in eastern Mexico. We analyzed the influence of temperature and precipitation in prompting changes to tree-ring width, as well as vessel frequency and diameter, of Mexican beech in eastern Mexico. We used growth rings and xylem vessels traits to infer the historical masting events of Mexican beech over the last 128 years. We obtained independent chronologies for Mexican beech in each of the studied sites, dating back 152–178 years. Precipitation was strongly associated with differences in tree-ring width between masting and non-masting years. Our study highlights the use of dendroecological research to detect climate-induced modifications in the vessel frequency and diameter of tree species inhabiting tropical montane cloud forests. This association also explained differences in vessel frequency and diameter recorded before, during, and after masting events. Our results revealed that Mexican beech undergoes masting events every 5.5 years on average, and that these events directly depend on minimum annual precipitation. In conclusion, our results advance our understanding on the plasticity of growth rings and vessels traits (frequency and diameter) in response to fluctuation in precipitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is likely to have serious impacts on the protective functions of tropical montane cloud forests worldwide (Webster 1995). Climatic fluctuations significantly influence the phenology, distribution and physiology of plants (Speer 2010; Ming-Lee et al. 2015). Although cycles of climate change extend over centuries and millennia (e.g., climatic variations), current global warming is expected to generate similar climatic fluctuations over the following decades. The rapid rate of change is expected to have a direct effect on the capacity of forest species to adapt to future climatic conditions (Helama et al. 2004; Tinoco-Rueda et al. 2009; Schoene and Bernier 2012; Rehm et al. 2015). More specifically, ongoing precipitation and temperature variations might have a severe impact on tropical montane cloud forests by influencing the phenology and distribution of several plant species in these communities.
Apart from deforestation, current climate change represents the greatest threat to tropical montane cloud forests, due to changes in the patterns of precipitation and cloud immersion (e.g., fog, mist and cloud water), which are associated with rising temperatures, habitat fragmentation, and increasing green-house gas emissions (Price et al. 2011; Ponce-Reyes et al. 2012). Reduced cloud immersion and increased evapotranspiration, resulting from global warming, directly influence several ecological and phenological processes in tropical montane cloud forests plant species, such as masting events (Price et al. 2011; Esperón-Rodríguez and Barradas 2015; FAO 2015).
Masting behavior occurs in many tree species in temperate regions (Harper 1977), serving as an adaptive strategy to climatic variation and/or as a strategy to avoid seed predation (Kelly 1994; Pearse et al. 2016). Several studies have shown that temperature, precipitation, and phenological events in masting years directly affect growth-ring width and vessel traits (García-González and Fonti 2008; Fonti et al. 2010; Speer 2010; González-González et al. 2013).
In recent decades (since 2001), dendroecological research has provided important tools for describing historical–ecological events and phenological variation in forest species (Schweingruber 1996; Speer 2010; D´Arrigo et al. 2014; Hacket-Pain et al. 2015; Amoroso et al. 2017). Such events are inferred from variation in tree-ring width and vessel traits, which, in turn, are used to reconstruct the forest history of relict and endemic tree species (Gareca et al. 2010; Génova and Moya 2012; Rita et al. 2015). Several authors have analyzed various environmental factors (e.g., maximum and minimum temperature and precipitation) associated with the timing of mass flowering in beech trees (Övergaard et al. 2007; Kon and Noda 2007; Sawada et al. 2008; Latte et al. 2015). Most of these authors suggest that flowering mostly occurs after years with benign environmental conditions, such as high temperatures during the summer months. These environmental conditions promote high rates of carbon assimilation by trees, leading to enhanced flower bud development and beechnut production. Several authors have studied different factors that trigger flowering and, in consequence, beechnut production. Masting events typically occur after 2 years with high temperature and low precipitation during the summer months, preceded by a year with low summer temperature and high precipitation (Matyas 1965; Norton and Kelly 1988; Piovensan and Adams 2005; Övergaard et al. 2007; Burns 2012; Etemad and Sefidi 2017).
Temperature represents one of the most important environmental factors affecting the growth of Fagus worldwide (Fang and Lechowicz 2006). In addition, several authors have suggested that high summer temperatures (June–July), in a particular year, are strongly associated with the onset of masting in Fagus during the following year (Ehnis 1981; Suzuki et al. 2005; Kon and Noda 2007; Bradshaw et al. 2010; Hacket-Pain et al. 2015). For instance, Bayramzadeh et al. (2008) and Noyer et al. (2017) reported that vessels of Fagus trees develop structural modifications in response to climatic and phenological events. These climatic events (e.g., high temperature and precipitation) are detected through temporal variations in tree-ring width in angiosperms and gymnosperms, partly due to the increased recovery-times of trees after such events (Speer 2010; Bryukhanova and Fonti 2013).
Fagus grandifolia subsp. mexicana (Mexican beech) is a Miocene relict species that is endemic to the tropical montane cloud forests of eastern Mexico. This species occurs at elevations of 1450–1987 m and is considered as endangered under Mexican law (Téllez-Valdés et al. 2006; SEMARNAT 2010; González-Espinosa et al. 2011). Reports suggest that Mexican beech diverged from Fagus grandifolia Ehrh, which inhabits the USA and Canada, approximately 7 million years ago (Manos and Stanford 2001; Denk and Grimm 2009). Mexican beech exhibits synchronic masting, which might be the result of autoecological reproductive strategies or a product of environmental changes, such as persistent droughts (Kelly 1994; Piovensan and Adams 2005).
The most extensive and least disturbed Mexican beech forests are located in the state of Hidalgo, in eastern Mexico (Rodríguez-Ramírez et al. 2013). These forests are characterized by the presence of masting events at every 2- to 8-year intervals (Ehnis 1981; Pérez-Rodríguez 1999). It was found that Mexican beech is susceptible to climatic variations, such as those associated with higher elevations, drought, and seasonal frosts (Ehnis 1981; Rodríguez-Ramírez et al. 2016, 2018). Natural disturbances, such as strong winds, hurricanes, and storms, regulate the development of Mexican beech forests (Peters 1992), causing suppression and release events in tree-ring width (Peters 1995).
The relationship between tree-ring width and vessel traits in masting events of Mexican beech trees and the climatic factors that trigger these processes remain largely unknown (Rodríguez-Ramírez et al. 2018). Because of this, our main aim is to reconstruct historical masting events of Mexican beech trees using dendroecological methods. The objectives of this study are: (1) to identify how precipitation and temperature are associated with masting events, and (2) to identify if any differences exist in tree-ring widths and vessel traits (e.g., diameter and frequency) between masting and non-masting years occurring between the years 1980 and 2012.
Materials and methods
Study area
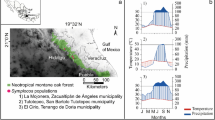
We selected three fragments of Mexican beech forests located in the Sierra Madre Oriental, which stretches in a north–south direction throughout eastern Mexico (Fig. 1). Temperate climate (Cb sensu García 1988) was characterized by mild temperatures (14.5–24.4 °C), including a dry cool season from November to January, a dry warm season from early February to May, cool summers (June–July) and a wet cool season from August to October. Humidity levels are in the range of 60–85% (Peters 1995; Williams-Linera et al. 2002). The soils of the sites are vitric (Tv) and humic (Th) andosols (FAO-UNESCO 1988) with light sandy-clay loam texture and pH values of 4 to 6 (Peters 1995).
The Mexican beech forests are characterized by dominant tree species with Holarctic affinities, such as Mexican beech (F. grandifolia subsp. mexicana), Martínez spruce (Picea martinezii T. F. Patterson), Mesoamerican yew (Taxus globosa Schltdl.), Magnolia (Magnolia schiedeana Schltdl.), Patula pine (Pinus patula Schltdl. & Cham.), Aztec pine (Pinus teocote Schltdl. & Cham.), several oak species (Quercus meavei Valencia-A., Sabás & Soto, Q. delgadoana S. Valencia, Nixon & L. M. Kelly and Q. trinitatis Trel.), sweetgum (Liquidambar styraciflua L.), and Mexican Clethra (Clethra mexicana DC.). These species are intermingled with evergreen tree species with Neotropical affinities, such as Zapotillo (Sideroxylon portoricense subsp. minutiflorum (Pittier) T.D. Penn.), Tarflower (Befaria aestuans L.), sweetwood (Nectandra spp.), wild avocado (Persea spp.), and Sabino (Podocarpus matudae Lundell) (Gual-Díaz and Rendón-Correa 2014; Rzedowski 2015).
Sample collection and chronology development
At each site, 20 dominant Mexican beech trees were selected (N = 60) based on the criteria established by Peters (1992) and Hukusima et al. (2013): (1) diameter at breast height (DBH) > 40 cm; (2) a height of 10–25 m; and (3) no evidence of scars or rot. For each tree, two cores were sampled at 1.3 m (breast height) with a Häglof® borer. We obtained a total of 120 wood cores from the three sites. At each site, we revised three complete cross-section discs from fallen trees as samples of growth patterns of Mexican beech in each locality and to detect ecological events (e.g., fire scars, defoliations, growth suppressions, and releases) affecting the forests (Fritts 1976). Sampled dominant Mexican beech trees were selected randomly within each site, ensuring that they encompassed the greatest possible variation in habitat characteristics (e.g., slopes ranging from 0.45° to 43.8° and distances of 30–500 m from water bodies).
The wood cores were dried at room temperature and were then mounted and polished with successively coarse grits (100 and 360) and fine grit sandpapers (400, 600, 3800, and 10,000) until the xylem cellular structure was visible in the transverse plane. Tree-ring series along the cores were dated by assigning calendar years to the rings through the identification of characteristic ring sequences (e.g., assigning to each ring the year in which growth started) as suggested by Stokes and Smiley (1968) and Rozas et al. (2015). This dating was verified with software COFECHA (Holmes 1983; Grissino-Mayer 2001). We measured tree-ring widths using a stereoscopic microscope and a Velmex tree-ring measuring system with 0.001 mm accuracy using the software TSAP-Win v. 4.67c (Rinn 2003). The computer software COFECHA allowed the identification of missing tree rings and cross-dating errors. For the analyses, we excluded 60 wood cores that were in poor condition (e.g., cores with evidence of decay, cores that were not properly mounted, or cores of short length) following the recommendations of Rozas (2001).
To obtain the average of detrended tree-ring width indices (RWI), we standardized raw ring-width series with autoregressive modeling to remove serial correlation using the ARSTAN computer program (Cook and Holmes 1995). We removed the non-climatic trends from each tree-ring series using a cubic spline with a 50% response at 10-year periods. This approach was flexible enough to accentuate high-frequency climatic information and to reduce white noise from non-climatic variance related to ontogenetic trends and/or local disturbances (e.g., droughts, strong winds, hurricanes and storms). Through this, we enhanced inter-annual variability, possibly related to masting events and the production of narrow tree rings (Dittmar and Elling 2007; Gareca et al. 2010; Drobyshev et al. 2014; Rodríguez-Ramírez et al. 2018).
We performed autoregressive modelling of each standardized series to remove temporal autocorrelation (Box and Jenkins 1976) and maximize the climatic signal. To produce a standardized chronology, the resulting indexed series were averaged using a bi-weight mean to reduce the influence of outliers (Cook and Holmes 1995). Temporal autocorrelation in chronologies was prevalent, due to the residual impact of growing conditions from previous years (Speer et al. 2016).
Historical records of Mexican beech masting
We gathered data of past Mexican beech masting events registered for each studied site for the years 1980, 1990, 1992, 1997, 2004, and 2012 (Ehnis 1981; Pérez-Rodríguez 1999; Godínez-Ibarra et al. 2007; Rodríguez-Ramírez et al. 2013). There are no records on masting events for other Mexican beech populations along the distribution range of this species.
Digitalization of tree-ring width and vessels traits
For each site, we randomly selected five cores to obtain tree-ring digital images for the recorded masting years, as well as for the two consecutive years before and after masting events. The wood cores were prepared using the finest grit sandpaper (10,000) and eliminating any dust with a hair drier. Since the ground tissue has very thick-walled fibers and parenchyma cells with dark deposits, the vessels lumen have a high contrast. In each digital image, we selected the area occupied by each tree-ring between two wood rays (an average of 7.5 mm width × 9.1 mm length). The area varied with respect to tree-ring width before, during, and after masting events [e.g., the widest and narrowest rings were 6.6 mm width × 8.3 mm length (ray to ray) and 2.5 mm width × 1.6 mm length (minimum area of 54.7 and 4 mm2, respectively; Fig. 2)]. These digital images were captured using a stereoscopic microscope (Axio Zoom.V16) with a 36 µm field of depth and saved in TIFF format with a digital camera (AxioCam MRc 5, Zeiss) to a 1.3 µm resolution. Figure 2 presents an example of a digitized cored with marked radial tree-ring width and vessel traits (number of vessels/mm2 and radial vessel diameter, µm); this technique has been used successfully with other species (Venegas-González et al. 2015). In each area, we quantified and measured all the vessels present using the software ImageJ v. 1.5 with manual detection (Java-based Image Processing, National Institute of Health).
Reconstruction of masting events: dendromastecology
Mast year reconstruction used two sources of data. We first delimited the historical masting events in the tree-ring digital image of trees from Medio Monte according to the historical records of Mexican beech masting. This region is considered as containing the most representative and best preserved Mexican beech forests studied (Rodríguez-Ramírez et al. 2013).
Finally, we compare variation in tree-ring width and vessel traits before, during and after masting year. We analyzed 10 randomly selected cores and captured tree-ring digital images, for the years ranging from 1847 to 2015 in Medio Monte to reconstruct historical masting events. The reason for restricting the analyses is because beech masting generally occurs in trees ≥ 40 years in age (Peters 1992; Drobyshev et al. 2010; Hukusima et al. 2013). Thus, we set 1886 as our initial year in the chronologies.
Earlier studies have suggested 2- to 8-year intervals between masting events (Rodríguez-Ramírez et al. 2013); therefore, we used this range of years to identify the tree-ring patterns around the recorded masting years. We used data on growth rings, before, during and after masting year to detect differences in tree-ring width and vessel traits.
Climate data
Several authors have suggested that mass flowering in beeches worldwide is triggered by two previous years with high summer temperature and low precipitation, preceded by a year with low summer temperature and high precipitation (Piovensan and Adams 2005; Kon and Noda 2007; Övergaard et al. 2007; Drobyshev et al. 2010; Ascoli et al. 2017).
To evaluate the association between temperature and precipitation with the masting data registered for Mexican beech trees, we gathered climate data for the period 1978–2011 (http://clicom-mex.cicese.mx/). These data included mean monthly values for minimum, average and maximum temperatures (Tmin, Tavg, and Tmax), as well as total annual precipitation (Prec) of a single year with the lowest rainfall. We considered climatic data 2 years before each masting event (e.g., 1978, 1979, 1988, 1989, 1990, 1991, 1995, 1996, 2002, 2003, 2010, and 2011), which were corroborated using information from Climate-data.org (http://es.climate-data.org/).
Variation ranges of the tree-ring widths and vessel traits
We analyzed data on mean maximum temperatures and Prec from climatic reconstructions for the State of Hidalgo (Cardoza-Martínez et al. 2013). To complete the missing information, we used the Drought-Net database (http://www.drought-net.org/) (Lemoine et al. 2016). This approach allowed the detection of narrow rings (≤ 1.00 mm) in the tree-ring digital images resulting from drought events, as suggested by Rozas et al. (2015).
We performed an Analyses of Variance (ANOVA) and Tukey multiple comparisons to assess if the values of tree-ring widths and vessel traits (frequency and diameter) differ significantly between drought years, non-masting years (NMY) and masting years (MY) in the studied forests. The analyses were performed using the R-library vegan in R (Version 2.14.0, http://www.r-project.org; Oksanen et al. 2016).
Influence of climate on tree-ring width and vessels traits
To test the relationship between climatic variables (Tmax, Tmin, Tavg and Prec) and tree-ring width and vessels traits, we performed three non-metric multidimensional scaling (NMDS) analyses. This approach allowed for the detection of differences between tree-ring width developed in MY and NMY, and to identify which climatic variables influence tree-ring width and vessel traits.
NMDS ordination was based on Bray-Curtis distances and 20 randomizations to determine the most stable solution. In addition, Wisconsin double standardization and R2 transformation were used as measures of ecological distance (Kenkel and Orlóci 1986). An advantage of this method is that the procedure is less dependent on data distribution than constrained methods, such as principal component analyses. The data were computed using the R-library vegan in the statistical software R.
To evaluate the NMDS, we used the stress-plot function and the Stress index to estimate R2 values between the vectors and values of the ordination (R). The vectors for climatic factors (Tmin, Tavg, Tmax, and Prec) and centroids were superimposed using the envfit function. We used the ordisurf function (within the vegan library) to draw the climatic variables in the space defined by two NMDS axes. Ordisurf fits smooth surfaces on the ordination using Generalized Additive Models (GAMs) with thin-plate splines (Wood 2000; Kindt and Coe 2005; Borcard et al. 2011). This approach allowed us to observe the relationship of climatic variables with tree-ring width and vessels traits. We used the Generalized Cross-Validation statistic (GCV score) to select the optimum model and minimize prediction error (Arlot and Celisse 2010).
Results
Dendromastecology of Mexican beech
The independent chronologies for Mexican beech extended up to 188 years for La Mojonera, 168 for Medio Monte, and 152 for El Gosco. A correlation between the three sites was detected, where the mean sensitivity to climatic variables was high and similar among sites (Table 1). Decreased radial growth was associated with several historical ENSO events (e.g., 1828–1830, 1850–1866, 1869–1890, 1905, 1913, 1918, 1929–1930, 1940, 1963, 1970, 1972, 1976, 1983, 1991, 1997, and 2012; Fig. 3a).

(modified from Cardoza-Martínez et al. 2013)
a Ring-width chronologies for Mexican beech forests. Black circles represent ENSO events and gray squares historical masting. Gray areas indicate the period (1978–2015) including ring-width chronologies for masting years. b Ring-width chronologies and masting events for the Medio Monte site. Black arrows represent recorded masting years and white arrows represent reconstructed masting years; and c reconstruction of annual precipitation in eastern Mexico for the period 1890–2015
The climatic reconstruction of Cardoza-Martínez et al. (2013) for the state of Hidalgo, together with data from Drought-Net, allowed us to recognize in the tree-ring digital images of trees from Medio Monte, 15 additional historical masting events for Mexican beech trees, to detect short 2-year periods (1955–1956, 1990–1992) between masting events (Fig. 3b). Notwithstanding, the dendrochronological reconstruction of historical masting events showed two 8-year periods (1926–1934, 2004–2012) between MY (Fig. 3b). Interestingly, these periods revealed precipitation values ranging from 1748 to 1798 mm, with a mean of ≥ 1750 mm (Fig. 3c). We estimated a mean of 5.5 years between MY recorded for Mexican beech in the state of Hidalgo. Likewise, we identify differences between tree-ring digital images and vessel traits formed during drought events, NMY and those associated with MY. ANOVA showed that statistically significant differences were present for vessel frequency and diameter in the Mexican beech forests studied (Table 2).
Vessel frequency and diameter were significantly higher in NMY and decreased in MY (P < 0.05). Both traits during the MY decreased one-fold or more compared to drought events and NMY, maintaining the negative scaling between these two variables (rsMY-0.27, P < 0.05). Notwithstanding, tree-ring widths did not differ significantly between NMY’s, drought years and MY’s (Fig. 4a); the same pattern was maintained for the reconstructed MY events (Fig. 4b).
Box plots showing the variation ranges of the tree-ring width, vessel frequency and diameter between drought years, NMY and MY. The upper and lower limits of the boxes represent the 75 and 25th percentiles, and whiskers represent the 90 and 10th percentile. Black circles show outliers. The solid lines within each box indicate statistically significant differences (P < 0.05). a Historical masting years; and b reconstruction of historical masting events. *Shows masting and drought events
Linking climate variation with tree-ring width and vessel traits
Ordination using NMDS showed that Prec was significantly associated with tree-ring width at the three sites (Fig. 5a; GCV score: 0.044; stress: 0.104). In turn, Prec had a considerable effect on vessel diameter and frequency (Fig. 5b, c; GCV score: 0.013, 0.044; stress: 0.120, 0.082). The NMDS2 values did not influence the Tmax for tree-ring width (Fig. 5; Table 3). Notwithstanding, NMDS2 was slightly conspicuous for Tmax with respect to vessel diameter and frequency (Fig. 5b, c).
Non-metric multidimensional scaling (NMDS) ordination, based on the relationship of climatic variables (Tmax, Tmin, Tavg and Prec) on the tree-ring width and xylem vessels. a Tree-ring width, b vessel frequency; and c vessel diameter in Mexican beech. Labels represent growth rings for each year and site. LM, La Mojonera; MM, Medio Monte; EG, El Gosco. Variable values are indicated with bold numbers and dotted lines
Discussion
Tropical montane cloud forests drive important ecological, hydrological, and climatological processes (Price et al. 2011). If tropical montane cloud trees begin to experience drought conditions resulting in cavitation, forest die-off might occur, leading to substantial changes in the growth and regeneration capacity of many tree species. This study shows that the radial growth of Mexican beech has been affected by specific climatic events such as drought at each Mexican beech forest studied (Fig. 3; Table 1). Compared to the other two sites (La Mojonera and Medio Monte), the Mexican beech trees at El Gosco were younger and had lower rates of tree-ring width. This result reinforced the observation that the Mexican beech forest at El Gosco has been affected by anthropogenic and natural disturbances in the recent past (Rodríguez-Ramírez et al. 2013). Possibly, this effect might correspond to the phase of canopy closure, with the convergence of individual crowns and initiation of intra-tree competition. This effect was observed by Podocarpus salignus D. Don. in Chile (Rozas et al. 2016). Climatic variations at each site might influence the tree-ring width of beech trees (Gual-Díaz and Rendón-Correa 2014), which is reflected by the presence of narrow rings (Fig. 3).
Our results suggest that Mexican beech trees undergo masting events, on average, every 5.5 years and that these events might be directly dependent on Prec. The results agree with those obtained by Drobyshev et al. (2014) for Fagus sylvatica from Europe. Climate change (e.g., high summer temperature, ENSO events) involving diminished precipitation might lead to the shortening of masting events (Fig. 3). Minimum annual precipitation plays a key role on tree-ring width of Mexican beech trees (Fig. 4; Table 2), rather than summer temperatures (Fig. 3), as for other species of Fagus (Drobyshev et al. 2014; Hacket-Pain et al. 2015). These climatic fluctuations (temperature and precipitation) are congruent with the patterns observed in other deciduous tree species in montane forests, such as Quercus ilex Lour., Olea europea L., and Ilex aquifolium L. (Abrantes et al. 2013; Rossi et al. 2013; Rita et al. 2015).
Our results revealed no significant associations between tree-ring width and Tmax (Table 3; Fig. 5), which has been proposed as the main factor affecting growth in other species of Fagus with northern distributions (Suzuki et al. 2005; Kon and Noda 2007; Bradshaw et al. 2010; Drobyshev et al. 2014; Hacket-Pain et al. 2015). The particular growth pattern of Mexican beech is the result of its southernmost distribution compared with other Fagus species worldwide. This phenomenon reflects the different plant associations and climatic conditions in which Mexican beech thrive (Fang and Lechowicz 2006; Rodríguez-Ramírez et al. 2016, 2018).
The observed differences in tree-ring width between MY and NMY in Mexican beech are indicative of the adaptive mechanisms of these trees to masting and climatic events (even ENSO events such as 2012, Figs. 2, 4; Table 2). The production of narrow rings in response to masting events, independent of climatic events (such as droughts), has also been reported in other Fagus species [e.g., F. grandifolia Ehrh in USA (Wason et al. 2017); F. crenata Blume in Japan (Sawada et al. 2008); and F. sylvatica L. in Europe (Hacket-Pain et al. 2015)]. Our results showed that tree-ring width within the MY had narrower variation than that of NMY or drought years. Therefore, instead of growth, resources are assigned to beechnut production during MY because of water deficits.
Our study revealed that vessel-related anatomical traits (frequency and diameter) adjust in response to drought events, NMY, and MY, corroborating the high plasticity mentioned for other Fagus species (Bayramzadeh et al. 2008; Pourtahmasi et al. 2011; Yin et al. 2016; Noyer et al. 2017). When a masting event occurs, Mexican beech develops fewer and narrower vessels, even narrower than in drought years (Fig. 4), maintaining their negative scaling. This finding is interesting because Mexican beech produces narrow vessels during MY to ensure hydraulic safety. Thus, vascular cambium modifications may be related with a trade-off between growth (narrow vessels during short periods of time) and beechnut production (Fig. 4). The plasticity in vessel frequency and diameter regulates water-transport efficiency, reflecting the ability of tropical montane cloud trees to adapt to climatic fluctuations as droughts and phenological events (Eller et al. 2017; von Arx et al. 2013; Rodríguez-Ramírez et al. 2018). Further anatomical studies of Fagus species worldwide are needed to understand vessel plasticity during masting events.
Structural modifications were indicated in differences in vessels traits during MY. These modifications might be related to hormonal changes (Chan and Cain 1967; Aloni 1987; Tyree and Zimmermann 2002; Rita et al. 2015) and environmental conditions, such as temperature, precipitation, wind, and inter-annual differences (Kelly 1994; Pearse et al. 2016). Similar modifications have been observed in other beech trees species such as Fagus orientalis in Middle East (Eşen 2000; Pourtahmasi et al. 2011); Fagus sylvatica in Europe (Hacket-Pain et al. 2015); and F. crenata in Japan (Kabeya et al. 2017). In these species, individual trees structurally modify their vessels before masting (Speer 2001). However, other biotic and abiotic factors (e.g., volatile organic chemicals, pathogens, fires, pollution, environmental factors) are needed to generate specific structural changes, with carbon distribution contributing to the modification of vessels traits and the resulting development of narrow tree rings (Sass and Eckstein 1995; Anderegg and Meinzer 2015). Such narrow tree rings appear to be essential for the onset of masting events (Övergaard et al. 2007).
We used several dendroecological techniques to detect differences in tree-ring width between MY and NMY (Fig. 5), which allowed for the reconstruction of historical masting events that are not on record. Our results suggest that reduced annual precipitation (814–998 mm) directly influences tree-ring width and vessel traits. This reconstruction suggests that masting events, both over short (2 years) and long (8 years) time intervals, have occurred repeatedly in the past when the required precipitation is reached (Fig. 5) as suggested by Peters (1995).
This study advances our understanding on the reproductive strategies of Mexican beech in the face of climatic fluctuations, which appear to have a strong influence on phenological events, such as masting synchrony (Vacchiano et al. 2016). The observed relationship of Prec with tree-ring width and vessels traits shows that this species has adapted to the southern part of its distribution range by developing narrow rings and using its resources for beechnut production.
Finally, we suggest that further research should focus on how climatic phenomena (e.g., El Niño and La Niña effects) and deforestation affect the masting behavior of trees (Burns 2012; Fletcher 2015). More specifically, future studies should address the possible reduction in reproductive potential and survival of Mexican beech in the tropical montane cloud forests of the Sierra Madre Oriental.
Author contribution statement
ECHRR contributed in the research by designing the experiment, writing the paper and running the data analyses; TT helped writing the paper, designing the experiment and running the data analyses; ILV supervised and revised all the project stages, including the manuscript writing.
References
Abrantes J, Campelo F, García-González I, Nabais C (2013) Environmental control of vessel traits in Quercus ilex under Mediterranean climate: relating xylem anatomy to function. Trees 27:655–662
Aloni R (1987) Differentiation of vascular tissues. Annu Rev Plant Physiol 38:179–204
Amoroso MM, Daniels LD, Baker PJ, Camarero JJ (2017) Dendroecology: tree-ring analyses applied to ecological studies. Springer, Switzerland
Anderegg WRL, Meinzer FC (2015) Wood anatomy and plant hydraulics in a changing climate. In: Hake U (ed) Functional and ecological xylem anatomy. Springer, Switzerland, pp 235–253
Arlot S, Celisse A (2010) A survey of cross-validation procedures for model selection. Stat Surv 4:40–79
Ascoli D, Vacchiano G, Turco M, Conedera M, Drobyshev I, Maringer J, Motta R, Hacket-Pain A (2017) Inter-annual and decadal changes in teleconnections drive continental-scale synchronization of tree reproduction. Nat Commun 8(2205):1–9
Bayramzadeh V, Funada R, Kubo T (2008) Relationships between vessel element anatomy and physiological as well as morphological traits of leaves in Fagus crenata seedlings originating from different provenances. Trees 22:217–224
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Use R! series. Springer, New York
Box GEP, Jenkins GM (1976) Time series analysis: forecasting and control. Holden-Day, San Francisco
Bradshaw RHW, Kito K, Gieseckre T (2010) Factors influencing the Holocene history of Fagus. For Ecol Manag 259:2204–2212
Bryukhanova M, Fonti P (2013) Xylem plasticity allows rapid hydraulic adjustment to annual climatic variability. Trees 27:485–496
Burns KC (2012) Masting in a temperate tree: evidence for environmental prediction. Austral Ecol 37:175–182
Cardoza-Martínez GF, Cerano-Paredes J, Villanueva-Díaz J, Cervantes-Martínez R, Guerra de la Cruz V, Estrada-Ávalos J (2013) Annual precipitation reconstruction of the Eastern region of Tlaxcala state. Rev Mex Cie Forest 5:110–127
Chan BC, Cain JC (1967) The effect of seed formation on subsequent flowering in apple. J Am Soc Hortic Sci 91:63–68
Climate-data.org (2016) Historical average temperature. http://climate-data.org/. Accessed 10 Oct 2016
Cook ER, Holmes RL (1995) Guide for computer program ARSTAN. In: Grissino-Mayer HD, Holmes RL, Fritts HC (eds) The International tree-ring data bank program library version 2.0 User’s Manual, Laboratory of Tree-Ring Research. University of Arizona, Arizona, pp 75–87
Cook ER, Holmes RL (1999) Program ARSTAN-chronology development with statistical analysis (users manual for program ARSTAN). Laboratory of Tree-Ring Research. University of Arizona, USA
D´Arrigo R, Davi N, Jacoby G, Wilson R, Wiles G (2014) Dendroclimatic studies: trees growth and climate change in northern forest. American Geophysical Union, Canada
Denk T, Grimm GW (2009) The biogeographic history of beech trees. Rev Palaeobot Palynol 158:83–100
Dittmar C, Elling W (2007) Dendroecological investigation of the vitality of Common Beech (Fagus sylvatica L.) in mixed mountain forests of the Northern Alps (South Bavaria). Dendrochronologia 2:37–56
Drobyshev I, Övergaard R, Saygin I, Niklasson M, Hickler T, Karlsson M, Sykes MT (2010) Masting behaviour and dendrochronology of European beech (Fagus sylvatica L.) in southern Sweden. For Ecol Manag 259:2160–2170
Drobyshev I, Niklasson M, Mazerolle MJ, Bergeron Y (2014) Reconstruction of a 253-year long mast record of European beech reveals its association with large scale temperature variability and no long-term trend in mast frequencies. Agric For Meteorol 192–193:9–17
Ehnis DE (1981) Fagus mexicana Martínez: su ecología e importancia. B. Sc. Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico City
Eller CB, Barros FV, Bittencourt PRL, Rowland L, Mencuccini M, Oliveira RS (2017) Xylem hidraulic safety and construction costs determine tropical tree growth. Plant Cell Environ 2018:1–15
Eşen D (2000) Ecology and control of Rhododendron (Rhododendron ponticum L.) in Turkish eastern beech (Fagus orientalis Lipsky) forest. Doctoral thesis. Virginia Polytechnic Institute and State University, Blacksburg, Virginia, USA
Esperón-Rodríguez M, Barradas VL (2015) Comparing environmental vulnerability in the montane cloud forest of eastern Mexico: a vulnerability index. Ecol Indic 52:300–310
Etemad V, Sefidi K (2017) Seed production and masting behaviour in Oriental beech (Fagus orientalis Lipsky) forests of northern Iran. Forest Ideas 23:65–76
Fang J, Lechowicz MJ (2006) Climatic limits for the present distribution of beech (Fagus L.) species in the world. J Biogeogr 33:1804–1819
FAO (2015) Global forest resources assessment 2015: how are the world´s forest changing? Food Agriculture Organization of the United Nations, Rome
FAO-UNESCO (1988) Soil map of the world. Revised legend. World soil resources report 60. FAO-UNESCO, Rome
Fletcher MS (2015) Mast seeding and the El Niño-Southern Oscillation: a long-term relationship? Plant Ecol 216:527–533
Fonti P, von Arx G, García-González I, Eilmann B, Sass-Klaassen U, Gärtner H, Eckstein D (2010) Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytol 185:42–53
Fritts HC (1976) Tree rings and climate. Academic Press, London
García E (1988) Modificaciones al sistema de clasificación climática de Köppen, México, Offset Larios. Mexico City
García-González I, Fonti P (2008) Ensuring a representative sample of earlywood vessels for dendroclimatological studies: an example from two ring-porous species. Trees 22:237–244
Gareca EE, Fernández M, Stanton S (2010) Dendrochronological investigation of the high Andean tree species Polylepis besseri and implications for management and conservation. Biodivers Conserv 19:1839–1851
Génova M, Moya P (2012) Dendroecological analysis of relict pine forests in the center of the Iberian Peninsula. Biodivers Conserv 21:2949–2965
Godínez-Ibarra O, Ángeles-Pérez G, López-Mata L, García-Moya E, Valdez-Hernández JV, Santos-Posadas H, Trinidad-Santos A (2007) Lluvia de semillas y emergencia de plántulas de Fagus grandifolia subsp. mexicana en La Mojonera, Hidalgo, México. Rev Mex Biodivers 78:117–128
González-Espinosa M, Meave JA, Lorea-Hernández FG, Ibarra-Manríquez G, Newton AC (2011) The Red List of Mexican cloud forest trees. Fauna & Flora International (FFI), Cambridge
González-González BD, Rozas V, García-González I (2013) Early vessels of the sub-Meditterranean oak Quercus pyrenaica have greater plasticity and sensitivity than those of the temperate Q. petrae at the Atlantic-Mediterranean boundary. Trees 28:237–252
Grissino-Mayer HD (2001) Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree Ring Res 57:205–221
Gual-Díaz M, Rendón-Correa A (2014) Bosques mesófilos de montaña de México: diversidad, ecología y manejo. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Mexico City
Hacket-Pain AJ, Friend AD, Lageard JGA, Thomas PA (2015) The influence of masting phenomenon on growth-climate relationships in trees: explaining the influence of previous summers´ climate on ring width. Tree Physiol 35:319–330
Harper JL (1977) Population biology of plants. Academic Press, London
Helama S, Lindholm M, Timonen M, Eronen M (2004) Detection of climate signal in dendrochronological data analysis: a comparison of tree-ring standardization methods. Theor Appl Climatol 79:239–254
Holmes RL (1983) Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull 43:69–78
Hukusima T, Matsui T, Nishio T, Pignatti S, Yang L, Lu SY et al (2013) Phytosociology of the beech (Fagus) forest in East Asia. Springer, Heidelberg
Kabeya D, Inagaki Y, Noguchi K, Han Q (2017) Growth rate reduction causes a decline in the annual incremental trunk growth in masting Fagus crenata trees. Tree Physiol 37:1444–1452
Kelly D (1994) The evolutionary ecology of mast seeding. Trees 9:465–470
Kenkel NC, Orlóci L (1986) Applying metric and nonmetric multidimensional scaling to ecological studies: some new results. Ecology 67:919–928
Kindt R, Coe R (2005) Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. World Agroforestry Centre (ICRAF), Nairobi
Kon H, Noda T (2007) Experimental investigation on weather cues for mast seeding of Fagus crenata. Ecol Res 22:802–806
Latte N, Lebourgeois F, Claessens H (2015) Increased tree-growth synchronization of beech (Fagus sylvatica L.) in response to climate change in northwestern Europe. Dendrochronologia 33:69–77
Lemoine N, Sheffield J, Dukes JS, Knapp AK, Smith MD (2016) Terrestrial precipitation analysis (TPA): a resource for characterizing long-term precipitation regimes and extremes. Methods Ecol Evol 7:1396–1401
Manos PS, Stanford AM (2001) The historical biogeography of Fagaceae: tracking the tertiary history of temperate and subtropical forests of the northern hemisphere. Int J Plant Sci 162:S77–S93
Matyas V (1965) Some ecological factors affecting the periodicity of fruit in oak and beech. Erdesz Kutatas Budapest 61:99–121 (in Hungarian with German summary)
Ming-Lee T, Markowitz EM, Howe PD, Ko CY, Leiserowitz AAA (2015) Predictors of public climate change awareness and risk perception around the world. Nat Clim Change 5:1014–1020
Norton DA, Kelly D (1988) Mast seeding over 33 years by Dacrydium cupressinum Lamb. (rimu) (Podocarpaceae) in New Zealand: the importance of economies of scale. Funct Ecol 2:399–408
Noyer E, Lachenbruch B, Dlouhá J, Collet C, Ruelle J, Ningre F, Fournier (2017) Xylem traits in European beech (Fagus sylvatica L.) display a large plasticity in response to canopy release. Ann For Sci 76:46
Oksanen J, Blanchet FG, Kindt R, Legendre P, Michin PR, Hara RBO´, Simpson GL, Solymos P, Stevens MHH, Wagner H (2016) Vegan: community ecology package. R package version 2.3-3. http://cran.r-project.org. Accessed 20 Nov 2016
Övergaard R, Gemmel P, Karlsson M (2007) Effects of weather conditions on mast year frequency in beech (Fagus sylvatica L.) in Sweden. Forestry 80:555–565
Pearse IS, Koenig WD, Kelly D (2016) Mechanisms of mast seeding resources, weather, cues, and selection. New Phytol 212:546–562
Pérez-Rodríguez PM (1999) Las hayas de México, monografía de Fagus grandifolia spp. mexicana. Universidad Autónoma de Chapingo, Chapingo, Mexico City
Peters R (1992) Ecology of beech forests in the northern Hemisphere. Doctoral Thesis, Wageningen Agricultural University, Wageningen, Germany
Peters R (1995) Architecture and development of Mexican beech forest. Vegetation science in forestry. In: Box EO, Peet RK, Masuzawa T, Yamada I, Fujiwara K, Maycock PF (eds) Vegetation science in forestry. Kluwer Academic Publishers, Dordrecht, pp 325–343
Piovensan G, Adams JM (2005) The evolutionary ecology of masting: does the environmental prediction hypothesis also have a role in mesic temperate forests? Ecol Res 20:739–743
Ponce-Reyes R, Reynoso-Rosales VH, Watson JEM, Van Der Wal J, Fuller RA, Pressey RL, Possingham HP (2012) Vulnerability of cloud forest reserves in Mexico to climate change. Nat Clim Change 2:448–452
Pourtahmasi K, Lotfiomran N, Bräuning A, Parsapajouh D (2011) Tree-ring width and vessel characteristics of Oriental beech (Fagus orientalis) along an altitudinal gradient in the Caspian forests, Northern Iran. IAWA J 32:461–473
Price MF, Gratzer G, Duguma LA, Kohler T, Maselli D, Rosalaura R (2011) Mountain forests in a changing world-realizing values, addressing challenges. FAO/MPS and SDC, Rome
Rehm EM, Olivas P, Stroud J, Feeley KJ (2015) Losing your edge: climate change and the conservation value of range-edge populations. Ecol Evol 5:4315–4326
Rinn F (2003) TSAP-Win. Time series analysis and presentation for dendrochronology and related applications for Microsoft Windows, version 4.64. http://www.rinntech.de/content/view/17/48/lang,english/index.html. Accessed 15 Dec 2016
Rita A, Cherubini P, Leonardi S, Todaro L, Borghetti M (2015) Functional adjustments of xylem anatomy to climatic variability: insights from long-term Ilex aquifolium tree-ring series. Tree Physiol 35:817–828
Rodríguez-Ramírez EC, Sánchez-González A, Ángeles-Pérez G (2013) Current distribution and coverage of Mexican beech forests Fagus grandifolia subsp. mexicana in Mexico. Endanger Species Res 20:205–216
Rodríguez-Ramírez EC, Sánchez-González A, Ángeles-Pérez G (2016) Relationship between vegetation structure and microenvironment in Fagus grandifolia subsp. mexicana forest relicts in Mexico. J Plant Ecol 138:1–11
Rodríguez-Ramírez EC, Luna-Vega I, Rozas V (2018) Tree-ring research of Mexican beech (Fagus grandifolia subsp. mexicana) a relict tree endemic to eastern Mexico. Tree Ring Res 74:1
Rossi L, Sebastiani L, Tognetti R, d´Andria R, Morelli G, Cherubini P (2013) Tree-ring wood anatomy and stable isotopes show structural and functional adjustments in olive trees under different water availability. Plant Soil 372:567–579
Rozas V (2001) Detecting the impact of climate and disturbances on tree-rings of Fagus sylvatica L. and Quercus robur L. in a lowland forest in Cantabria, Northern Spain. Ann For Sci 58:237–251
Rozas V, Camarero JJ, Sangüesa-Barreda G, Souto M, García-González I (2015) Summer drought and ENSO-related cloudiness distinctly drive Fagus sylvatica growth near the species rear-edge in norther Spain. Agric For Meteorol 201:153–164
Rozas V, Le Quesne C, Muñoz A, Puchi P (2016) Climate and growth of Podocarpus salignus in Valdivia. Chile Dendrobiol 76:3–11
Rzedowski J (2015) Catálogo preliminar de las especies de árboles silvestres de la Sierra Madre Oriental. In: Flora del Bajío y de regiones adyacentes, fascículo complementario XXX. Instituto de Ecología. A.C. Centro Regional del Bajío Pátzcuaro, Michoacán, Mexico City
Sass U, Eckstein D (1995) The variability of vessel size in beech (Fagus sylvatica L.) and its ecophysiological interpretation. Trees 9:247–252
Sawada H, Kaji M, Oomura K, Igarashi Y (2008) Influences of mast seedling on tree growth dynamics of Fagus crenata and Fagus japonica in central Honshu, Japan. J Jpn For Soc 90:129–136
Schoene DHF, Bernier PY (2012) Adapting forestry and forest to climate change: a challenge to change the paradigm. For Policy Econ 24:12–19
Schweingruber FH (1996) Tree ring and environment: dendroecology. Paul Haupt AG Berne, Switzerland
SEMARNAT, Secretaría del Medio Ambiente y Recursos Naturales (2010) Norma Oficial Mexicana NOM-059-SEMARNAT-2010. Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Diario Oficial de la Federación. Segunda Sección, México, Distrito Federal [online]. http://www.profepa.gob.mx/innovaportal/file/435/1/NOM_059_SEMARNAT_2010.pdf. Accessed 06 Apr 2016
Speer JH (2001) Oak mast history from dendrochronology: a new technique demonstrated in the southern Appalachian region. Dissertation, University of Tennessee, Knoxville, USA
Speer JH (2010) Fundamentals of tree ring research. University of Arizona Press, Tucson
Speer JH, Bräuning A, Zhang Q, Pourtahmasi K, Gaire NP, Dawadi B et al (2016) Pinus roxburghii stand dynamics at a heavily impacted site in Nepal: research through an educational fieldweek. Dendrochronologia 41:2–9
Stokes MA, Smiley TL (1968) An introduction to tree-ring dating. University of Chicago Press, Chicago
Suzuki W, Osumi K, Masaki T (2005) Mast seeding and its spatial scale in Fagus crenata in northern Japan. For Ecol Manag 205:105–116
Téllez-Valdés O, Dávila-Aranda P, Lira-Saade R (2006) The effects of climate change on the long-term conservation of Fagus grandifolia var. mexicana, an important species of the cloud forest in eastern Mexico. Biodivers Conserv 15:1095–1107
Tinoco-Rueda JA, Toledo-Medrano ML, Carrillo-Negrete IJ, Monterroso-Rivas I (2009) Clima y variabilidad climática en los municipios de Hidalgo con presencia de bosque mesófilo de montaña. In: Monterroso-Rivas AJ (ed) El bosque mesófilo en el estado de Hidalgo. Perspectiva ecológica frente al cambio climático. Universidad Autónoma Chapingo, Mexico City, pp 71–98
Tyree MT, Zimmermann MH (2002) Xylem structure and the ascent of sap. Springer, Berlin
Vacchiano G, Hacket-Pain A, Turco M, Motta R, Maringer J, Conedera M, Drobyshev I, Ascoli D (2016) Spatial patterns and broad-scale weather cues of beech mast seeding in Europe. New Phytol 215:595–608
Venegas-González A, von Arx G, Chagas MP, Filho MT (2015) Plasticity in xylem anatomical traits of two tropical species in response to intra-seasonal climate variability. Trees 29:423–435
von Arx G, Kueffer C, Fonti P (2013) Quantifying plasticity in vessel grouping added value from the image analysis tool Roxas. IAWA J 34:433–445
Wason JW, Dovciak M, Beier CM, Battles JJ (2017) Tree growth is more sensitive than species distributions to recent changes in climate and acidic deposition in the northeastern United States. J Appl Ecol 54:1648–1657
Webster GL (1995) The panorama of Neotropical cloud forest. In: Churchill SP, Balslev H, Forero E, Luteyn JL (eds) Biodiversity and conservation of Neotropical Montane Forests. The New York Botanical Garden, New York, pp 53–57
Williams-Linera G, Rowden A, Newton AC (2002) Distribution and stand characteristics of relict populations of Mexican beech (Fagus grandifolia var. mexicana). Biol Cons 109:27–36
Wood SN (2000) Modelling and smoothing parameter estimation with multiple quadratic penalties. J R Stat Soc Ser B 62:413–428
Yin J, Fridley JD, Smith MS, Bauerle TL (2016) Xylem vessel traits predict the leaf phenology of native and non-native understorey species of temperate deciduous forests. Funct Ecol 30:206–214
Acknowledgements
We wish to thank Osvaldo Franco-Ramos and Lorenzo Vázquez-Selem for their help with tree-ring measurements and for lending the necessary equipment; Susana Guzmán Gómez and María del Carmen Loyola Blanco (Laboratorio de Microscopía y Fotografía de la Biodiversidad II, Instituto de Biología, UNAM) for technical assistance with the digital photographs; Othón Alcántara-Ayala and Rodrigo Ortega García for their support during field work; Ana Paola Martínez-Falcón for assistance with the statistical analyses; Santiago Ramírez-Barahona and Carlos Solís Hay for his critical observations. This research was financed by the project PAPIIT IN223218. The first author thanks the financial support granted by the postdoctoral fellowship DGAPA-UNAM 2015-2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by A. Bräuning.
Rights and permissions
About this article
Cite this article
Rodríguez-Ramírez, E.C., Terrazas, T. & Luna-Vega, I. The influence of climate on the masting behavior of Mexican beech: growth rings and xylem anatomy. Trees 33, 23–35 (2019). https://doi.org/10.1007/s00468-018-1755-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-018-1755-3