Abstract
Two-year-old olive trees (Olea europaea L., cv. Coratina) were subjected to a 15-day period of water deficit, followed by 12 days of rewatering. Water deficit caused decreases in predawn leaf water potential (Ψw), relative water content and osmotic potential at full turgor (Ψπ100) of leaves and roots, which were normally restored upon the subsequent rewatering. Extracts of leaves and roots of well-watered olive plants revealed that the most predominant sugars are mannitol and glucose, which account for more than 80% of non-structural carbohydrates and polyols. A marked increase in mannitol content occurred in tissues of water-stressed plants. During water deficit, the levels of glucose, sucrose and stachyose decreased in thin roots (with a diameter <1 mm), whereas medium roots (diameter of 1–5 mm) exhibited no differences. Inorganic cations largely contribute to Ψπ100 and remained stable during the period of water deficit, except for the level of Ca2+, which increased of 25% in water-stressed plants. The amount of malate increased in both leaves and roots during the dry period, whereas citrate and oxalate decreased. Thin roots seem to be more sensitive to water deficit and its consequent effects, while medium roots present more reactivity and a higher osmotic adjustment. The results support the hypothesis that the observed decreases in Ψw and active osmotic adjustment in leaves and roots of water-stressed olive plants may be physiological responses to tolerate water deficit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Olive tree (Olea europaea L.) is a woody species typically cultivated in most Mediterranean countries where plants are often exposed to long periods of water deficit during the dry season (Connor and Fereres 2005). Among fruit tree species, olive tree is able to tolerate a broad range of adverse environmental factors, including the low availability of water in soil (Bacelar et al. 2007), salinity (Tattini et al. 1996), chilling and high temperature stress (Bongi and Long 1987) and high irradiance levels (Sofo et al. 2004).
The uncommon capability of adaptation of olive tree against water deficit is due to a variety of morphological and physiological adaptations, such as the regulation of stomata aperture and transpiration (Nogués and Baker 2000), the regulation of gas exchange (Xiloyannis et al. 2004), the appearance of leaf anatomical alterations (Chartzoulakis et al. 1999), the ability of extracting water from the soil due to a deep root system and to a high water potential gradient between canopy and roots (Fernández et al. 1997), and a very developed osmotic adjustment (Chartzoulakis et al. 1999; Dichio et al. 2006). This last contribution describes changes in the osmotic potential of leaves and roots due to accumulation of organic osmolytes confined to the cytoplasmic compartment and vacuoles of cells (Munns 1988).
Osmotic adjustment reduces the osmotic component of the total water potential and allows plant tissues to maintain water even at low xylem and soil water potentials, so maintaining turgor and metabolic activity, and indirectly growth and productivity during prolonged water deficit (Hanson and Hits 1982; Rhodes and Samaras 1994). The recognized metabolic benefits of osmolyte accumulation may depend on either active augmentation of them within cells (i.e., active osmotic adjustment) or loss of water from the plant tissues and the consequent concentration of solutes (i.e., passive osmotic adjustment) or both.
The contribution of passive osmotic adjustment in olive tree varies on the basis of plant physiological conditions and genotypes but it represents approximately a half of the total osmotic adjustment, whereas the other half is due to ex novo synthesis of osmotically active compounds (Dichio et al. 2006). The levels of specific sugar compounds and the extent of their involvement in osmotic adjustment depend on the plant species, genotype within the same species and environmental stress conditions (McCue and Hanson 1990). In particular, studies performed on peach (Prunus persica [L.] Batsch.) (Lo Bianco et al. 2000) have demonstrated the important role of sorbitol and sucrose in plants subjected to water deficit. Osmotic adjustment of Rosaceous fruit trees, such as apple (Malus domestica [L.] Borkh.) (Wang and Stutte 1992) and cherry (Prunus cerasus L. and P. avium x pseudocerasus) (Ranney et al. 1991) during water deficit is mainly accompanied by the accumulation of sorbitol formed by glucose reduction. Mannitol, another alditol, is the most widely distributed hexitol in nature (Williamson et al. 2002) and has been identified as the major active solute, which increases its level in leaves of Fraxinus excelsior L. under water deficit conditions (Guicherd et al. 1997). Other important osmolytes in plants are organic acids (such as malic, oxalic and citric acids), inorganic cations, inorganic anions (mainly sulphate, phosphate and chloride) and some amino acids, such as proline and glycine betaine (Ingram and Bartels 1996; Nuccio et al. 1999).
Understanding the mechanisms by which olive plants tolerate water deficit is essential for selecting more tolerant plant cultivars. The objective of this work was to study the influence of water deficit and a following rewatering on osmolyte contents in olive plants and the contribution of osmolyte accumulation to osmotic adjustment. As there are few reports concerning the carbohydrate and polyols composition and their physiological role in woody plant tissues other than leaves, changes in the osmolyte contents at increasing levels of water deficit were also investigated in olive roots.
Materials and methods
Plant material and water-stress conditions
A group of 50 olive trees (Olea europaea L. cv. Coratina), two year old and own rooted, was transplanted on March 2000 in 18-L pots containing approximately a 2:1 mixture of soil (83.1% sand, 8.8% silt and 9.0% clay) and peat. Cultivar Coratina is indigenous of southern Italy characterized by a high degree of tolerance against water deficit if compared to other olive cultivars and by expanded leaves with an elliptic–lanceolate shape (Xiloyannis et al. 2004). Until 5 July, when water-stress period was initiated, plants were grown outside under natural conditions in Metaponto at ‘Pantanello’ Agricultural Experimental Station (40°24′N, 16°48′E) and were supplied with 3–4 g of slow nitrogen release complex fertilizer (Nitroposka Gold 15N−9P−16K + 2Ca + 7Mg; Compo Agricoltura, Cesano Maderno, MI, Italy) every 25–30 days. Plants were subjected to 15 days of water deficit followed by 12 days of rewatering conditions. Before the onset of water-stress period, each pot was covered with plastic films and aluminium sheets to minimize evaporation from soil surface and to avoid exposure of soil to direct sunlight, respectively. During the plant growth, the soil water content was constantly maintained at about 85% of field water capacity by adding at the end of daily sunlight the amount of water lost through transpiration.
During the period of water deficit, ten plants were used as controls (control plants, CP) and irrigated daily to maintain an optimum soil water content; the remaining plants were stressed (stressed plants, SP) by withholding water as to achieve four stress levels at following predawn leaf water potential (Ψw) values: −0.45 MPa (CP; day 0 from the beginning of water deficit), −1.5 MPa (day 7), −3.9 MPa (day 11) and −6.0 MPa (day 15). CP were maintained at a predawn Ψw of −0.45 MPa during the whole experimental period. The water soil conditions of well-watered plants were restored during the subsequent 12 days of rewatering.
Plant water status
Throughout the periods of water deficit and rewatering, plant water status was determined by concomitant measurements of relative water content (RWC) and Ψw, as they are two significant indicators of the degree of water deficit. The values of Ψw were measured at predawn (04:30 h) on expanded leaves taken from each plant along the median segment of new-growth shoots. Each excised leaf was immediately put inside a polyethylene bag for Ψw measurement with a Sholander pressure chamber (Model 600, PMS Instruments Co. Corvallis, OR, USA). In order to obtain the RWC values of olive leaves and roots, after harvest, the plant tissues were weighed for fresh mass (FM) determination and then placed in beakers sealed with parafilm and hydrated in the dark for 12–24 h. After measurement of saturated mass (SM), the plant samples were dried at 80°C for 48 h to determine dry mass (DM). The RWC values were evaluated as:
Throughout the drought and rewatering treatments, tissues were hydrated for 12–24 h before determination of osmotic potential at full turgor (Ψπ100). Cell contents were extracted using plastic syringes to squeeze homogeneously the tissue and to extrude 100 μL cell-content samples. Each sample was used to determine the osmolarity with a vapour pressure osmometer (Wescor model 2000, Logan, UT, USA) calibrated against a salt solution. The values of Ψπ100 of expressed sap were calculated from the Van’t Hoff relation as given by Nobel (1983):
Active osmotic adjustment (AΔΨπ100), as a result of the net accumulation of osmolytes in the symplast, was defined as the difference between the value of Ψπ100 in stressed plants (SP) and the respective value in control plants (CP).
Plant material preparation
During the periods of water deficit and rewatering, leaves and roots were sampled before dawn to minimize variation in solute accumulation during light exposure. For root sampling, the selected plants were removed from pots, gently separated from the attached soil and carefully washed with distilled water. After sampling, roots were divided in two groups: medium roots (maturation zone; with a diameter (∅) of 1–5 mm) and thin roots (elongation zone; ∅ <1 mm). All plant tissues were immediately stored at −80°C until lyophilization. Samples were collected from three plants having the same Ψw, with each sample containing five fully expanded leaves from the mid-section of current-year shoots and 10–15 g FM of roots. The lyophilized tissues were ground with an IKA analytical mill Model A10 (Aldrich, Milan, Italy) and stored in airtight vials at room temperature until analysis. All the osmolyte concentrations were expressed as mmol per kg of dry mass (DM).
Low molecular weight non-structural carbohydrates and polyols
Low molecular weight non-structural carbohydrates and polyols (NSC–P) were extracted as described by Cataldi et al. (2000). A weighed amount (approximately 50 mg) of lyophilized tissue, added of internal standard 3-O-methylglucopyranose at the concentration of 40 μM, was suspended in 16 mL of deionized water (Milli Q grade, Millipore, Bedford, MA, USA). The suspension was then shaken for 15 min and centrifuged at 3,000 rpm for 10 min (Model 4225, ALC s.r.l., Italy). Prior to analysis, the aqueous extract was filtered through single use 0.22 μm-pore-size nylon filter (Aldrich, St Louis, MO) and passed on a cartridge On Guard A (Dionex, Sunnyvale, CA) to remove anion contaminants. Excellent recoveries were obtained for each sugar constituent in both analyzed olive tissues, which on average ranged from 97 ± 1% for stachiose to 104 ± 3% for glucose.
Carbohydrates and polyols were analyzed by high-performance anion-exchange chromatography (HPAEC) in conjunction with pulsed amperometric detection (PAD) using a DX-500 liquid chromatograph (Dionex). Soluble sugars were separated on a Dionex CarboPac PA1 column (250 × 2 mm id) equipped with a guard column (50 × 2 mm id) and eluted with NaOH 12 mM + Ba(AcO)2 1 mM at a flow rate of 0.4 mL min−1. Column and pre-column were maintained at a temperature of 22 ± 1°C by a water jacket coupled with a circulating water bath model WK4DS (Colora, Messtechnik GmbH, Germany). The following triple-potential waveform was used to detect carbohydrates and polyols as described previously (Cataldi et al. 2000): detection potential, E DET = +0.25 V (t DEL = 240 ms, t INT = 200 ms), oxidation potential E OX = +0.80 V (t OX = 180 ms), reduction potential E RED = −0.25 V (t RED = 360 ms). Low molecular weight non-structural carbohydrates and polyols were identified by comparison with retention times of sugar standards injected under the same experimental conditions. As mannitol and glucose were present at relatively high levels in olive tissue extracts, a 16-fold dilution was usually accomplished before injection.
Inorganic and organic anions
Inorganic and organic anions were extracted with water according to Russo and Karmarkar (1998) and analyzed by ion chromatography with conductivity detection (IC-CD) using a DX-500 liquid chromatograph (Dionex). Inorganic anions were separated on an IonPac AS12A column (Dionex), whereas both inorganic and organic anions were separated on a microbore IonPac AS11-HC column (Dionex). The AS12A column was eluted with Na2CO3 2.7 mM + NaHCO3 0.3 mM at 22 ± 1°C at a flow rate of 1.0 mL min−1. The AS11-HC column was eluted with 10–30 mM NaOH gradient in order to reduce the base line distortions at 30 ± 1°C at a flow rate of 0.38 mL min−1. The NaOH eluent was prepared daily starting from a 50% (w/w) NaOH solution under helium pressure by using a Dionex EO1 eluent/solvent organizer. The quantification of anions was performed using the calibration curves obtained for each anion by plotting the peak area ratios of each compound to an internal standard (IS) against the concentrations of each analyte identified. The internal standard used was nitrite, whose chromatographic peak was markedly separated by other peaks and not present in the plant samples examined.
Inorganic cations
Inorganic cations were extracted by an acid digestion, according to Walinga et al. (1995). A weighed amount (approximately 400 mg) of lyophilized tissue was added to 10 mL of 95% H2SO4 and 4 mL of HNO3 concentrated. After mineralization for 2 h at 370°C, the material was suspended in pure water and then filtered through paper (Whatman International Ltd, Maidstone, England). Prior to analysis, the aqueous extract was 50× diluted to be included in the linearity ranges defined for each cation. The levels of Na+, K+, Ca2+ and Mg2+ were determined by flame atomic absorption spectrophotometry using a Spectra AA-30 flame spectrophotometer (Varian, Mulgrave, Victoria, Australia) with cathode lamps (Photron Pty. Ltd, Australia). The lamp amperage was 5.0 mA for Na+ and K+ analysis, and 3.5 mA for Ca2+ and Mg2+ analysis. A mixture of acetylene and air (flame temperature of 2,400–2,700°K) was used to volatilize and atomize samples. For Na+, K+, Ca2+ and Mg2+ analysis, the wave lengths selected were 589.0, 766.5, 422.7 and 285.2 nm, respectively, whereas the concentration ranges used were 0.15–0.60, 0.5–2.0, 1–4 and 0.1–0.4 μg mL−1, respectively.
Results
Effects of water deficit on plant water status
The values of Ψw and RWC of olive tissues were significantly affected by water availability (Fig. 1). The water potential decreased rapidly after the 7 days of water deficit and then increased after 15 days, when plants were rewatered (Fig. 1a). At the end of the rewatering period, SP reached the same Ψw value of CP (Fig. 1a). In parallel, RWC in SP decreased, reaching the value of 59 ± 3% in leaves after 15 days of water deficit (Fig. 1b), whereas slight differences of RWC were observed in medium and thin roots (Fig. 1c, d). After 12 days from the beginning of rewatering, RWC in all the tissues reached the values of CP (Fig. 1). During the whole experimental period, CP showed no major changes of Ψw and RWC (Fig. 1b, d).
Changes in leaf water potential (Ψw) and relative water content (RWC) in leaves, medium roots and thin roots of water-stressed (closed symbols) and control (open symbols) plants. Vertical dotted lines indicate the beginning of the rewatering period. Values are the means (±SE) of three measurements from three selected plants. Values with the asterisk are significantly different between control and water-stressed plants (P ≤ 0.05, according to Student’s t-test)
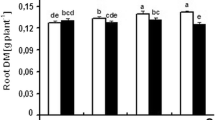
The values of Ψπ100 in leaves and medium roots decreased with decreasing Ψw, and they were lower in leaves than in roots during the whole experimental period (Fig. 2a). At the beginning of the experiment, the mean of Ψπ100 was −2.61 ± 0.02 MPa in leaves, −1.26 ± 0.11 MPa in medium roots and −0.57 ± 0.02 MPa in thin roots (Fig. 2a). Water deficit determined a decline in the values of AΔΨπ100, particularly marked in leaves and medium roots (−0.68 MPa and −1.69 MPa after 15 days, respectively) (Fig. 2b). At the end of the rewatering period, AΔΨπ100 values in leaves and medium roots were significantly lower than those observed at the beginning of the experiment, whereas no significant differences were found in thin roots (Fig. 2b).
a Osmotic potential at full turgor (Ψπ100) in leaves (gray columns), medium roots (black columns) and thin roots (white columns) of water-stressed plants during the experimental period. b Active osmotic adjustment (AΔΨπ100) in leaves, medium roots and thin roots of water-stressed plants. Values are the means (+SE) of three measurements from three plants having the same Ψw. Values followed by different letters (uppercase letters between tissue types and lowercase between sampling dates) are significantly different (P ≤ 0.05, according to Student’s t test)
Changes in low molecular weight non-structural carbohydrates and polyols during water deficit
Analyses of leaf extracts from CP revealed that mannitol and glucose were the predominant sugar compounds with a very similar content, representing 43.2 and 45.0%, respectively, of the total amount of NSC–P (Table 1). In leaf tissues, the levels of raffinose series oligosaccharides, namely raffinose and stachyose, accounted for less than 2.0% of total NSC–P, and the levels of the raffinose oligosaccharides precursors, namely galactose, sucrose and myo-inositol, were about fourfold higher than those of the raffinose series (Table 1). Fructose was present in leaves at a concentration of 9.0 ± 3.0 mmol kg−1 DM, which is approximately 24-fold lower than that of glucose (Table 1). In the roots of CP, the same amount of sugar compounds of leaves samples was found, even though the amounts of each carbohydrate and sugar alcohol changed according to the specific root size (Table 1). Likewise leaves, the most abundant sugar compounds in olive roots were mannitol and glucose, which represented together 82 and 70% of the total NSC–P in medium and thin roots, respectively (Table 1). In leaves of CP, the total amount of other identified carbohydrates and polyols, other than mannitol and glucose, was about 12% (Table 1).
Water deficit caused significant increases in mannitol content in all the olive tissue examined (Table 1). After 15 days of water deficit, the mannitol levels in leaves, medium and thin roots were 294 ± 28, 229 ± 8 and 219 ± 19 mmol kg−1 DM, respectively (Table 1). After 12 days of rewatering, the mannitol content of root tissues declined to control levels, whereas leaves exhibited a slightly lower value if compared to CP (Table 1). Glucose, the second most abundant sugar in olive tissues, increased sharply throughout the drought period (Table 1). No significant variation of glucose was revealed in medium roots of SP if compared to CP, whereas thin roots exhibited a reduction in all the levels of water deficit (Table 1). At the end of the rewatering period, glucose concentration in leaves of SP reached the same values of CP, whereas its content in medium and thin roots was slightly lower than that found in CP (Table 1). Sucrose levels in olive tissues were much lower than those of mannitol and glucose (Table 1). In SP, sucrose exhibited a significant quantitative decrease both in leaves and thin roots after 11 days, whereas no significant differences were found in medium roots (Table 1).
The differences in sugar content of other identified compounds in SP were minor, with two exceptions: fructose in leaves and stachyose in thin roots (Table 1). Although fructose content in olive tissues was much lower than that of glucose, leaves of SP exhibited a marked increase in this sugar up to a Ψw of −3.9 MPa (after 11 days of water deficit) and then a rapid fall at a Ψw of −6.0 MPa (after 15 days) (Table 1). The level of stachyose at the maximum level of water deficit showed a significant decrease in thin roots of SP with decreasing water potential, reaching a value of about one-tenth of that found at the beginning of the experiment (Table 1).
The contribution of NSC–P to Ψπ100 in leaves, medium roots and thin roots was −0.97, −0.55 and −0.19 MPa in CP, and −0.96, −0.98 and −0.31 MPa in SP, respectively (Table 2). The osmotic contribution of mannitol in all the tissues increased in water-stressed plants, whereas glucose showed an opposed trend (Table 2). Contribution to total Ψπ100 by other NSC–P (fructose, myo-inositol, sucrose, raffinose and stachyose) did not show singnificant differences in plants at the maximum level of stress if compared to CP (Table 2).
Effects of water deficit on the amounts of cations and anions
The total content of inorganic cations in leaves of CP (42.9% of total osmolytes) was not significantly different from that of NSC–P (37.1% of total osmolytes) (Fig. 3a). In leaves of CP, Na+, K+, Ca2+, Mg2+ represented 3 ± 1, 52 ± 6, 37 ± 5 and 8 ± 2% of total cations, respectively (Fig. 3a). In leaves of SP, the levels of Na+, K+ and Mg2+ were not affected by water deficit, whereas Ca2+ levels increased of 25% at the maximum level of water deficit if compared to CP (Fig. 4).
a Percentage concentrations of cations, carbohydrates and polyols, and total anions in leaves of control plants. Values are the means (+SE) of 23 measurements from different plants having the same Ψw. b Percentage concentrations of organic and inorganic anions in leaves (gray columns), medium roots (black columns) and thin roots (white columns) of control plants. Values are the means (+SE) of 3–8 measurements from three plants having the same Ψw. Values followed by different letters are significantly different (P ≤ 0.05, according to Student’s t-test)
Levels of inorganic cations in leaves of water-stressed (full symbols) and control (empty symbols) plants during the experimental period. Data represent the means (+SE) of 3–8 measurements from three plants having the same Ψw. Values with the asterisk are significantly different between control and water-stressed plants (P ≤ 0.05, according to Student’s t-test)
In CP, the total content of anions in leaves—which represent 13% of total osmolytes—was significantly lower than those of NSC–P and cations (Fig. 3a). Malate, citrate and oxalate in leaves of CP accounted for 59 ± 7% of total anions (Fig. 3b). During the drought period, no considerable differences in anions level were found in leaves of SP (Figs. 5a, 6a), with the exception of malate, whose concentration after 11 days of water deficit was 2.5-fold higher than that of CP (Fig. 6a). By contrary, in the leaves of SP at the maximum level of water deficit, the level of oxalate was significantly lower than that found in CP (Fig. 6a). During the rewatering period, anions level in leaves reached the values observed at the beginning of the experiment (Figs. 5a, 6a), with the only exception of oxalate, whose concentration after 11 days of water recovery was 30% lower than that of CP (Fig. 6a). In roots of CP, chloride and oxalate were the most abundant anions (87 and 81% of total anions in medium and thin roots, respectively) (Figs. 5b, c, 6b, c). As in leaves, at the maximum level of water deficit, the level of malate showed a significant sixfold increase in medium roots and a threefold increase in thin roots if compared to CP (Fig. 6b, c). The concentration of oxalate in medium and thin roots after 11 days of water deficit was 28 and 18% lower than that of CP, respectively (Fig. 6b, c). During the rewatering period, anions level in both medium and thin roots reached values similar to those observed at the beginning of the experiment (Figs. 5b, c, 6b, c).
Levels of inorganic anions in leaves (a), medium roots (b) and thin roots (c) during the experimental period. Data represent the means (+SE) of 3–8 measurements from three plants having the same Ψw. Values followed by different letters (uppercase letters between tissue types and lowercase between sampling dates) are significantly different (P ≤ 0.05, according to Student’s t-test)
Levels of organic acids in leaves (a), medium roots (b) and thin roots (c) during the experimental period. Data represent the means (+SE) of 3–8 measurements from three plants having the same Ψw. Values followed by different letters (uppercase letters between tissue types and lowercase between sampling dates) are significantly different (P ≤ 0.05, according to Student’s t-test
Discussion
Among the organic solutes responsible to increase the osmotic pressure of many woody plants in severe abiotic stresses, non-structural sugar compounds have been demonstrated to play an important role (Parker and Pallardy 1987; Abrams et al. 1990). In our experiment, the solutes significantly contributing to osmotic potential and the osmotic adjustment in both roots and leaves were examined in olive trees exposed to a progressive water deficit.
The most abundant sugar compounds in the roots of well-watered olive plants (CP) were mannitol, glucose and sucrose, in decreasing order of concentration (Table 1). The levels of the other sugars identified in CP were not significantly different between the thin and medium roots, except for stachyose, whose concentration in thin root extracts was four times higher than that measured in medium roots (Table 1). During the period of water deficit, the changes in Ψπ100 and AΔΨπ100 in medium roots (Fig. 2) point out that these tissues can maintain a higher capacity of turgor maintenance, required to drive advective flow in the xylem. Moreover, the increase in mechanical strength through enhanced turgor better enables medium roots to explore drying soil, which typically offers greater mechanical resistance. In thin roots, the increased levels of mannitol were accompanied by decreases in glucose, sucrose and stachyose concentrations at all the levels of water deficit, whereas the levels of these sugars in medium roots were much less affected (Table 1). These trends confirm the different behavior of root tissues in olive tree: thin roots, mainly involved in water uptake, seem to be more sensitive to water deficit and its consequent effects, while medium roots, primarily involved in long-distance transport, present more reactivity and a higher osmotic adjustment.
As described for root samples, mannitol and glucose were the main sugars in olive leaves, representing almost 90% of the total amount of NSC–P in well-watered plants (CP) (Table 1). By contrast, the levels of raffinose and stachyose in CP were only 0.4 and 1.4% of the total sugar content, respectively (Table 1). Such minor contributions are probably due to the fact that raffinose and stachyose are not stored in leaf mesophyll, but, upon synthesis in the intermediary cells of the minor veins of olive source leaves, they are immediately exported into the phloem (Flora and Madore 1993). These oligosaccharides are the main translocatable sugars in olive plants, in contrast to other species that mainly synthesize polyols (e.g., alditols), that represent the most important form of carbon transported in the phloem (Loescher 1987; Moing et al. 1992). Despite the fact that sucrose is also transported in the phloem, its concentration in the leaves of CP was four times higher than that of stachyose (Table 1), probably because this disaccharide, like fructose and mannitol, is also a temporary storage compound (Smith 1999).
As in some other woody plants (Ranney et al. 1991; Lo Bianco et al. 2000), mannitol is preferentially accumulated in olive leaves in response to water deficit (Table 1). At the maximum level of water deficit, mannitol content was 1.3 times higher in leaves of SP than in CP, confirming that this alditol plays a key role in osmotic adjustment of leaf tissues during water deficit. The increased foliar content of mannitol in olive leaves could be related to an increased activity of mannose-6-phosphate reductase (M6PR) and a decreased activity of mannitol dehydrogenase (MDH), which are the two key enzymes involved in the biosynthesis and catabolism of this sugar (Tattini et al. 1996). In many osmotically stressed plants, M6PR concentrations increase in the leaves while MDH levels drop in sink tissues, and these changes are accompanied by both increases in mannitol concentrations in both sink and source tissues (Williamson et al. 2002). A recent study of Sickler et al. (2007) on Arabidopsis plants transformed with celery’s mannose-6-phosphate reductase (M6PR) gene, demonstrated that mannitol can be considered not only as a compatible solute, but also as an antioxidant able to protect chloroplasts and allow higher photosynthetic rates under water and salt stress. Furthermore, tobacco plants that synthesize and accumulate mannitol and engineered by the introduction of a bacterial gene that encodes mannitol-1-phosphate dehydrogenase have an increased ability to tolerate high salinity (Tarczynski et al. 1993). This information could be of relevant importance for olive plants experiencing the stress conditions typical of Mediterranean climates, as in this study. Unlike the situation seen for mannitol, a marked decrease in glucose concentration was observed in leaves of SP at the maximum level of water deficit (Table 1). As reported by Gucci et al. (1998) for leaves of salt-treated olive plants, this decrease may be due to a lower biosynthesis of monosaccharides induced by water deficit.
The levels of K+ and Ca2+ found in leaves of CP (Fig. 4) are in accordance with those found by Peltier et al. (1997) in six species of Oleaceae. In olive leaves, water deficit did not determine changes in the levels of Na+, K+ and Mg2+ (Fig. 4). As these cations are transported in tree species through xylem vessels (Arndt et al. 2000), the stability of their foliar levels during water deficit likely depends on the fact that olive tree is able to maintain a high transpirative flux also in drought conditions (Xiloyannis et al. 2004). By contrast, the increase in Ca2+ detected in the leaves of SP (Fig. 4) could be also related not only to its function as an osmolyte but also to the role that this ion plays in membranes stability and protection during water deficit (Palta 1996).
The levels of inorganic anions and organic acids did not show clear correlations with the levels of water deficit reached by olive plants, with the exception of malate, whose levels increased in all the tissues examined (Fig. 6). Malate in plants is involved in many aspects of metabolism, signaling, nutrition and resistance against abiotic stresses and, for all these reasons, the accumulation of this ion in plants is tightly regulated (Martinoia and Rentsch 1994; Schulze et al. 2002). In addition to these findings, our results demonstrated that malate also have an important role as an osmolyte during adaptation of olive plants to drought.
The obtained results support the hypothesis that the decreases in Ψw and AΔΨπ100 in leaf and roots of olive trees subjected to water deficit are due to the accumulation of osmolytes within cells, such as mannitol, Ca2+ and malate. Moreover, the maintenance of negative values of AΔΨπ100 at the end of the rewatering period suggests that this strategy may provide a long-term protection against future periods of water deficit. The implication of our results is that, although stress tolerance in olive tree is undeniably dependent on complex interactions between several metabolic pathways (Connor and Fereres 2005), increasing osmolyte accumulation can be a strategy to enhance tolerance against water deficit in this species.
References
Abrams MD, Kubiske NE, Steiner KC (1990) Drought adaptations and responses in five genotypes of Fraxinus pennsylvanica Marsh: photosynthesis, water relations and leaf morphology. Tree Physiol 6:305–315
Arndt ST, Wanek W, Clifford SC, Popp M (2000) Contrasting adaptations to drought stress in field-grown Ziziphus mauritiana and Prunus persica trees: water relations, osmotic adjustment and carbon isotope composition. Aust J Plant Physiol 27:985–996
Bacelar EA, Moutinho-Pereira JM, Lopes JI, Gonçalves BC, Ferreira TC, Correia CM (2007) Changes in growth, gas exchange, xylem hydraulic properties and water use efficiency of three olive cultivars under contrasting water availability regimes. Environ Exp Bot 60:183–192. doi:10.1016/j.envexpbot.2006.10.003
Bongi G, Long SP (1987) Light-dependent damage to photosynthesis in olive leaves during chilling and high temperature stress. Plant Cell Environ 10:241–249
Cataldi TRI, Margiotta G, Iasi L, Dichio B, Xiloyannis C, Bufo SA (2000) Determination of sugar compounds in olive plant extracts by anion-exchange chromatography with pulsed amperometric detection. Anal Chem 72:3902–3907. doi:10.1021/ac000266o
Chartzoulakis K, Patakas A, Bosabalidis AM (1999) Changes in water relations, photosynthesis and leaf anatomy induced by intermittent drought in two olive cultivars. Environ Exp Bot 42:113–120. doi:10.1016/S0098-8472(99)00024-6
Connor DJ, Fereres E (2005) The physiology of adaptation and yield expression in olive. Hortic Rev (Am Soc Hortic Sci) 31:155–229
Dichio B, Xiloyannis C, Sofo A, Montanaro G (2006) Osmotic adjustment in leaves and roots of olive tree (Olea europaea L.) during drought stress and rewatering. Tree Physiol 26:179–185
Fernández JE, Moreno F, Girón IF, Blázquez OM (1997) Stomatal control of water use in olive tree leaves. Plant Soil 190:179–192. doi:10.1023/A:1004293026973
Flora LL, Madore MA (1993) Stachyose and mannitol transport in olive (Olea europaea L.). Planta 189:484–490. doi:10.1007/BF00198210
Gucci R, Moing A, Gravano E, Gaudillere JP (1998) Partitioning of photosynthetic carbohydrates in leaves of salt-stressed olive plants. Aust J Plant Physiol 25:571–579
Guicherd P, Peltier JP, Gout E, Bligny R, Marigo G (1997) Osmotic adjustment in Fraxinus excelsior L.: malate and mannitol accumulation in leaves under drought conditions. Trees Struct Funct 11:155–161
Hanson AD, Hits ED (1982) Metabolic responses of mesophytes to plant water deficits. Annu Rev Plant Physiol 33:163–203. doi:10.1146/annurev.pp.33.060182.001115
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47:377–403. doi:10.1146/annurev.arplant.47.1.377
Lo Bianco R, Rieger M, Sung SJ (2000) Effect of drought on sorbitol and sucrose metabolism in sinks and sources of peach. Physiol Plant 108:71–78. doi:10.1034/j.1399-3054.2000.108001071.x
Loescher WH (1987) Physiology and metabolism of polyols in higher plants. Physiol Plant 70:553–557. doi:10.1111/j.1399-3054.1987.tb02857.x
Martinoia E, Rentsch D (1994) Malate compartmentation-responses to a complex metabolism. Annu Rev Plant Physiol 45:447–467
McCue KF, Hanson AD (1990) Drought and salt tolerance: towards understanding and application. Trends Biotechnol 8:358–362. doi:10.1016/0167-7799(90)90225-M
Moing A, Carbonne F, Rashad MH, Gaudillere JP (1992) Carbon fluxes in mature peach leaves. Plant Physiol 100:1878–1884
Munns R (1988) Why measure osmotic adjustment? Aust J Plant Physiol 15:717–726
Nobel PS (1983) Biophysical plant physiology and ecology. WH Freeman and Company, San Francisco
Nogués S, Baker NR (2000) Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J Exp Bot 51:1309–1317. doi:10.1093/jexbot/51.348.1309
Nuccio ML, Rhodes D, McNeil SD, Hanson AD (1999) Metabolic engineering of plants for osmotic stress resistance. Curr Opin Plant Biol 2:128–134. doi:10.1016/S1369-5266(99)80026-0
Palta JP (1996) Role of calcium in plant responses to stresses: linking basic research to solution of practical problems. HortScience 31:51–57
Parker WC, Pallardy SG (1987) Leaf and root osmotic adjustment in water-stressed Quercus alba, Q macrocarpa and Q stellata seedlings. Can J For Res 18:1–5. doi:10.1139/x88-001
Peltier JP, Marigo D, Marigo G (1997) Involvement of malate and mannitol in the diurnal regulation of the water status in members of Oleaceae. Trees Struct Funct 12:27–34
Ranney TG, Bassuk NL, Whitlow TH (1991) Osmotic adjustment and solute constituents in leaves and roots of water-stressed cherry (Prunus) trees. J Am Soc Hortic Sci 116:684–688
Rhodes D, Samaras Y (1994) Genetic control of osmoregulation in plants. In: Stange K (ed) Cellular and molecular physiology of cell regulation. CRC Press, Boca Raton, pp 347–361
Russo VM, Karmarkar SV (1998) Water extraction of plant tissues for analysis by anion chromatography. Commun Soil Sci Plant Anal 29:245–253
Schulze J, Tesfaye M, Litjens RHMG, Bucciarelli B, Trepp G, Miller S et al (2002) Malate plays a central role in plant nutrition. Plant Soil 247:133–139. doi:10.1023/A:1021171417525
Sickler CM, Edwards GE, Kiirats O, Gao Z, Loescher W (2007) Response of mannitol-producing Arabidopsis thaliana to abiotic stress. Funct Plant Biol 34:382–391. doi:10.1071/FP06274
Smith CJ (1999) Carbohydrate biochemistry. In: Lea PJ, Leegood RC (eds) Plant biochemistry and molecular biology, 2nd edn. Wiley, London, pp 87–99
Sofo A, Dichio B, Xiloyannis C, Masia A (2004) Lipoxygenase activity and proline accumulation in leaves and roots of olive tree in response to drought stress. Physiol Plant 121:58–65. doi:10.1111/j.0031-9317.2004.00294.x
Tarczynski MC, Jensen RG, Bohnert HJ (1993) Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 259:508–510. doi:10.1126/science.259.5094.508
Tattini M, Gucci R, Romani A, Baldi A, Everard JD (1996) Changes in non-structural carbohydrates in olive (Olea europaea) leaves during root zone salinity stress. Physiol Plant 98:117–124. doi:10.1111/j.1399-3054.1996.tb00682.x
Walinga I, Van der Lee JJ, Houba VJG, Van Vark W, Novozamsky I (1995) Plant Analysis Manual. Kluwer Academic Publishers, Dordrecht, The Netherlands
Wang Z, Stutte GW (1992) The role of carbohydrates in active osmotic adjustment in apple under water stress. J Am Soc Hortic Sci 117:816–823
Williamson JD, Jennings DB, Guo WW, Pharr DM, Ehrenshaft M (2002) Sugar alcohols, salt stress, and fungal resistance: Polyols-Multifunctional plant protection? J Am Soc Hortic Sci 127:467–473
Xiloyannis C, Gucci R, Dichio B (2004) Irrigazione. In: Fiorino P (ed) Olea: Trattato di Olivicoltura. Il Sole 24 ORE Edagricole S.r.l., Verona, pp 365–389
Acknowledgments
The authors gratefully acknowledge Dr. Giuseppe Montanaro, Mr. Antonio Ditaranto and Mr. Mario Pompeo for field assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Buckley.
Rights and permissions
About this article
Cite this article
Dichio, B., Margiotta, G., Xiloyannis, C. et al. Changes in water status and osmolyte contents in leaves and roots of olive plants (Olea europaea L.) subjected to water deficit. Trees 23, 247–256 (2009). https://doi.org/10.1007/s00468-008-0272-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-008-0272-1