Abstract
Key message
The total NSC concentration in the roots declined more significantly than in the above-ground tissues/organs under drought treatment, and the level did not return to that of the control after re-watering.
Abstract
Non-structural carbohydrates (NSC) reflect the relative balance between C-gain (photosynthesis) and C-loss (respiration) and play a pivotal role in carbon cycling in a forest ecosystem. However, little is known regarding the effects of extreme drought and re-watering on the NSC status in different tissues/organs. This study examined the variation in NSC concentrations in different tissues/organs and the total NSC pool sizes in Chinese fir (Cunninghamia lanceolata) saplings after drought and re-watering. Results showed that significant differences were observed in the concentrations of total NSC and its components in the different tissues/organs. For example, the NSC concentrations were nine times higher in bark than in stemwood. Moreover, the responses of NSC and its components to extreme drought also varied in different tissues/organs. Drought either significantly increased or maintained the total NSC concentration in the above-ground tissues/organs. By contrast, drought reduced the total NSC concentration in the sapling roots. Furthermore, the results also showed that extreme drought leads to sapling death, which is supported by the result of needle staining and the failure of the total NSC concentration to recover after re-watering. The concentrations of NSC and its components further decreased, and a more pronounced decline was observed in the roots than in the above-ground tissues/organs after re-watering. We speculated that drought can cause failure in carbon translocation between the above- and below-ground tissues/organs and thus cause varied responses of different tissues/organs to extreme drought and re-watering. Overall, these findings suggest the need to investigate the potential differential responses of various tissues/organs to climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-structural carbohydrates (NSC) play a pivotal role in carbon cycling in forest ecosystems; in particular, NSC acts as a buffer for insufficient source activity in trees (Hoch et al. 2003; Zhu et al. 2012; Yang et al. 2015), thereby allowing plant survival under stressful conditions (Poorter and Kitajima 2007; O’Brien et al. 2014) and facilitating recovery after disturbances (Iwasa and Kubo 1997). The concentration and pool size of NSC reflect the relative balance between C-gain (photosynthesis) and C-loss (respiration) (Chapin et al. 1990). Therefore, a comprehensive understanding about the variation in NSC status and the response of NSC concentration to stressful conditions is critical in predicting the future carbon cycling as part of the effort to address global climate change (McDowell et al. 2008; Li et al. 2013).
The frequency and severity of drought events continue to increase because of climate change and unreasonable human activities (IPCC 2007). The response of carbon cycling in forest ecosystems to drought has recently received much attention (Zhao and Running 2010; Zeppel et al. 2011). During drought, trees downregulate the carbon assimilation rates by reducing stomatal conductance (Farquhar and Sharkey 1982, Zang et al. 2014), but drought does not significantly reduce maintenance respiration (Meir et al. 2008). Therefore, stored carbon reserves may be remobilized to fuel the maintenance respiration (McDowell et al. 2008; McDowell and Sevanto 2010). However, contradictory results on the responses of NSC to experimental drought have been observed. Some studies have reported that the NSC concentration increases during drought (Würth et al. 2005; Galvez et al. 2011; Liu et al. 2015), whereas other reports have found that the NSC concentration decreases (Sayer and Haywood 2006; Anderegg 2012; Adams et al. 2013) or remains constant (Pizarro and Bisigato 2010, Gruber et al. 2012) under the same condition.
These discrepancies can be partly attributed to the species-specific ability to reach different depths of water resources (Nardini et al. 2016), the severity of drought (Zhang et al. 2015) or the potential differential responses across different tissues/organs (e.g. leaves, bark, stemwood and roots) to drought (Hartmann et al. 2013a). Hartmann et al. (2013a) reported that in Norway spruce saplings, the effect of drought on the NSC concentration is less prominent in the above-ground tissues/organs than in the roots. Dichio et al. (2009) found that the NSC concentration in thin roots of olive saplings is more sensitive to drought than that in medium roots. However, the majority of recent studies focused on the effect of drought on the NSC status in individual tissues/organs, such as leaves (Rodríguez-Calcerrada et al. 2011; Adams et al. 2013; Liu et al. 2015) or roots (Galvez et al. 2011). To date, few studies have investigated the drought response of NSC concentration in different tissues/organs (Hartmann et al. 2013a), particularly to extreme drought and re-watering.
Chinese fir (Cunninghamia lanceolata) is a fast-growing timber species that has been widely planted to meet increasing timber demand. In recent decades, drought occurred frequently in subtropical China (Zhai et al. 2010; Lu et al. 2011; Yang et al. 2012), where Chinese fir plantations have been widely established. However, little is known about the effects of extreme drought on the NSC status in Chinese fir. Therefore, the present study examined the variation in NSC concentrations among different tissues/organs and the total NSC pool size in Chinese fir saplings after exposure to extreme drought and re-watering treatments. This study aims to investigate the difference in NSC concentrations among different tissues/organs and to analyze the effects of drought and re-watering on the NSC concentrations in these tissues/organs. Our specific objectives are to test the following hypotheses: (1) there are significant differences in the NSC concentrations among different tissues/organs; and (2) the NSC concentration in roots is more sensitive to drought than above-ground tissues/organs.
Materials and methods
Study site and experimental design
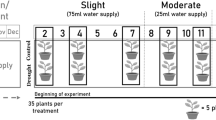
This study was conducted in the Huitong Experimental Station of Forest Ecology (26°40′–27°09′ N and 109°26′–110°08′ E) of the Chinese Ecological Research Network in Huitong County, Hunan Province, China. The mean annual temperature of the site is 16.5 °C, with a monthly mean temperature ranging from 1.9 °C in January to 29.0 °C in July. The site receives an annual rainfall of approximately 1200 mm and 1 month to 2 months of summer drought that usually begins in late July (Wang et al. 2013). In late March 2013, 50 one-year-old Chinese fir saplings with local provenance were obtained from a nursery garden and transferred into plastic pots (diameter 35 cm; height 30 cm). These plastic pots were perforated at the bottom to allow water drainage. Plastic pots were filled with homogenized surface soil obtained from a Chinese fir plantation. A translucent roof with a height of 2.0 m was installed over the potted saplings to prevent throughfall. All pots were manually watered to field capacity for 2–3 days based on the amount of water lost via evapotranspiration, which is determined using the change in individual pot weight. All pots were randomly arranged daily to ensure the exposure of all saplings to homogeneous conditions.
In early July 2013, a total of 40 healthy saplings were selected in this drought and re-watering experiments. The selected sample saplings were relatively uniform based on sapling height and ground diameter. On July 20, 2013, eight randomly chosen saplings were harvested carefully, stored immediately in a cool box, and then transported into the laboratory for dry mass and NSC analyses. Afterward, drought was initiated. Two water regimens were imposed, namely, control and drought (suspension of watering). Up to 16 saplings per treatment were used for 30 days. On August 20, 2013, eight randomly chosen saplings in each treatment were harvested for laboratory dry mass and NSC analyses. The eight remaining saplings in the drought treatment were watered and maintained at or near field capacity for re-watering treatment (drought-CK). The eight remaining saplings in the control treatment served as control (CK–CK). All saplings in the different treatments were harvested on September 10, 2013 for laboratory analysis. To minimize the diurnal variation in carbohydrate concentration (Morin et al. 2011), the saplings were harvested between 8:00 and 9:00 at each occasion. Throughout the drought and soil re-watering periods, volumetric water content was determined using a handheld time–domain reflectometer (TRIME-PICO TDR, Imko Company, Germany).
Biomass and NSC analyses
In the laboratory, all saplings harvested on July 20, August 20, and September 10, 2013 were immediately separated into the needles, stems (including the axis of twigs) and roots. The needles were further categorized into current-year needles (CN) and 1-year-old needles (ON). Bark (BA) also was separated manually from stemwood (SW) for each stem sample. The roots were divided in two groups: coarse roots (with a diameter ≥2 mm; CR) and fine roots (with a diameter <2 mm; FR). All samples obtained from each tissue/organ were placed in the microwave at 600 W for 90 s to denature the enzymes (Hoch et al. 2003) and were subsequently oven heated at 70 °C for at least 72 h until a consistent weight was obtained. The biomass (dry mass) of each tissue was then recorded. Each dried sample was milled and stored at 4 °C prior to NSC analyses.
NSC analyses were conducted using four replicates for each treatment (4 randomly selected saplings from a total of 8 saplings). The NSC are defined here as soluble sugar (sucrose, glucose and fructose) plus starch. Soluble sugar and starch analysis was performed following the method described by Yang et al. (2015). About 0.1 g milled sample was suspended in 10 mL of aqueous ethanol (80 % v/v) and incubated in a water bath shaker at 80 °C for 10 min. The sample was centrifuged at 3000 rpm for 10 min. Supernatant and residues were obtained for soluble sugar and starch analyses. Soluble sugar was measured enzymatically by the K-SUFRG Kit (Megazyme, Wicklow, Ireland) (Bergmeyer et al. 1988; McCleary et al. 1997). d-glucose concentration was quantified using hexokinase and glucose-6-phosphate-dehydrogenase. Nicotinamide adenine dinucleotide phosphate (NADPH) absorbance was measured at 340 nm using a spectrophotometer (Hitachi Ltd, Tokyo, Japan). Subsequently, d-fructose concentration was determined following isomerization by phosphoglucose isomerase. Sucrose concentration is the difference in d-glucose content before and after sucrose hydrolysis by β-fructosidase (invertase). If the amount of soluble sugars per sample is higher than the threshold concentration of NADPH, the sample solution must be diluted. Starch was measured enzymatically by the K-TSTA Kit (Megazyme, Wicklow, Ireland) (Bergmeyer et al. 1988; McCleary et al. 1997). Starch was hydrolyzed to maltodextrin by α-amylase and was further hydrolyzed to d-glucose by amyloglucosidase. Starch concentration was then determined by absorbance measurement at 510 nm after adding a glucose oxidase/peroxidase reagent.
Data analysis
One-way ANOVA was used to test the differences in the soluble sugar, starch, and total NSC concentrations among the different tissues/organs before drought treatment. Two-way ANOVA was used to test the effect of drought treatment, different tissues/organs, and their interaction on the total NSC and its components concentrations during the drought and re-watering periods. Whole-sapling NSC pool sizes (gram per sapling) were calculated as the sum of the NSC contents (the products of NSC concentrations and tissue biomasses.) in different tissues/organs. NSC fraction indicates the relative allocation of NSC content to different tissues/organs. All analyses were performed using SPSS software (SPSS 17.0 for Windows, SPSS Inc., Chicago, IL, USA).
Results
Soil water content
No significant difference in soil moisture was observed before drought treatment (P > 0.05). After 12 days of drought treatment, soil moisture decreased progressively with drought time, dropping by 51.8 and 56.2 % in drought and drought-CK treatments, respectively (Fig. 1). After 26 days of drought, the corresponding soil moisture was 80.8 and 81.6 % in drought and drought-CK treatments, respectively (Fig. 1). After re-watering, soil moisture returned to the control level (Fig. 1).
Differences in NSC concentrations among tissues/organs
Significant difference in soluble sugar concentration was observed among the tissues/organs (P < 0.05) harvested on July 20, 2013 (before drought treatment). The soluble sugar concentrations in different tissues/organs ranged from 13.91 mg/g to 135.74 mg/g and were found in the following increasing sequence: SW < FR < CR < CN < BA < ON (Fig. 2a). Significant difference in starch concentration was also observed among the tissues/organs (P < 0.05). However, the sequence of increasing starch concentration (SW < CN < ON < FR < BA < CR) was different from that of soluble sugar concentration (Fig. 2b). A similar pattern of soluble sugar concentration was observed in total NSC concentration of the different tissues/organs because of the negligible contribution of starch to the total NSC. Similar to the soluble sugar concentration, we found that the total NSC concentration in BA was nearly nine times higher than that in SW (Fig. 2c).
Soluble sugar (a), starch (b) and total NSC concentrations (c) in different tissues/organs of Chinese fir saplings before drought treatment (July 20, 2013). Values are mean ± SE (n = 4). Different lowercase letters indicate significant differences among tissues/organs (P < 0.05). CN current-year needles, ON 1-year-old needles, BA barks, SW stemwoods, CR coarse roots, FR fine roots
Response of NSC concentration to drought and re-watering
A significant interactive effect of drought and tissues/organs on soluble sugar concentrations was observed (Fig. 3a; Table 1), thereby indicating that the responses of soluble sugars of the different tissues/organs to drought significantly varied. No statistical difference (P > 0.05) in soluble sugar concentration in CN and ON was observed between CK and drought treatment. However, the soluble sugar concentration increased significantly in BA and SW and decreased significantly in CR and FR after drought treatment (Fig. 3a). A significant interactive effect of drought and tissues/organs on starch concentrations was observed (Fig. 3b; Table 1). In contrast to the soluble sugar concentration, the starch concentration in CN and ON showed a significant and pronounced decline (Fig. 3b). Moreover, the responses of the total NSC concentration to drought significantly varied among the different tissues/organs (Fig. 3c). The reduction in the total NSC concentration was more remarkable in the roots than in the needles and barks (Fig. 3c).
Responses of soluble sugar (a), starch (b), and total NSC concentrations (c) in different tissues/organs of Chinese fir saplings to drought (August 20, 2013). Values are mean ± SE (n = 4). The asterisks indicate significant differences between the control and drought treatments in individual tissue. *P < 0.05; **P < 0.01; ***P < 0.001. CN current-year needles, ON one-year-old needles, BA barks, SW stemwoods, CR coarse roots, FR fine roots
After re-watering, the soluble sugar, starch, and total NSC concentrations in the drought-CK treatment did not recover compared with the control levels (Fig. 4). The soluble sugar concentrations in all tissues/organs under the drought-CK treatment remained significantly lower (P < 0.01) than those under the CK–CK treatment, except in SW (P > 0.05), as indicated by a significant interactive effect of re-watering and tissues/organs (Table 2). Moreover, the change in the starch concentration was generally less dynamic in needles than in other tissues/organs (Fig. 4b; P < 0.05). The total NSC concentrations of all the tissues/organs further decreased after re-watering compared with those under drought treatment. Furthermore, the decline in the total NSC concentration was more pronounced in the roots than in the above-ground tissues/organs (Fig. 4c).
Responses of soluble sugar (a), starch (b), and total NSC concentrations (c) in different tissues/organs of Chinese fir saplings to drought recovery treatments (September 10, 2013). Values are mean ± SE (n = 4). The asterisks indicate the significant differences between the control (CK–CK) and drought recovery (drought-CK) treatments in individual tissue. *P < 0.05; **P < 0.01; ***P < 0.001. CN current-year needles, ON one-year-old needles, BA barks, SW stemwoods, CR coarse roots, FR fine roots
Total NSC pool and NSC fraction
Drought and re-watering showed a negligible effect on biomass (Fig. 5a). Compared with the control treatment, the total NSC pool size in the drought treatment obviously decreased by 24.5 %. However, no significant difference was found in the total NSC pool size between the control and drought treatments (Fig. 5b). After re-watering, the total NSC pool size further decreased, and a significant difference was detected between the CK–CK and drought-CK treatments (P < 0.05). NSC fractions (relative allocation of NSC content) in different tissues/organs varied significantly under the control treatment (P < 0.05) and were found in the following decreasing sequence: BA > ON > CN > CR > FR > SW (Fig. 5c). The pattern of NSC allocation in the drought and drought-CK treatments differed from that in the control treatment. The NSC allocation to below-ground tissues/organs decreased significantly in the drought treatment (Fig. 5c). After re-watering, the NSC allocation to below-ground tissues/organs did not recover to the control level. Moreover, the total NSC allocation to the needles decreased (Fig. 5c).
Responses of the biomass (a), total NSC pool size (b) and NSC fraction (c) to drought and re-watering. NSC fraction indicates the relative allocation of NSC content to different tissues/organs. Values are mean ± SE (n = 4). The lowercase letters indicate significant differences in the different treatments (P < 0.05)
Discussion
Differences in NSC concentrations in different tissues/organs
Significant differences were observed in the NSC concentrations among different tissues/organs most likely due to the differences in the physiological functions of each tissue and in the variations in NSC components. Large variations in the NSC concentrations among different tissue types have been reported (Li et al. 2001). Needles are the physical “platform” for photosynthesis, i.e., the manufacture of sugars. Soluble sugars, especially glucose, are the major NSC component in needles. No significant differences in the NSC concentrations were found among needles of saplings in different age classes. This result is contrary to the report of Li et al. (2001), who found that current-year needles display significantly lower NSC concentrations than the two-year-old needles of Pinus cembra. This discrepancy may largely be attributed to the differences between the plants used. We used saplings, whereas Li et al. (2001) used adult trees. The effect of needle age on needle traits depends on the light availability in the canopy of adult trees (Eimil-Fraga et al. 2015). In our study, a lack of significant differences in the NSC concentrations among needles of saplings in different age classes is most likely due to the similar light availability for saplings, especially those grown in pots. The NSC concentrations were significantly higher in the bark or phloem of P. cembra (Li et al. 2002), Populus tremuloides (Landhäusser and Lieffers 2003), P. cembra (L.), and Larix decidua (Gruber et al. 2013) than those in xylem wood, confirming the significant differences we found in the NSC concentrations in barks and sapwoods. Zhang et al. (2014) also reported that the NSC concentrations were significantly higher in the bark than in the woods of 12 temperate tree species. The higher NSC concentrations in the bark than in xylem wood can be ascribed to the high metabolic activity of the barks and to their function in long-distance transport of carbohydrates, as indicated by the high sucrose concentration in the present study. Roots, especially CR, are important storage reserve of NSC. Such function reflects the buffering capacity of trees against various stresses. This characteristic might have caused higher NSC concentrations in CR than in FR as revealed in our study. Fan and Guo (2010) reported that the NSC concentrations were significantly higher in higher-order roots (large diameter) than in lower-order roots (small diameter); these results are similar to ours in this study.
Response of NSC to drought and re-watering
The responses of NSC and its components to drought varied among the different tissues/organs. The total NSC concentrations in the needles decreased under drought. Coincidentally, the soluble sugar concentration increased or remained unchanged, whereas starch concentration decreased. This outcome is consistent with previous results obtained (Lee et al. 2008; Hartmann et al. 2013a). Similar to needles, the bark demonstrated increased soluble sugar concentrations at the expense of starch under drought treatment. This phenomenon may largely be explained by the mechanism of osmotic adjustment to increase drought tolerance (Wang and Stutte 1992) because monosaccharides, being the most important osmoticum for adjustment, can significantly increase the cell osmotic pressure. Our work did not find a reduction but rather detected a significant increase in the total NSC concentrations in SW under drought treatment. Moreover, SW demonstrated greater responses to drought than BA. This finding is contrary to the view that phloem or bark demonstrates high activity and that their carbohydrate availability may reflect the altered carbon sink–source balance under stress. Recent studies have focused on the difference in NSC reserves between phloem and xylem (Anderegg et al. 2012; Landhäusser and Lieffers 2003; Li et al. 2002; Gruber et al. 2013), whereas little attention has been given to the response of phloem and xylem NSC contents to drought. Therefore, the underlying response mechanisms must be investigated further. The total NSC concentration in the roots under drought treatment declined more significantly than in the above-ground tissues/organs, with a reduction of approximately 56 %. Similar to our findings, recent results have shown that severe drought decreases root NSC concentrations (Hartmann et al. 2013a, b). Moreover, drought is likely to reduce carbon assimilation (Brodribb and McAdam 2011) and impede carbon translocation (Hartmann et al. 2013a, b). This phenomenon explains the progressive depletion of in situ carbon reserves in the roots observed in the present study, as indicated by the concurrent pronounced decrease in starch and soluble sugar concentrations.
Sapling death occurred in the drought-CK treatment as confirmed by the result of needle staining. The outcome was further supported by the failure of the total NSC concentration in the drought-CK treatment to recover after re-watering. Interestingly, we found that NSC concentration significantly declined both in the roots and above-ground tissues/organs, except in SW, after re-watering. However, this effect was less prominent in the above-ground tissues/organs than in the roots, as indicated by the significant interactive effect of drought and tissues/organs on NSC concentration. Hartmann et al. (2013a, b) found a different response of NSCs in the above-ground tissues/organs and roots of Norway spruce saplings to lethal drought, and their finding is consistent with our results. They suggested that declining plant water potential caused by drought can result in xylem cavitation and failure in phloem function (carbon translocation), thereby causing local depletion of root carbon reserves. The current study did not measure the predawn leaf water potential, phloem turgor pressure, and xylem cavitation. Therefore, testing the carbon translocation failure from the above-ground tissues/organs to the roots was impossible. Carbon starvation occurs when the supply of available carbohydrate falls below metabolic requirements, which results from stomatal closure to avoid water deficiency after extreme drought (McDowell et al. 2008, Sevanto et al. 2014). Our study found that the NSC concentration considerably declined in the roots and either increased or remained the same in the above-ground tissues/organs during drought treatment. Moreover, the NSC concentration both in the above-ground tissues/organs and roots did not return to the control level after re-watering. Our results also indicated that carbon starvation may occur only in the roots and not in the above-ground tissues/organs. However, mortality of Chinese fir sapling is not only caused by carbon starvation in roots. Carbohydrate metabolism and plant hydraulics are mutually dependent (McDowell et al. 2011; Anderegg and Callaway 2012; Mitchell et al. 2013; De la Serrana et al. 2015; Nardini et al. 2016). Further studies are required to simultaneously study carbohydrate metabolism and plant hydraulics in the above- and below-ground tissues/organs in response to drought (Hartmann et al. 2013a).
Conclusions
The differences in the physiological functions of each tissues/organs caused significant differences in the NSC concentrations in various tissues/organs. We confirmed the effect of extreme drought on the varying NSC concentrations in the above- and below-ground tissues/organs. The NSC concentration significantly declined both in the above- and below-ground tissues/organs after re-watering, and a more pronounced decline in NSC concentration was observed in the roots. We speculated that the drought-induced failure of the phloem functions resulted in the uncoupling of the root system from the above-ground tissues/organs; thus, the aboveground tissues/organs and the roots responded differently to drought stress. However, the degree by which the results we obtained from the saplings will apply to mature trees is unknown. Further studies are needed to validate the coupling of the above- and below-ground tissues/organs and the response of this coupling to extreme drought in natural ecosystems or field experiments.
Author contribution statement
Qingpeng Yang participated in the experimental design, data analyses, and writing of the paper. Weidong Zhang participated in statistical analyses, discussion, and writing of the paper. Renshan Li carried out most of the experiments and participated in data analyses. Ming Xu participated in the experimental design and revised the manuscript. Silong Wang designed and directed the study.
References
Adams HD, Germino MJ, Breshears DD, Barron-Gafford GA, Guardiola-Claramonte M, Zou CB, Huxman TE (2013) Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol 197:1142–1151
Anderegg WRL (2012) Complex aspen forest carbon and root dynamics during drought. Clim Change 111:983–991
Anderegg WRL, Callaway ES (2012) Infestation and hydraulic consequences of induced carbon starvation. Plant Physiol 159:1866–1874
Anderegg WRL, Berry JA, Smith DD, Sperry JS, Anderegg LDL, Field CB (2012) The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc Natl Acad Sci USA 109:233–237
Bergmeyer HU, Bergmeyer J, Grassl M (1988) Methods of enzymatic analysis. VCH Publishers (UK) Ltd. Cambridge, UK
Brodribb TJ, McAdam SAM (2011) Passive origins of stomatal control in vascular plants. Science 331:582–585
Chapin FS III, Schulze ED, Mooney HA (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21:423–447
De la Serrana RG, Vilagrosa A, Alloza JA (2015) Pine mortality in southeast Spain after an extreme dry and warm year: interactions among drought stress, carbohydrates and bark beetle attack. Trees 29:1791–1804
Dichio B, Margiotta G, Xiloyannis C, Bufo SA, Sofo A, Cataldi TRI (2009) Changes in water status and osmolyte contents in leaves and roots of olive plants (Olea europaea L.) subjected to water deficit. Trees 23:247–256
Eimil-Fraga C, Sánchez-Rodríguez F, Álvarez-Rodríguez E, Rodríguez-Soalleiro R (2015) Relationships between needle traits, needle age and site and stand parameters in Pinus pinaster. Trees 29:1103–1113
Fan PP, Guo DL (2010) Slow decomposition of lower order roots: a key mechanism of root carbon and nutrient retention in the soil. Oecologia 163:509–515
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Galvez DA, Landhäusser SM, Tyree MT (2011) Root carbon reserve dynamics in aspen seedlings: does simulated drought induce reserve limitation? Tree Physiol 31:250–257
Gruber A, Pirkebner D, Florian C, Oberhuber W (2012) No evidence for depletion of carbohydrate pools in Scots pine (Pinus sylvestris L.) under drought stress. Plant Biology 14:142–148
Gruber A, Pirkebner D, Oberhuber W (2013) Seasonal dynamics of mobile carbohydrate pools in phloem and xylem of two alpine timberline conifers. Tree Physiol 33:1076–1083
Hartmann H, Ziegler W, Kolle O, Trumbore S (2013a) Thirst beats hunger—declining hydration during drought prevents carbon starvation in Norway spruce saplings. New Phytol 200:340–349
Hartmann H, Ziegler W, Trumbore S (2013b) Lethal drought leads to reduction in nonstructural carbohydrates in Norway spruce tree roots but not in the canopy. Funct Ecol 27:413–427
Hoch G, Richter A, Körner C (2003) Non-structural carbon compounds in temperate forest trees. Plant Cell Environ 26:1067–1081
IPCC (2007) Climate change 2007: the physical sciences basis: contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Iwasa Y, Kubo T (1997) Optimal size of storage for recovery after unpredictable disturbances. Evol Ecol 11(1):41–65
Landhäusser SM, Lieffers VJ (2003) Seasonal changes in carbohydrate reserves in mature northern Populus tremuloides clones. Trees 17:471–476
Lee BR, Jin YL, Jung WJ, Avice JC, Morvan-Bertrand A, Ourry A, Park CW, Kim TH (2008) Water-deficit accumulates sugars by starch degradation-not by de novo synthesis—in white clover leaves (Trifolium repens). Physiol Plantarum 134:403–411
Li MH, Hoch G, Körner C (2001) Spatial variability of mobile carbohydrates within Pinus cembra trees at the alpine treeline. Phyton 41:203–213
Li MH, Hoch G, Körner C (2002) Source/sink removal affects mobile carbohydrates in Pinus cembra at the Swiss treeline. Trees 16:331–337
Li MH, Cherubini P, Dobbertin M, Arend M, Xiao WF, Rigling A (2013) Responses of leaf nitrogen and mobile carbohydrates in different Quercus species/provenances to moderate climate changes. Plant Biology 15:177–184
Liu CG, Wang YJ, Pan KW, Jin YQ, Liang J, Li W, Zhang L (2015) Photosynthetic carbon and nitrogen metabolism and the relationship between their metabolites and lipid peroxidation in dwarf bamboo (Fargesia rufa Yi) during drought and re-watering. Trees 29:1633–1647
Lu E, Luo Y, Zhang R, Wu Q, Liu L (2011) Regional atmosphere anomalies responsible for the 2009–2010 severe drought in China. J Geophys Res 116:D2114
McCleary BV, Gibson TS, Mugford DC (1997) Measurement of total starch in cereal products by amyloglucosidase-α-amylase method: collaborative study. J AOAC Int 80:571–579
McDowell NG, Sevanto S (2010) The mechanisms of carbon starvation: how, when, or does it even occur at all? New Phytol 186:264–266
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M (2011) The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Eco Evol 26:523–532
Meir P, Metcalfe DB, Costa ACL, Fisher RA (2008) The fate of assimilated carbon during drought: impacts on respiration in Amazon rainforests. Phil Trans R Soc B 363:1849–1855
Mitchell PJ, O′Grady AP, Tissue DT, White DA, Ottenschlaeger ML, Pinkard EA (2013) Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol 197:862–872
Morin C, Bélanger G, Tremblay GF, Bertrand A, Castonguay Y, Drapeau R, Michaud R, Berthiaume R, Allard G (2011) Diurnal variations of nonstructural carbohydrates and nutritive value in Alfalfa. Crop Sci 51:1297–1306
Nardini A, Casolo V, Dal Borgo A, Savi T, Stenni B, Bertoncin P, Zini L, McDowell NG (2016) Rooting depth, water relations and non-structural carbohydrate dynamics in three woody angiosperms differentially affected by an extreme summer drought. Plant Cell Environ 39:618–627
O’Brien MJ, Leuzinger S, Philipson CD, Tay J, Hector A (2014) Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat clim change 4:710–714
Pizarro LC, Bisigato AJ (2010) Allocation of biomass and photoassimilates in juvenile plants of six Patagonian species in response to five water supply regimes. Ann Bot-london 106:297–307
Poorter L, Kitajima K (2007) Carbohydrate storage and light requirements of tropical moist and dry forest tree species. Ecology 88:1000–1011
Rodríguez-Calcerrada J, Shahin O, Carmen del Rey M, Rambal S (2011) Opposite changes in leaf dark respiration and soluble sugars with drought in two Mediterranean oaks. Funct Plant Biol 38:1004–1015
Sayer MAS, Haywood JD (2006) Fine root production and carbohydrate concentrations of mature longleaf pine (Pinus palustris P. Mill.) as affected by season of prescribed fire and drought. Trees 20:165–175
Sevanto S, McDowell NG, Turin Dickman L, Pangle R, Pockman WT (2014) How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ 37:153–161
Wang Z, Stutte GW (1992) The role of carbohydrates in active osmotic adjustment in apple under water stress. J Am Soc Hortic Sci 117:816–823
Wang QK, He TX, Wang SL, Liu L (2013) Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest. Agr Forest Meteorol 178–179:152–160
Würth MKR, Pelaez-Riedl S, Wright SJ, Körner C (2005) Non-structural carbohydrate pools in a tropical forest. Oecologia 143:11–24
Yang J, Gong DY, Wang WS, Hu M, Mao R (2012) Extreme drought event of 2009/2010 over southwestern China. Meteorol Atmos Phys 115:173–184
Yang QP, Liu LL, Zhang WD, Xu M, Wang SL (2015) Different responses of stem and soil CO2 efflux to pruning in a Chinese fir (Cunninghamia lanceolata) plantation. Trees 29:1207–1218
Zang U, Goisser M, Grams TEE, Häberle K, Matyssek R, Matzner E, Borken W (2014) Fate of recently fixed carbon in European beech (Fagus sylvatica) saplings during drought and re-watering. Tree Physiol 34:29–38
Zeppel MJB, Adams HD, Anderegg WRL (2011) Mechanistic causes of tree drought mortality: recent results, unresolved questions and future research needs. New Phytol 192:800–803
Zhai J, Liu B, Hartmann H, Su B, Jiang T, Fraedrich K (2010) Dryness/wetness variations in ten large river basins of China during the first 50 years of the 21st century. Quatern Int 226:101–111
Zhang HY, Wang CK, Wang XC (2014) Spatial variations in non-structural carbohydrates in stems of twelve temperate tree species. Trees 28:77–89
Zhang T, Cao Y, Chen YM, Liu GB (2015) Non-structural carbohydrate dynamics in Robinia pseudoacacia saplings under three levels of continuous drought stress. Trees 29:1837–1849
Zhao MS, Running SW (2010) Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 329:940–943
Zhu WZ, Cao M, Wang SG, Xiao WF, Li MH (2012) Seasonal dynamics of mobile carbon supply in Quercus aquifolioides at the upper elevational limit. PLoS One 7(3):e34213. doi:10.1371/journal.pone.0034213
Acknowledgments
This research was funded by the National Basic Research Program of China (973 Program, Grant No. 2012CB416905) and the National Natural Science Foundation of China (Grant Nos. 31570402 and 31200302). We thank two anonymous reviewers for their constructive comments and suggestions. We also thank Lanlan Liu, Bing Fan, Xiuyong Zhang, Zhengqi Shen, Xiaojun Yu, and Ke Huang for their invaluable assistance in the laboratory and the field experiments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by A. Nardini.
Rights and permissions
About this article
Cite this article
Yang, Q., Zhang, W., Li, R. et al. Different responses of non-structural carbohydrates in above-ground tissues/organs and root to extreme drought and re-watering in Chinese fir (Cunninghamia lanceolata) saplings. Trees 30, 1863–1871 (2016). https://doi.org/10.1007/s00468-016-1419-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-016-1419-0