Abstract
Background
Continuous kidney replacement therapy (CKRT) is often used for acute kidney injury (AKI) or fluid overload (FO) in children ≤ 10 kg. Intensive care unit (ICU) mortality in children ≤ 10 kg reported by the prospective pediatric CRRT (ppCRRT, 2001–2003) registry was 57%. We aimed to evaluate characteristics associated with ICU mortality using a contemporary registry.
Methods
The Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK) registry is a retrospective, multinational, observational study of children and young adults aged 0–25 years receiving CKRT (2015–2021) for AKI or FO. This analysis included patients ≤ 10 kg at hospital admission. Primary and secondary outcomes: ICU mortality and major adverse kidney events at 90 days (MAKE-90) defined as death, persistent kidney dysfunction, or dialysis within 90 days, respectively.
Results
A total of 210 patients were included (median age 0.53 years (IQR, 0.1, 0.9)). ICU mortality was 46.5%. MAKE-90 occurred in 150/207 (72%). CKRT was initiated at a median 3 days (IQR 1, 9) after ICU admission and lasted a median 6 days (IQR 3, 16). On multivariable analysis, pediatric logistic organ dysfunction score (PELOD-2) at CKRT initiation was associated with increased odds of ICU mortality (aOR 2.64, 95% CI 1.68–4.16), and increased odds of MAKE-90 (aOR 2.2, 95% CI 1.31–3.69). Absence of comorbidity was associated with lower MAKE-90 (aOR 0.29, 95%CI 0.13–0.65).
Conclusions
We report on a contemporary cohort of children ≤ 10 kg treated with CKRT for acute kidney injury and/or fluid overload. ICU mortality is decreased compared to ppCRRT. The extended risk of death and morbidity at 90 days highlights the importance of close follow-up.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) and pathologic fluid overload (FO) are common in critically ill children ≤ 10 kg and are associated with adverse outcomes [1,2,3]. Some children with severe AKI may require kidney replacement therapy, delivered through peritoneal dialysis or extracorporeal therapy, most commonly continuous kidney replacement therapy (CKRT) [1]. Historically, CKRT utilization in infants and small children (weighing ≤ 10 kg) with AKI has been lower than that in older children, as the use of machines designed for older children and adults makes CKRT delivery technically challenging [4]. While newer devices have been developed (Carpediem™) or adapted (Aquadex™) for neonates and infants, the majority of children ≤ 10 kg continue to receive CKRT utilizing devices approved for larger children or adults [5, 6].
There are limited multicenter reports on the use of CKRT in infants and small children [4, 7]. The largest multicenter study, the Prospective Pediatric Continuous Renal Replacement Therapy (ppCRRT) Registry, performed almost 20 years ago, reported 57% intensive care unit (ICU) mortality among infants and small children ≤ 10 kg [4]. Since 2005, there have been changes in the care of these patients, including increased awareness and recognition of AKI and FO, and availability of newer CKRT filters and devices. However, the impact of those changes in a multicenter population have not been reported. In order to continue to improve outcomes in critically ill infants and young children treated with CKRT, a better understanding of the epidemiology and outcomes in a contemporary, multicenter cohort is greatly needed.
The Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK) is a multinational investigator group that was formed to study the epidemiology, practices, and clinical and patient-centered outcomes of children receiving CKRT for AKI and FO [8]. In this planned secondary analysis of the WE-ROCK registry, we specifically evaluated infants and small children weighing ≤ 10 kg who received CKRT. We aimed to describe (1) demographic and clinical characteristics; (2) practice variations (CKRT dosing, blood flow, anticoagulation); and (3) outcomes (ICU mortality, major adverse kidney events at 90 days (MAKE-90)) in this cohort. We hypothesized that there would be (1) lower ICU mortality in the contemporary cohort, (2) wide variation in CKRT practice, and (3) a significant burden of morbidity and mortality beyond ICU discharge.
Methods
Study population
Details of the WE-ROCK study methods and demographics of the overall cohort have been reported previously [8, 9]. Briefly the WE-ROCK study included children and young adults (0–25 years old) receiving CKRT for AKI or FO in an ICU from January 2015 to December 2021. This current analysis includes a subgroup of patients with an admission weight ≤ 10 kg. Exclusions include: CKRT for a non-AKI or non-FO indication, dialysis-dependent kidney failure, a severe congenital anomaly of the kidney and urinary tract likely to progress to kidney failure, concomitant use of extracorporeal membrane oxygenation (ECMO), and those treated with the Carpediem™ device, due to the presence of an existing registry focusing on the device [8]. Children receiving ECMO were excluded because of the significant morbidity and mortality seen in this cohort, and the desire to understand the effect of CKRT alone on outcomes. The study was performed in line with the principles of the Declaration of Helsinki. The Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC) and each participating site approved this study, with a waiver of informed consent in view of its retrospective nature. Data sharing agreements were instituted between each site and the data coordinating site (CCHMC).
Demographic and CKRT technique data
Demographic data including sex, age, and time from ICU admission to CKRT initiation were collected for all patients. Data at CKRT initiation included kidney function, presence of sepsis [10] within 24 h of ICU admission, Pediatric Logistic Organ Dysfunction 2 (PELOD-2) [11] score in the 24 h prior to CKRT initiation, fluid balance, and loop diuretic challenge. Details of CKRT prescription, including device, modality, filter, prescribed dose, and anticoagulation, were collected daily for the first 7 days the patient received CKRT, or until procedure termination if less than 7 days. CKRT dose was calculated as the prescribed effluent dose. While CKRT dose is the amount of blood cleared of solute over a unit of time, effluent flow is regarded as an acceptable surrogate of solute clearance for prescribing the dose [12]. CKRT dose was a key exposure variable. Fluid balance was defined using intake and output as has been previously described [13].
Outcomes of interest
The primary outcome was ICU mortality. Secondary outcomes included MAKE-90, CKRT duration, and ICU length of stay. MAKE-90, defined as a composite of death, dialysis-dependence, or persistent kidney dysfunction (> 25% decline in kidney function from baseline) [14], has been recommended by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workgroup on clinical trials in AKI. We used this, as it allows for assessment of a greater percentage of patients with a clinically meaningful poor outcome and overcomes the limitation of competing risks (for example, mortality and persistent kidney dysfunction) [15]. We chose to assess MAKE at 90 days because this is the time when chronic kidney disease is diagnosed after AKI [16].
Statistical analysis
Continuous variables are reported as median with interquartile range (IQR) and were compared using Wilcoxon rank sum tests. Categorical variables are reported as proportion with percent and were compared using Chi-square tests. To examine the association between ICU mortality and CKRT dose, a univariate logistic regression model was used to model the probability of ICU mortality as a function of CKRT dose at initiation. CKRT dose at initiation was flexibly modeled using restricted cubic spline terms with four knots (5th, 35th, 65th, and 95th percentiles) to allow for potential non-linear associations [17]. A cubic spline plot (i.e., model-based predicted values) was generated to visualize the association between CKRT dose and ICU mortality. Predicted probabilities of ICU mortality as a function of CKRT dose were obtained via an inverse logit transformation of the log odds.
Multivariable logistic regression models were used to estimate adjusted odds ratio (aOR) and 95% confidence intervals (CI) to identify the risk factors associated with ICU mortality and MAKE-90. A priori relevant covariates were selected for each outcome based on the existing literature and clinical practice. For continuous covariates, linear associations with outcomes were assumed and odds ratios are presented as a comparison of the 75th versus 25th percentile (i.e., odds ratios per IQR increase). In all analyses, a p-value < 0.05 was considered statistically significant. All statistical analyses were performed using R (V4.3.1, https://www.r-project.org/). The rms package (version 6.7.1) was used to perform regression analyses.
Results
Patient characteristics
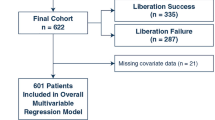
A total of 210 infants and small children from 32 centers in 7 countries were included in this analysis. Selected patient characteristics are displayed in Table 1. The weight range was 1.9–10 kg, median of 6.47 kg (IQR 3.73–8.69), and 75 (32%) weighed ≤ 5 kg. The age range was 1 day–4.4 years, median 0.53 years (IQR 0.1–0.9), with 47 (22%) younger than 1 month. The most common reason for admission was shock/infection/trauma (31%), followed by respiratory failure (22%); 80 (38%) had sepsis at ICU admission. Comorbidities were seen in 81%, with cardiac (27%), and gastrointestinal (26%) being most common, although only 18% had no comorbidity.
CKRT initiation
CKRT was initiated a median of 3 days (IQR 1, 9) after ICU admission and lasted a median of 6 days (IQR 3, 16). In the 24 h prior to CKRT initiation, the median PELOD-2 score was 7 (IQR 5,10) and VIS was 7 (IQR 0, 20). The median FO at CKRT initiation was 16.4% (IQR 6.3, 32.8) with 91 (43%) children having > 20% FO (Table 1).
CKRT technique
The most common CKRT modality was continuous veno-venous hemodiafiltration (CVVHDF) in 146 (69%) patients, and polysulfone filters were most commonly used (80%) (Table 1). Anticoagulation strategies included citrate in 110 (52%), heparin in 64 (31%), no anticoagulation in 21 (10%) and other in 15 (7%, epoprostenol and bivalirudin). The most common catheter placement location was internal jugular vein (n = 156, 76%) with size ranging from 6 to 10 Fr. Median blood flow per body weight was 8 mL/kg/min (IQR 5.9, 11.3).
CKRT dosing
CKRT dose was calculated as the prescribed effluent dose. The median prescribed CKRT dose at initiation was 2104 mL/1.73 m2/h (IQR 1564, 3027) or 63.8 mL/kg/h (IQR 49.2, 88.1), with 85% having a CKRT dose prescription of > 40 mL/kg/h. The median hourly dose increased over the first 7 days of treatment. There was wide variation between centers, with the median dose at initiation ranging from 27.8 to 187.8 mL/kg/h (861–4244 mL/1.73 m2/h) (Supplementary Fig. 1). There was also a wide range of initial prescribed dose within centers that enrolled at least 5 patients in the study (Supplementary Fig. 2).
Mortality prior to ICU discharge
Overall, 97 (46.1%) died prior to ICU discharge. Of those, 41 patients died during their CKRT course (Fig. 1). A description of patient characteristics by ICU mortality is presented in Table 1. Those who died in the ICU had lower weights (5.9 kg vs. 7.3 kg, p = 0.022), with those weighing ≤ 5 kg having higher mortality (59% compared to 40% mortality in those > 5 kg, p < 0.009). There was no significant difference in mortality by age categories, with those younger than 1 month age (55%), 1 month to 1 year (43%) and older than 1 year (47%) (Table 2). Patients who died had higher PELOD-2 scores (8 vs. 6, p < 0.001) and VIS (13.5 vs. 3, p < 0.001) in the 24 h prior to CKRT initiation. Those with respiratory failure as the primary reason for admission, and those with immunologic co-morbidities (including patients with hematopoietic stem cell transplants), were more likely to die in the ICU (Table 1).
Timeline up to 90 days from CKRT initiation. CKRT was initiated at a median (IQR) 3 (1–9) days after ICU admission. Open diamonds represent those who died during their CKRT course (n = 41). Closed triangles represent those who died after coming off CKRT (8 patients died after their CKRT course beyond the 90 day mark). (Created with BioRender.com)
There were no differences between CKRT modality, filter type, or mode of anticoagulation by ICU mortality status. CKRT dose at initiation was similar between survivors and non-survivors. By day 3 of therapy, it was higher in those who did not survive with a median of 84.3 mL/kg/h (IQR 54, 114.8) compared to 63.6 mL/kg/h (IQR 43.3, 90.8) in those who survived, p = 0.017. Considering the possibility that higher clearances in those who did not survive could reflect regional citrate use and accumulation, we evaluated anticoagulation type and found no significant difference between proportion of patients receiving citrate compared to other anticoagulants.
The predicted probability of ICU mortality decreased slowly until a CKRT dose of 47 mL/kg/h and increased with further increases in dose (p = 0.349) (Fig. 2). In the evaluation of CKRT dose at initiation by tertiles (< 53 mL/kg/h, 53–80 mL/kg/h, and > 80 mL/kg/h), the smallest and youngest infants received the highest dose. Median weight and age for those with a prescription > 80 mL/kg/h were 5.2 kg (IQR 3, 7.7) and 0.3 years (IQR 0.06, 0.6) compared to 8 kg (IQR 4.4, 9.6) and 0.6 years (0.1, 1), respectively, for those < 53 mL/kg/h. Those receiving a dose > 80 mL/kg had the highest rate of KRT dependence at hospital discharge and longest duration of mechanical ventilation (Supplementary Table 1), but there was no difference in ICU or hospital mortality.
In multivariable logistic regression analysis which included weight, percent fluid overload, PELOD-2 score, and CKRT dose at initiation, dose was not associated with increased odds of death prior to ICU discharge (aOR 1.07, 95% CI 0.88–1.29). Only higher PELOD-2 score (aOR 2.64, 95% CI 1.68–4.16) at CKRT initiation remained statistically significantly associated with increased odds of death prior to ICU discharge (Table 3).
MAKE-90
MAKE-90 data were available in 207 patients of whom 150 (72.4%) fulfilled criteria, with death in 100 (67%), and persistent kidney dysfunction (> 25% decline from baseline kidney function or dialysis dependence) in 50 (33%) (Table 2). The cohort of 50 patients with persistent kidney dysfunction included 16 who were dialysis dependent at 90 days. In multivariable logistic regression analysis which included weight, percent fluid overload, CKRT dose at initiation, CKRT duration, and PELOD-2 Score at CKRT initiation, and the presence or absence of any comorbidity, PELOD-2 was associated with increased odds of MAKE-90 (aOR 2.20, 95% CI 1.31–3.69). Absence of any comorbid condition had a protective effect, with lower odds of MAKE-90 (aOR 0.29, 95%CI 0.13–0.65) (Table 4).
Discussion
In this secondary analysis of the multicenter international WE-ROCK registry, we describe the clinical characteristics, CKRT treatment, and outcomes in a large cohort of children weighing ≤ 10 kg requiring CKRT. This study shows that infants and small children with AKI and FO who weigh ≤ 10 kg at CKRT initiation have higher mortality prior to ICU discharge compared to children weighing > 10 kg (46% in children ≤ 10 kg vs. 36% for the entire WE-ROCK cohort). We also report that there remains a significant burden of morbidity and mortality at 90 days, with nearly half the survivors having persistent kidney dysfunction. Additionally we show that there are important and large variations in prescribed dialysis dosing, both within and between centers.
While CKRT has become the modality of choice in critically ill older children with severe AKI and FO, there is a paucity of data on infants and small children treated with CKRT ≤ 10 kg. Most studies of CKRT in this population are single-center and small [18, 19]. In a retrospective cohort of 85 infants < 10 kg from 5 United States centers from 1993 to 2001, Symons et al. reported ICU mortality of 62% ICU [7]. Among a similar but prospective cohort in the ppCRRT registry, which enrolled patients from 2001 to 2005, Askenazi et al. reported 56% mortality in 84 patients [4]. In a more contemporary retrospective cohort of 51 infants weighing ≤ 10 kg, 47% died in the hospital [6]. Many of these studies included infants with severe congenital kidney disease and those who received CKRT for a non-AKI/FO indication (i.e., ingestion or inborn errors of metabolism), groups which have usually had better outcomes compared to those with AKI and/or FO [4, 6, 7]. In the current study, we focused on a less heterogenous population, and limited enrollment to only those treated with CKRT for AKI and/or FO, and excluding concurrent ECMO use that has a substantial negative effect on survival. After excluding those with inborn errors of metabolism, the mortality in the ppCRRT cohort was 62% compared to 46% in our study [4]. While there are significant differences between these studies, we may be seeing some improvement in the outcomes of infants receiving CKRT over time, which are likely related to overall improvements in ICU care, along with better recognition of AKI and FO [9, 20, 21].
As recognition and ICU survival of critically ill children of all ages with AKI have improved, there has been a shift to include the outcomes of persistent kidney dysfunction captured in MAKE-90. However there are limited follow-up data on neonates and infants treated with CKRT, particularly focusing on MAKE-90 or other long-term outcomes. Most studies include all children < 18 years, and have been from either small single center studies or large claims-based data [22, 23]. Using province-wide health administrative databases of children aged 0–18 years hospitalized in Ontario, Canada, Robinson et al. reported that those who survived an episode of pediatric AKI requiring dialysis were at significantly increased risk of a composite outcome of kidney failure or death versus an age-matched control population that did not have AKI requiring dialysis [23]. At a median follow-up of 9.6 months, death occurred in 6.7% and kidney failure in 2.6%, along with hypertension, chronic kidney disease, and repeat episodes of AKI. More recently, Gulcek et al. reported on 109 patients weighing < 15 kg who received various modalities of acute KRT including CKRT, HD, and PD [24]. ICU mortality was seen in 64 (58.7%). At a mean follow-up of 2.9 ± 2.1 years, 34 patients (including 3 who received CKRT) were evaluated, and 22 patients (64.7%) were reported to have ≥ 1 kidney risk factor including elevated blood pressure/hypertension, abnormal eGFR, and/or proteinuria. We show high rates of MAKE-90 in children ≤ 10 kg, with 50% of survivors having abnormal kidney function at 90 days, including many still requiring dialysis. The high rates of persistent kidney dysfunction highlight the need for close follow-up in this at-risk population, as has been previously recommended [25,26,27].
In the current study, we also looked at sub-populations and patient characteristics associated with adverse outcomes. While the severity of illness at CKRT initiation was associated with worse MAKE-90, the absence of any comorbidity was associated with lower odds of developing MAKE-90. Over 80% of those in our cohort had at least 1 comorbidity. This reflects the increasing medical complexity of infants and small children admitted to ICUs, where the proportion of children with chronic comorbidities has increased significantly in the last decade [20]. These children experience higher mortality and longer ICU stays than children without chronic medical conditions [20].
To continue improving outcomes in infants and small children treated with CKRT, a critical step is understanding the characteristics and heterogeneity in CKRT prescription and delivery. One of the most interesting findings we report is the variation in CKRT dosing. Pediatric CKRT prescriptions have been extrapolated from adults [28, 29], with a dose of 2000 mL/1.73 m2/h approximating adult weight-based doses (25–30 mL/kg/h for a 70 kg patient) [30]. However, there are wide variations reported in clinical practice, ranging from < 1000 to > 4000 mL/1.73 m2/h, which equated to 20–150 mL/kg/h [31]. We report similar variation not only between centers, but also within the same center. The dose variance in this population takes on particular importance when one considers that weight-based dosing and BSA-based dosing diverge at lower weights. This nonlinear relationship between weight and BSA results in a disproportionately higher dose in neonates and infants. The current study shows that most patients are prescribed a dose > 40 mL/kg/h, which would be considered a high dose in adults. While high-dose CKRT has been studied extensively in adults without consistent evidence of benefit, little is known about its impact on outcomes in small children. The potential consequences of such high doses are loss of proteins, amino acids, phosphorus, and other micronutrients [32]. However, we acknowledge that details of individual CKRT treatments, notably delivered dose, reasons for dose increase, and the change in clinical status and severity of illness scores over the duration of therapy was not collected. This limits our ability to assess differences in prescribed and delivered dose, or the reasons in changes in CKRT dose over time. While we did not see a significant relationship between dose and outcomes, this highlights the opportunity for the development of standardized protocols for dosing in this population and the systematic study of dosing in this population.
The primary strength of this study is it represents a large contemporary report of infants weighing ≤ 10 kg receiving CKRT, including data from 32 centers across 7 countries. Nonetheless, we acknowledge several important limitations. Registry data are subject to center and patient selection bias. Given the study design and its retrospective nature, we only collected information on those who started CKRT for AKI and FO. The study also lacks information on those with severe AKI/FO who may not have received CKRT due to their size, or severity of illness, or lack of appropriate resources, or may have received peritoneal dialysis or kidney support with Carpediem™. While CKRT use is becoming increasingly common in pediatric patients with critical illness, peritoneal dialysis remains the most common modality of KRT in infants and small children worldwide [33]. All sites included in WE-ROCK are tertiary or quaternary care centers in North America, Western Europe, the United Kingdom, and Australia; therefore, the findings may only be applicable to centers with similar practice models and resources. Sites were permitted to participate in WE-ROCK by including 10 or more consecutive patients. While this was done to promote inclusion and participation from sites that do not have significant research resources due to lack of funding for WE-ROCK, we recognize this may have resulted in site selection bias as only sites with some research resources were able to participate.
We highlight that survival in infants and small children who weigh ≤ 10 kg at CKRT initiation is improving. Furthermore, we describe that many survivors have kidney sequelae at 90 days. There is significant practice variation in CKRT dosing with marked differences between and within centers. When dosing was adjusted for weight, 85% of neonates received a dose higher than the “high dose” CKRT described in adult studies. This study highlights the need for evidence-based guidelines for CKRT prescription in infants and small children, as well as a standardized approach to follow-up for those that survive to discharge to ensure monitoring for medium- and long-term kidney-related complications.
Data availability
De‐identified summary data are available upon request through the WE-ROCK collaborative. Under the current approval by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC), and the data use agreements signed by the individual participating sites and CCHMC, analyses are done by the data coordinating center at CCHMC. The data from the WE-ROCK collaborative will be made available to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal following an application process and execution of a data use agreement as required by the IRB at the CCHMC.
Abbreviations
- CKRT:
-
Continuous kidney replacement therapy
- ICU:
-
Intensive care unit
- ppCRRT registry:
-
Prospective pediatric continuous renal replacement therapy registry
- WE-ROCK:
-
Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease
- AKI:
-
Acute kidney injury
- FO:
-
Fluid overload
- MAKE-90:
-
Major adverse kidney events at 90 days
- PELOD-2:
-
Pediatric logistic organ dysfunction score
- KRT:
-
Kidney replacement therapy
- IRB:
-
Institutional Review Board
- CCHMC:
-
Cincinnati Children’s Hospital Medical Center
- VIS:
-
Vasoactive-inotrope score
- eGFR:
-
Estimated glomerular filtration rate
- NIDDK:
-
National Institute of Diabetes and Digestive and Kidney Diseases
- IQR:
-
Interquartile range
- aOR:
-
Adjusted odds ratio
- CI:
-
Confidence intervals
- CVVHDF:
-
Continuous veno-venous hemodiafiltration
References
Jetton JG, Boohaker LJ, Sethi SK et al (2017) Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1:184–194. https://doi.org/10.1016/S2352-4642(17)30069-X
Kaddourah A, Basu RK, Bagshaw SM et al (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376:11–20. https://doi.org/10.1056/NEJMoa1611391
Alten JA, Cooper DS, Blinder JJ et al (2021) Epidemiology of acute kidney injury after neonatal cardiac surgery: a report from the multicenter neonatal and pediatric heart and renal outcomes network. Crit Care Med 49:e941–e951. https://doi.org/10.1097/CCM.0000000000005165
Askenazi DJ, Goldstein SL, Koralkar R et al (2013) Continuous renal replacement therapy for children ≤10 kg: a report from the prospective pediatric continuous renal replacement therapy registry. J Pediatr 162:587-592.e3. https://doi.org/10.1016/j.jpeds.2012.08.044
Menon S, Broderick J, Munshi R et al (2019) Kidney support in children using an ultrafiltration device: a multicenter, retrospective study. Clin J Am Soc Nephrol 14:1432–1440. https://doi.org/10.2215/CJN.03240319
Kedarnath M, Alexander EC, Deep A (2023) Safety and efficacy of continuous renal replacement therapy for children less than 10 kg using standard adult machines. Eur J Pediatr 182:3619–3629. https://doi.org/10.1007/s00431-023-05007-y
Symons JM, Brophy PD, Gregory MJ et al (2003) Continuous renal replacement therapy in children up to 10 kg. Am J Kidney Dis 41:984–989. https://doi.org/10.1016/s0272-6386(03)00195-1
Menon S, Krallman KA, Arikan AA et al (2023) Worldwide exploration of renal replacement outcomes collaborative in kidney disease (WE-ROCK). Kidney Int Rep 8:1542–1552. https://doi.org/10.1016/j.ekir.2023.05.026
Starr MC, Gist KM, Zang H et al (2024) Continuous kidney replacement therapy and survival in children and young adults: findings from the multinational WE-ROCK collaborative. Am J Kidney Dis S0272–6386:00610–00613. https://doi.org/10.1053/j.ajkd.2023.12.017
Miranda M, Nadel S (2023) Pediatric sepsis: a summary of current definitions and management recommendations. Curr Pediatr Rep 11:29–39. https://doi.org/10.1007/s40124-023-00286-3
Leteurtre S, Duhamel A, Salleron J et al (2013) PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med 41:1761–1773. https://doi.org/10.1097/CCM.0b013e31828a2bbd
Bagshaw SM, Chakravarthi MR, Ricci Z et al (2016) Precision continuous renal replacement therapy and solute control. Blood Purif 42:238–247. https://doi.org/10.1159/000448507
Gorga SM, Selewski DT, Goldstein SL, Menon S (2024) An update on the role of fluid overload in the prediction of outcome in acute kidney injury. Pediatr Nephrol 39:2033–2048. https://doi.org/10.1007/s00467-023-06161-z
Billings FT, Shaw AD (2014) Clinical trial endpoints in acute kidney injury. Nephron Clin Pract 127:89–93. https://doi.org/10.1159/000363725
Palevsky PM, Molitoris BA, Okusa MD et al (2012) Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol 7:844–850. https://doi.org/10.2215/CJN.12791211
Lameire NH, Levin A, Kellum JA et al (2021) Harmonizing acute and chronic kidney disease definition and classification: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int 100:516–526. https://doi.org/10.1016/j.kint.2021.06.028
Harrell FE (2015) Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. Springer International Publishing, Cham
Lee ST, Cho H (2016) Fluid overload and outcomes in neonates receiving continuous renal replacement therapy. Pediatr Nephrol 31:2145–2152. https://doi.org/10.1007/s00467-016-3363-z
Diane Mok TY, Tseng M-H, Chiang M-C et al (2018) Renal replacement therapy in the neonatal intensive care unit. Pediatr Neonatol 59:474–480. https://doi.org/10.1016/j.pedneo.2017.11.015
Killien EY, Keller MR, Watson RS, Hartman ME (2023) Epidemiology of intensive care admissions for children in the US from 2001 to 2019. JAMA Pediatr 177:506–515. https://doi.org/10.1001/jamapediatrics.2023.0184
Starr MC, Kula A, Lieberman J et al (2020) The impact of increased awareness of acute kidney injury in the neonatal intensive care unit on acute kidney injury incidence and reporting: results of a retrospective cohort study. J Perinatol 40:1301–1307. https://doi.org/10.1038/s41372-020-0725-y
Frisby-Zedan J, Barhight MF, Keswani M et al (2023) Long-term kidney outcomes in children following continuous kidney replacement therapy. Pediatr Nephrol 38:565–572. https://doi.org/10.1007/s00467-022-05579-1
Robinson CH, Jeyakumar N, Luo B et al (2021) Long-term kidney outcomes following dialysis-treated childhood acute kidney injury: a population-based cohort study. J Am Soc Nephrol 32:2005–2019. https://doi.org/10.1681/ASN.2020111665
Gülçek ÖN, Gülhan B, Kesici S et al (2023) Long-term kidney follow-up after pediatric acute kidney support therapy for children less than 15 kg. Pediatr Nephrol 38:3811–3821. https://doi.org/10.1007/s00467-023-06013-w
Harer MW, Selewski DT, Kashani K et al (2021) Improving the quality of neonatal acute kidney injury care: neonatal-specific response to the 22nd Acute Disease Quality Initiative (ADQI) conference. J Perinatol 41:185–195. https://doi.org/10.1038/s41372-020-00810-z
Goldstein SL, Akcan-Arikan A, Alobaidi R et al (2022) Consensus-based recommendations on priority activities to address acute kidney injury in children: a modified delphi consensus statement. JAMA Netw Open 5:e2229442. https://doi.org/10.1001/jamanetworkopen.2022.29442
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120:c179–c184. https://doi.org/10.1159/000339789
VA/NIH Acute Renal Failure Trial Network; Palevsky PM, Zhang JH et al (2008) Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359:7–20. https://doi.org/10.1056/NEJMoa0802639
RENAL Replacement Therapy Study Investigators; Bellomo R, Cass A et al (2009) Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 361:1627–1638. https://doi.org/10.1056/NEJMoa0902413
Maxvold NJ, Smoyer WE, Custer JR, Bunchman TE (2000) Amino acid loss and nitrogen balance in critically ill children with acute renal failure: a prospective comparison between classic hemofiltration and hemofiltration with dialysis. Crit Care Med 28:1161–1165. https://doi.org/10.1097/00003246-200004000-00041
Ricci Z, Guzzi F, Tuccinardi G, Romagnoli S (2016) Dialytic dose in pediatric continuous renal replacement therapy patients. Minerva Pediatr 68:366–373
Vijayan A, Palevsky PM (2012) Dosing of renal replacement therapy in acute kidney injury. Am J Kidney Dis 59:569–576. https://doi.org/10.1053/j.ajkd.2011.11.035
Nourse P, Cullis B, Finkelstein F et al (2021) ISPD guidelines for peritoneal dialysis in acute kidney injury: 2020 Update (paediatrics). Perit Dial 41:139–157. https://doi.org/10.1177/0896860820982120
Acknowledgements
WE-ROCK collaborative author list: The following individuals served as collaborators and investigators for the WE-ROCK studies. They collaborated in protocol development and review, data analysis, and participated in drafting or review of the manuscript, and their names should be citable by PubMed.
Emily Ahern CPNP, DNP1, Ayse Akcan Arikan MD2, Issa Alhamoud MD3, Rashid Alobaidi MD, MSc4, Pilar Anton-Martin MD, PhD5, Shanthi S Balani MD6, Matthew Barhight MD, MS7, Abby Basalely MD, MS8, Amee M Bigelow MD, MS9, Gabriella Bottari MD10, Andrea Cappoli MD10, Eileen A Ciccia MD11, Michaela Collins BA12, Denise Colosimo MD13, Gerard Cortina MD14, Mihaela A Damian MD, MPH15, Sara De la Mata Navazo MD16, Gabrielle DeAbreu MD8, Akash Deep MD17, Kathy L Ding BS18, Kristin J Dolan MD2, Sarah N Fernandez Lafever MD, PhD16, Dana Y Fuhrman DO, MS19, Ben Gelbart MBBS20, Katja M Gist , DO MSc12, Stephen M Gorga MD, MSc21, Francesco Guzzi MD22, Isabella Guzzo MD10, Taiki Haga MD23, Elizabeth Harvey MD24, Denise C Hasson MD25, Taylor Hill-Horowitz BS8, Haleigh Inthavong BS, MS2, Catherine Joseph MD2, Ahmad Kaddourah MD, MS26, Aadil Kakajiwala MD, MSCI27, Aaron D Kessel MD, MS8, Sarah Korn DO28, Kelli A Krallman BSN, MS12, David M Kwiatkowski MD Msc29, Jasmine Lee MSc24, Laurance Lequier MD4, Tina Madani Kia BS4, Kenneth E Mah MD, MS15, Eleonora Marinari MD10, Susan D Martin MD30, Shina Menon MD31, Tahagod H Mohamed MD9, Catherine Morgan MD MSc4, Theresa A Mottes APRN7, Melissa A Muff-Luett MD32, Siva Namachivayam MBBS20, Tara M Neumayr MD11, Jennifer Nhan Md, MS27, Abigail O’Rourke MD8, Nicholas J Ollberding PhD12, Matthew G Pinto MD33, Dua Qutob MD26, Valeria Raggi MD10, Stephanie Reynaud MD34, Zaccaria Ricci MD13, Zachary A Rumlow DO3, María J Santiago Lozano MD, PhD16, Emily See MBBS20, David T Selewski MD, MSCR35, Carmela Serpe MSc, PhD10, Alyssa Serratore RN, MsC20, Ananya Shah BS18, Weiwen V Shih MD1,18, H Stella Shin MD36, Cara L Slagle MD12, Sonia Solomon DO33, Danielle E Soranno MD37, Rachana Srivastava MD38, Natalja L Stanski MD12, Michelle C Starr MD, MPH37, Erin K Stenson MD1,18, Amy E Strong MD, MSCE3, Susan A Taylor MSc17, Sameer V Thadani MD2, Amanda M Uber DO32, Brynna Van Wyk ARNP, MSN3, Tennille N Webb MD, MSPH39, Huaiyu Zang PhD12, Emily E Zangla DO6, Michael Zappitelli MD, MSc24
1Children’s Hospital Colorado, University of Colorado School of Medicine, Aurora, CO, USA
2Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA
3University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA, USA
4Univeristy of Alberta, Edmonton, Canada
5Le Bonheur Children’s Hospital, Memphis, TN, USA
6University of Minnesota, Minneapolis, MN, USA
7Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, USA
8Cohen Children’s Medical Center, Zucker School of Medicine, New Hyde Park, NY, USA
9Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH, USA
10Bambino Gesù Children Hospital, IRCCS, Rome, Italy
11Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, MO, USA
12Cincinnati Children’s Hospital Medical Center; University of Cincinnati College of Medicine, Cincinnati, OH, USA
13Meyer Children’s Hospital, IRCCS, Florence, Italy
14Medical University of Innsbruck, Innsbruck, Austria
15Stanford University School of Medicine, Palo Alto, CA, USA
16Gregorio Marañón University Hospital, School of Medicine, Madrid, Spain
17King’s College Hospital, London, England
18University of Colorado, School of Medicine, Aurora, CO, USA
19University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA
20Royal Children’s Hospital, University of Melbourne, Murdoch Children’s Research Institute, Melbourne, Victoria, Australia
21University of Michigan Medical School, C.S. Mott Children’s Hospital, Ann Arbor, MI, USA
22Santo Stefano Hospital, Prato, Italy
23 Osaka City General Hospital, Osaka, Japan
24Hospital for Sick Children, Toronto, Ontario, Canada
25NYU Langone Health, Hassenfeld Children’s Hospital, New York, NY, USA
26 Sidra Medicine and Weil Cornel Medicine, Qatar, Doha, Qatar
27Children’s National Hospital, Washington, DC, USA
28Westchester Medical Center, Westchester, NY, USA
29Lucile Packard Children’s Hospital, Palo Alto, CA, USA
30Golisano Children’s Hospital at University of Rochester Medical Center, Rochester, NY, USA
31Seattle Children’s Hospital, University of Washington, Seattle, WA, USA
32University of Nebraska Medical Center, Children’s Hospital & Medical Center, Omaha, NE, USA
33Maria Fareri Children’s Hospital at Westchester Medical Center, Valhalla, NY, USA
34Hopital Bicetre, APHP Université Paris-Saclay, Kremlin-Bicetre, Val de Marne, France
35Medical University of South Carolina, Charleston, SC, USA
36Children’s Healthcare of Atlanta, Emory University, Atlanta, GA, USA
37Indiana University School of Medicine, Riley Hospital for Children, Indianapolis, IN, USA
38Mattel Children’s Hospital at UCLA, Los Angeles, Ca, USA
39Children’s of Alabama/University of Alabama at Birmingham, Birmingham, AL, USA
Additional acknowledgements
We are grateful to the following: T. Christine E. Alvarez MHI RN1, Elizabeth Bixler BS2, Erica Blender Brown MA, CRA3, Cheryl L Brown BS1, Ambra Burrell BA4, Anwesh Dash BS5, Jennifer L Ehrlich RN MHA6, Simrandeep Farma HBSc7, Kim Gahring RN BSN, CCRN8, Barbara Gales RN9, Madison R Hilgenkamp10, Sonal Jain MS11, Kate Kanwar BA MS4, Jennifer Lusk BSN RN, CCRN8, Christopher J. Meyer BA AA1, Katherine Plomaritas BSN RN12, Joshua Porter BS5, Jessica Potts BSN RN13, Alyssa Serratore BNurs, GDipNP(PIC), RN, MsC14, Elizabeth Schneider BS5, Vidushi Sinha BS5, PJ Strack RN,BSN,CCRN15, Sue Taylor RN16, Katherine Twombley MD3, Brynna Van Wyk MSN, ARNP CPNP6, Samantha Wallace MS17, Janet Wang BS5, Megan Woods BS5, Marcia Zinger RN18, Alison Zong BS5
1Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA
2Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA
3Medical University of South Carolina, Charleston, SC, USA
4Nationwide Children’s Hospital, Columbus, OH, USA
5University of Tennessee Health Science Center College of Medicine, Memphis, TN, USA
6University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA, USA
7Hospital for Sick Children, Toronto, ON, Canada
8Children’s Hospital Colorado, Aurora, CO, USA
9Mattel Children Hospital at UCLA, Los Angeles, CA, USA
10University of Nebraska Medical Center, Children’s Hospital & Medical Center, Omaha, NE, USA
11Seattle Children’s Hospital, Seattle, WA, USA
12University of Michigan, C.S. Mott Children’s Hospital, Ann Arbor, MI, USA
13Children’s of Alabama/University of Alabama at Birmingham, Birmingham, AL, USA
14Royal Children’s Hospital, Melbourne, VIC, Australia
15Children’s Mercy Hospital, Kansas City, MO, USA
16King’s College Hospital, London, England
17Indiana University School of Medicine, Riley Hospital for Children, Indianapolis, IN, USA
18Cohen Children’s Medical Center, New Hyde Park, NY, USA
Funding
This study was funded in part by the Gerber Foundation (K Gist, S Menon). REDCap at Cincinnati Children’s Hospital Medical Center is funded and supported by the Center for Clinical and Translational Science and Training grant support (UL1TR001425).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no real or perceived conflicts of interest that could affect the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit for publication. For full disclosure, we provide here an additional list of other authors’ commitments and funding sources that are not directly related to this study: Katja Gist is a consultant for Bioporto Diagnostics and Potrero Medical. Shina Menon is a consultant for Medtronic, Inc. and Nuwellis, Inc. Theresa A. Mottes is a consultant for Medtronic Inc. Melissa Muff-Luett is a consultant for Medtronic Inc. Michael Zappitelli has completed consultant work for Bioporto Diagnostics Inc. No other competing interests were reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A complete list of WEROCK investigators appears in the Acknowledgments. They collaborated in protocol development and review, data analysis, and participated in drafting or review of the manuscript, and their names should be citable by PubMed.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Menon, S., Starr, M.C., Zang, H. et al. Characteristics and outcomes of children ≤ 10 kg receiving continuous kidney replacement therapy: a WE-ROCK study. Pediatr Nephrol (2024). https://doi.org/10.1007/s00467-024-06438-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00467-024-06438-x