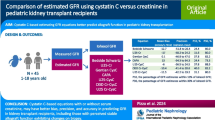
Abstract
Background
Evaluating glomerular filtration rate (GFR) remains challenging in pediatrics; new formulas were developed to increase performance of GFR estimation (eGFR). We aimed to evaluate the recently published formulas as applied to another pediatric population.
Methods
A retrospective study was conducted in a cohort of 307 patients with a “kidney risk” (mean age 12.1 ± 4.5 years, sex ratio 1/1) assessed in a tertiary pediatric nephrology center and a mean measured GFR (mGFR) using plasma iohexol clearance of 85.5 ± 25.3 mL/min/1.73 m2; creatinine levels were measured by IDMS-standardized enzymatic method and cystatin C by immunonephelometry. The following eGFRs were calculated: Schwartz2009, Schwartz-Lyon, CKiDU25creat, and EKFC for eGFR using creatinine (eGFR-creat), CKiDU25cys and FAScys for eGFR using cystatin (eGFR-cys) as well as combined SchwartzCreat-Cys, average (CKiDU25creat-CKiDU25cys), and average (EKFC-FAScys) for eGFR using both biomarkers. The performance of the different formulas was evaluated compared to mGFR by absolute bias measurement and accuracy (p10%, p30%). Results are expressed as mean ± SD.
Results
Creatinine-based formulas and especially the new CKiDU25 and EKFC overestimate GFR, even in children with normal kidney function. However, the bias is constant with these two formulas whatever the age group or gender, contrary to the previously published formulas. In contrast, cystatin C-based equations and combined formulas showed good performance in all age groups and all medical conditions with an acceptable bias and p30%.
Conclusions
In our pediatric population, the performance of all creatinine-based formulas is inadequate with significant GFR overestimation, mainly in subjects with mGFR > 75 mL/min/1.73 m2. Conversely, cystatin C-based or combined formulas have acceptable performance in patients followed in a tertiary pediatric nephrology unit.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The determination of glomerular filtration rate (GFR) is a major component of kidney function monitoring. Whereas measurements of clearance of exogenous substances are still considered as reference methods, their use in daily routine is not possible. Thus, alternative bedside methods are required to evaluate kidney function, such as creatinine and/or cystatin C based-formulas, allowing the calculation of an estimated GFR (eGFR).
Numerous equations have been proposed, but the reliability of these formulas remains debated. Moreover, new formulas have been recently proposed for children and teenagers, notably the EKFC (European Kidney Function Consortium) equation [1] and the CKiDU25 (Chronic Kidney Disease in Children Under 25) formula, based both on creatinine and/or cystatin C [2]. Promising results have been obtained in a wide but general population of children and young adults [3]. Thus, our hypothesis is that performance of these equations might be worse in a tertiary pediatric nephrology population. The aim of this study is to evaluate the performance of these recently published formulas compared to the gold standard iohexol clearance (mGFR) in our population.
Methods
We retrospectively included all children with a plasmatic iohexol clearance measurement with a concomitant creatinine and/or a cystatin C determination obtained during their follow-up in our center between June 2020 and January 2022. The following data were collected: age, body weight, height, body surface area (according to the formula of Dubois (BSA = height0.725 × weight0.425 × 0.007184) [4], medical condition (i.e., indication for mGFR assessment), systolic and diastolic blood pressure (BPs, BPd), plasma creatinine, serum cystatin C, and urinary protein. Percentile of height and blood pressure were calculated using age-based pediatric blood pressure reference charts from the Baylor College of Medicine [5]. BMI was calculated with actual weight as obesity was not a frequent condition in our population. Hypertension (HBP) was defined as BPs and/or BPd > 95th percentile. Percentile of weight and BMI were determined using the World Health Organization (WHO) charts [6].
Biochemical evaluations were performed by our centralized laboratory (Service de biochimie et biologie moléculaire Laboratoire de biologie médicale multisite, Hospices Civils de Lyon, France). All plasma creatinine values were obtained by an enzymatic technique (Architect ©, Abbott Diagnostics) traceable to the National Institute of Standards and Technology (NIST SRM 967 and NIST SRM 914). Laboratory imprecision for creatinine is 1.8%, 1.24%, and 1.37% at 57 µM, 174 µM, and 500 µM, respectively. Cystatin C was measured with a standardized immunonephelometry method (nephelometric Siemens assay on Atellica Neph 630) with a method traceable to the International Federation of Clinical Chemistry Working Group for Standardization of serum cystatin C and the IRMM certified reference materials. Total imprecision of the method was 2.2% and 2.3% at 1.2 and 2.3 mg/L, respectively.
Iohexol plasma clearance (mGFR) was performed according to a standardized technique that used a single-bolus IV injection of iohexol (Omnipaque 300 mg/mL; GE Healthcare SAS, Vélizy-Villacoublay, France). The dose injected was determined by measuring the difference between the weights of the syringe before and after the administration. A multiple sample method was performed and blood samples were drawn from the contralateral arm after 120, 180, and 240 min. Iohexol biochemical assays were performed at the Medical Biology Reference Laboratory (LBMR) (Service de biochimie et biologie moléculaire Laboratoire de biologie médicale multisite, Hospices Civils de Lyon, France). Serum iohexol concentrations were determined using high-performance liquid chromatography ultraviolet light detection (HPLC–UV). The iohexol assay has an analytical imprecision of < 5% (laboratory imprecision for iohexol is 4.9%, 2.94%, and 1.91% at 41 µM, 160 µM, and 317 µM, respectively). The external quality control is provided by Equalis (Uppsala, Sweden) every 3 months. Measured GFR was determined using a slope intercept GFR model, and the results were expressed per 1.73 m2 of body surface area (BSA) calculated according to the formula of Dubois [4]. The GFR was calculated from the slope of plasma concentrations using a 1-compartment model corrected using the Bröchner-Mortensen formula.
As summarized in Table 1, different formulas were used to calculate eGFR: Schwartz2009 [7], Schwartz-Lyon [8], CKiDU25creat [2], and EKFC [1] for the creatinine-based eGFR, as well as CKiDU25cys [2] and FAScys [9] for the cystatin C-based eGFR, and combined Schwartz [7], the mean of CKiDU25creat and CKiDU25cys, and the mean of EKFC and FAScys for combined eGFR. Abnormal albuminuria was defined by urinary albumin/creatinine ratio > 3 mg/mmol.
Variables were tested for normality using the Kolmogorov–Smirnov test. The agreement between mGFR and eGFR was assessed by the calculation of absolute bias (eGFR–mGFR, mL/min/1.73 m2), and accuracy with p30% and p10% (i.e., proportion of eGFR within ± 30% and 10% of mGFR, respectively). We investigated the performance of the different eGFR formulas in subgroups according to the underlying medical condition (namely, transplanted children or not), gender, and the stage of chronic kidney disease (CKD).
Differences between groups were studied by parametric tests (Student’s t-test and one-way analysis of variance (ANOVA) followed by Fisher post hoc test for continuous data), and dichotomized variables were compared using the Pearson χ2 test. Regression graphs and Bland–Altman plots with quantile regression lines (2.5th, median and 97.5th percentile lines) are proposed. Bland–Altman plots and regression graphs were built using the gold standard mGFR [10]. Regression analyses were also performed to assess the evolution of absolute bias with age. All analyses were performed using Statview© software. A value of p < 0.05 was considered statistically significant. Results are presented as mean ± SD. Acceptable performance is defined by an absolute bias ≤ 10% and a p30% ≥ 90% [11, 12]. This retrospective review has been approved by the local IRB (Comité Ethique des Hospices Civils de Lyon, session June 10, 2022, registration number 22_890).
Results
In total, 307 children were analyzed, at a mean age of 12.1 ± 4.5 years, body mass index of 19.3 ± 5.0 kg/m2 (74% of patients having a normal BMI), average percentile of BPs 63.0 ± 28.6, average percentile of BPd 61.3 ± 25.9, mean creatinine of 57.2 ± 27.1 µmol/L, mean mGFR of 85.5 ± 25.3 mL/min/1.73 m2 [15–175], and a mean urinary albumin/creatinine ratio of 9.7 ± 34.4 mg/mmol (among them 31% with abnormal albuminuria). All these characteristics are displayed in Table 2.
Patients were referred for suspected or established kidney dysfunction or kidney risk (for example follow-up of urological abnormalities, metabolic disease potential drug toxicity, and other factors 29%), or after kidney transplantation (31%) or non-kidney (i.e., lung, heart, liver, and bone marrow) transplantation (40%).
The performance of each equation is detailed in Table 3 with three key parameters: absolute bias and accuracy with p30% and p10%. As illustrated in Table 3, in our cohort, only the cystatin C-based formulas have acceptable performance. The combined Schwartz formula also has an excellent performance with an absolute bias at − 0.04 ± 12.0 mL/min/1.73 m2, with a convincing accuracy (p30% at 95% and p10% at 52%) whatever CKD stage. In contrast, all creatinine-based formulas have insufficient performance for a reliable estimation of GFR, whatever the severity of CKD. A significant overestimation of GFR is observed with Schwartz2009 in the group with mGFR > 75 mL/min/1.73 m2, whereas GFR is significantly underestimated by CKiDU25cys in this group of patients. When mGFR is below 75 mL/min/1.73 m2, GFR is overestimated with CKiDU25creat, EKFC, FAScys, mean EKFC-FAScys, and mean CKiDU25creat-CKiDU25cys, as well.
Performance of equations was evaluated according to gender (Table 4). Performance of Schwartz 2009, FAScys, and average EKFC-FAScys was decreased for females. For all other formulas, no significant difference based on mean bias was found.
In the current study, the performance of creatinine-based formulas is better in kidney transplant recipients as compared to other organ transplant recipients and to patients with CKD. Notably, Schwartz2009, Schwartz-Lyon, and CKiDU25creat have a mean absolute bias of 8.0 ± 15.6, 1.6 ± 13.1, and 6.2 ± 12.7 mL/min/1.73 m2 in kidney transplant patients, respectively, while the mean absolute bias of each of the previous formulas is over 10 mL/min/1.73 m2 in the total population (Table 5). In contrast, the CKiDU25cys, FAScys, combined Schwartz formula, average CKiDU25creat-CKiDU25cys, and average EKFC-FAScys have an excellent performance whatever the initial disease with a mean absolute bias lower than 10: − 4.2 ± 8.1, 3.9 ± 9.6, − 0.9 ± 8.6, 1.0 ± 8.4, and 7.0 ± 9.3 mL/min/1.73 m2, respectively, in kidney transplant patients and − 7.0 ± 13.3, 2.8 ± 14.1, − 0.04 ± 12.0, 3.6 ± 12.6, and 7.0 ± 13.0 mL/min/1.73 m2 in the total population. We find no significant differences between the disease subgroups regarding EKFC, combined Schwartz, and average EKFC-FAScys.
The absolute bias depending on age and gender is shown in Figs. 1 and 2 for each formula: the Schwartz 2009 and Schwartz-Lyon overestimate eGFR in the younger age groups, whereas GFR is underestimated in adolescents/young adults both in girls and boys. Similarly, the absolute bias of SchwartzCys is not constant with age in girls (p = 0.002), with an underestimation of eGFR in girls below 10 years. In girls, FAScys and combined Schwartz underestimate eGFR in the youngest age groups. In contrast, in boys, SchwartzCys, FAScys, and combined Schwartz do not significantly vary with age. Overall, the absolute bias is constant in all age groups in both genders for CKiDU25creat, EKFC, and CKiDU25cys. In addition, the absolute bias of eGFR formulas is not constant with GFR. Indeed, for all eGFR formula except Schwartz2009, eGFR is overestimated in severe CKD whereas eGFR formulas underestimate mGFR for normal or high mGFR (Fig. 3).
Absolute bias between cystatin and creatinine-cystatin-based eGFR equations according to age and gender. A FAScys in girls, B FAScys in boys, C CKiDU25cys in girls, D CKiDU25cys in boys, E combined Schwartz in girls, F combined Schwartz in boys, G mean CKiDU25creat- CKiDU25cys in girls, H mean CKiDU25creat- CKiDU25cys in boys, I mean EKFCcreat-FAScys in girls, and J mean EKFCcreat-FAScys in boys
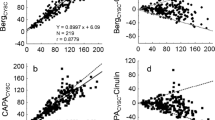
Regression graphs and Bland–Altman plots of the different creatinine based eGFR estimating equations compared with the measured GFR (mGFR) determined with the reference standard, iohexol clearance. A Schwartz2009, B SchwartzLyon, C CKiDU25creat, and D EKFC. Plain lines represent the regression line with the 95% limits of agreement (broken lines)
Discussion
In this study, we show that creatinine-based formulas and especially the new CKiDU25 and EKFC formulas overestimate GFR in our selected pediatric population, whatever the severity of CKD (Figs. 3 and 4). Thus, using such formulas, we may underestimate the severity of kidney disease on one hand but also miss a diagnosis of early CKD on the other hand. However, the bias is constant with these two formulas whatever the age group or gender, contrary to the previously published pediatric formulas. Interestingly, cystatin C-based equations and combined formulas, namely, FAScys, CKiDU25cys, combined Schwartz, average CKiDU25creat-CKiDU25cys, and average EKFC-FAScys, showed a good performance in all age groups and all medical conditions. In our population, the performance of creatinine-based formulas is poor with a significant overestimation of GFR and a p30% < 80% mainly in patients with a GFR > 75 mL/min/1.73 m2. In contrast, cystatin C-based or combined formulas have acceptable performance with a p30% ≥ 90% even in kidney transplant patients. It is worth noting that although the combined Schwartz formula was established in 2012 using the pre-IFCC calibrated cystatin C measurement, its performance is comparable to other formulas combining creatinine and cystatin (average CKiDU25creat-CKiDU25cys and average EKFC-FAScys).
Regression graphs and Bland–Altman plots of the different cystatin and creatinine-cystatin-based eGFR equations compared with the measured GFR (mGFR) determined with the reference standard, iohexol clearance. A FAScys, B CKiDU25cys, C combined Schwartz, D mean CKiDU25 (mean CKiDU25creat-CKiDU25cys), and E mean EKFCcreat-FAScys. Plain lines represent the regression line with the 95% limits of agreement (broken lines)
Leion et al. showed in a cohort of 702 children between 2 and 18 years who were referred for mGFR measurement (i.e., iohexol and inulin clearance in 440 and 262 children, respectively) an acceptable performance for 6 out of 10 creatinine-based formulas (including Schwartz2009 IDMS) and for 4 out of 5 cystatin C-based formulas (including FAScys) with a bias ≤ 10% and p30% ≥ 75% [13]; this is better than our results. However, even though a quite similar creatinine enzymatic IDMS standardized method was used in the two studies, the use of two different methods of mGFR assessment in Leion’s paper could partly explain this discrepancy.
To compare our results with Den Bakker’s results, we studied the relative bias of the different formulas, in particular FAScys. In contrast to their results, we found an overestimation rather than an underestimation with FAScys (6.0% vs. − 4.7%, respectively) [14]. However, the reference method for mGFR assessment was inulin clearance which could explain quite different results as compared to iohexol clearance. Moreover, the assays used for creatinine measurement were also not exactly the same, namely, IDMS corrected Jaffé method before 2008 and then IDMS standardized enzymatic method, respectively [14]. Lastly, our current results are in accordance with Salvador’s results showing in a cohort of 96 children an unacceptable bias over 10% and low p30% with creatinine-based equations with iohexol clearance, while demonstrating a good performance of combined Schwartz and SchwartzCys with low bias and p30% at 90% [15].
Nyman et al. [3] conducted an external validation of the CKiDU25 equation in the European cohort of the EKFC (European Function Consortium), which included 2293 children with a mean mGFR of 97 mL/min/1.73 m2 with similar characteristics, such as age (11.9 years) and BMI (18 kg/m). They found that CKiDU25creat and EKFC exhibited minimal bias (1.3 and − 1.6 mL/min/1.73 m2, respectively) and an accuracy of > 80% (P30), that is much better performance than in our study. In contrast, Nyman et al. observed that cystatin C-based equations (CKiDU25cys and FAScys) underestimated mGFR, while only CKiDU25cys showed underestimation in our study. It is worth noting that, while creatinine determination was standardized to IDMS in both studies, there were differences in GFR determination and the study populations. Nyman et al. used data from various European centers, suggesting a more diverse pediatric population, whereas our study focused on patients from a tertiary nephrology center [3]. Additionally, although general characteristics, including BMI, were comparable between the two studies, it is possible that altered body composition in our patients may have contributed to the differences in GFR estimation. This highlights the importance of not relying solely on creatinine levels for estimating GFR in specific populations.
In the present study, we used iohexol plasma clearance. Iohexol (molecular weight 0.821 kDa) is indeed considered as an ideal marker of glomerular filtration: it is a nonionic, low-osmolar contrast agent, freely filtered through the glomerulus and neither secreted, metabolized, nor reabsorbed by the kidney [16,17,18]. Iohexol has < 2% plasma protein binding and a nearly negligible extrarenal clearance [16, 19]. Although several authors demonstrated that results of urinary inulin clearance measurement and plasmatic iohexol clearance were comparable [20,21,22], small differences can nevertheless be found.
Depending on age and gender, children, adolescents, and young adults will experience significant variations in body composition, particularly in muscle mass and fat mass, which can influence the performance of creatinine-based equations. Some formulas have thus been developed to address these challenges, particularly the CKiDU25 or EKFC/FAS formulas. Therefore, we compared performance of the various equations according to gender. Whereas global performance in terms of mean bias is poor in the whole group with Schwartz2009, which has no specific coefficient according to age, a worse performance was found in female patients. A slightly poorer performance was observed with FAScys and consequently with average EKFC-FAScys in the female group in our population. For all other formulas, no difference in mean bias was observed, confirming the importance of adapting formulas according to gender and age in the pediatric/adolescent/young adult population.
Using a theoretical approach, Pottel showed that the CKiDU25creat formula provides a constant bias in all age groups and genders between 1 and 25 years, as opposed to Schwartz2009 [23]. Here, we confirm these results with a constant bias both with EKFC and CKiDU25. However, with the cystatin-based equation, we obtained a constant bias only with CKiDU25cys which is significantly different than FAScys in girls. Finally, the better performance (lower mean bias and better accuracy (p30% and p10%) of EKFC, CKiDU25creat, CKiDU25cys, and combined creat-cys equations (combined Schwartz, average CKiDU25creat-CKiDU25cys, and average EKFC-FAScys) associated with a quite constant bias among age groups and gender, allowing a more reliable estimation of GFR in clinical practice. Conversely, the Schwartz2009 and Schwartz-Lyon formulas overestimated the eGFR in a variable way according to age and do not allow a sufficiently reliable estimate over time for the patient.
It is well known that performance of GFR-estimating equations depends on CKD stage and the different evaluated cohorts [15, 24,25,26]. In accordance with Salvador et al. [15], creatinine-based equations perform better in CKD patients (GFR < 75 mL/min/1.73 m2) contrary to cystatin C ones in which bias is significantly lower when GFR is over 75 mL/min/1.73 m2. Finally, combined Schwartz, average CKiDU25creat-CKiDU25cys, and the mean of EKFC and FAScys provide an acceptable performance whatever CKD level, which is of main importance in clinical practice.
In specific populations, it has been demonstrated that the use of cystatin C increases the performance of GFR estimating formulas [27,28,29], but cystatin C is not available in all laboratories and remains expensive compared to creatinine. When only creatinine is available, EKFC and CKiDU25creat give satisfactory results, with a constant bias with age. However, the use of CKiDU25creat requires height measurement, thus preventing all laboratories to estimate GFR as suggested by the recommendations; moreover, it does not prevent the problem of change of formula at the age of 25 years [2]. The performance of the EKFC is satisfying; the bias is constant, allows estimation of GFR without height, and avoids the change of eGFR formula at the time of the adolescent-adult transition period with an improbable change in eGFR between the formulas used in children and adults [1].
The strengths of this study are the use of the same reference GFR measurement method by iohexol clearance as well as a unique enzymatic IDMS standardized creatinine determination method (reference method) for all patients. As for weaknesses, this cohort studies only a White/Caucasian population, with no Black race, and there is no proposed adjustment by ethnicity for children and young adults. Another weakness is the lack of measurement of body composition in our patients to estimate abnormal muscle mass in our population. In addition, GFR measurement is expressed in mL/min/1.73 m2 based on a calculation of body surface area using a formula established in normal-weighted patients. However, this approach may not be suitable for overweight or obese patients, potentially leading to inaccurate final results in mGFR [30, 31]. This issue is likely more prevalent in a tertiary pediatric nephrology population.
Conclusion
New creatinine-based equations CKiDU25creat and EKFC allow a better estimation of eGFR especially in adolescents and teenagers compared to Schwartz2009. However, in patients followed in a tertiary pediatric nephrology unit, creatinine remains an insufficient parameter for estimating GFR with a significant overestimation of GFR which could result in misdiagnosis of CKD. The cystatin C or creatinine-cystatin C combination allows better estimation of GFR in this population and should be preferred when cystatin C determination is possible.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to confidential data but are available from the corresponding author on reasonable request.
References
Pottel H, Björk J, Courbebaisse M, Couzi L, Ebert N, Eriksen BO et al (2021) Development and validation of a modified full age spectrum creatinine-based equation to estimate glomerular filtration rate: a cross-sectional analysis of pooled data. Ann Intern Med 174:183–191
Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ (2021) Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int 99:948–956
Nyman U, Björk J, Berg U, Bökenkamp A, Dubourg L, Goffin K et al (2022) The modified CKiD study estimated GFR equations for children and young adults under 25 years of age: performance in a European multicenter cohort. Am J Kidney Dis 80:807–810
Du Bois D, Du Bois EF (1989) A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5:303–311
Shypailo RJ (2018) Age-based pediatric blood pressure reference charts. Baylor College of Medicine, Children’s Nutrition Research Center, Body Composition Laboratory. http://www.bcm.edu/bodycomplab/BPappZjs/BPvAgeAPPz.htm. Accessed 15 Dec 2023
World Health Organization (2004) The WHO multicentre growth reference study (MGRS). https://www.who.int/tools/child-growth-standards/who-multicentre-growth-reference-study. Accessed 15 Dec 2023
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA et al (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
De Souza VC, Rabilloud M, Cochat P, Selistre L, Hadj-Aissa A, Kassai B et al (2012) Schwartz formula: is one k-coefficient adequate for all children? PLoS One 7:e53439
Pottel H, Delanaye P, Schaeffner E, Dubourg L, Eriksen BO, Melsom T et al (2017) Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant 32:497–507
Krouwer JS (2008) Why Bland-Altman plots should use X, not (Y+X)/2 when X is a reference method. Stat Med 27:778–780
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 3(Suppl):1–150
Stevens LA, Zhang Y, Schmid CH (2008) Evaluating the performance of equations for estimating glomerular filtration rate. J Nephrol 21:797–807
Leion F, Hegbrant J, den Bakker E, Jonsson M, Abrahamson M, Nyman U et al (2017) Estimating glomerular filtration rate (GFR) in children. The average between a cystatin C- and a creatinine-based equation improves estimation of GFR in both children and adults and enables diagnosing Shrunken Pore Syndrome. Scand J Clin Lab Invest 77:338–344
den Bakker E, Gemke RJ, van Wijk JA, Hubeek I, Stoffel-Wagner B, Bökenkamp A (2020) Evidence for shrunken pore syndrome in children. Scand J Clin Lab Invest 80:32–38
Salvador CL, Tøndel C, Rowe AD, Bjerre A, Brun A, Brackman D et al (2019) Estimating glomerular filtration rate in children: evaluation of creatinine- and cystatin C-based equations. Pediatr Nephrol 34:301–311
Bäck SE, Krutzén E, Nilsson-Ehle P (1988) Contrast media as markers for glomerular filtration: a pharmacokinetic comparison of four agents. Scand J Clin Lab Invest 48:247–253
Gaspari F, Perico N, Ruggenenti P, Mosconi L, Amuchastegui CS, Guerini E et al (1995) Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol 6:257–263
Olsson B, Aulie A, Sveen K, Andrew E (1983) Human pharmacokinetics of iohexol. A new nonionic contrast medium. Invest Radiol 18:177–182
Krutzén E, Bäck SE, Nilsson-Ehle I, Nilsson-Ehle P (1984) Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med 104:955–961
Berg UB, Bäck R, Celsi G, Halling SE, Homberg I, Krmar RT et al (2011) Comparison of plasma clearance of iohexol and urinary clearance of inulin for measurement of GFR in children. Am J Kidney Dis 57:55–61
Dubourg L, Lemoine S, Joannard B, Chardon L, de Souza V, Cochat P et al (2021) Comparison of iohexol plasma clearance formulas vs. inulin urinary clearance for measuring glomerular filtration rate. Clin Chem Lab Med 59:571–579
Brown SC, O’Reilly PH (1991) Iohexol clearance for the determination of glomerular filtration rate in clinical practice: evidence for a new gold standard. J Urol 146:675–679
Pottel H, Björk J, Delanaye P, Nyman U (2022) Evaluation of the creatinine-based chronic kidney disease in children (under 25 years) equation in healthy children and adolescents. Pediatr Nephrol 37:2213–2216
Björk J, Nyman U, Berg U, Delanaye P, Dubourg L, Goffin K et al (2019) Validation of standardized creatinine and cystatin C GFR estimating equations in a large multicentre European cohort of children. Pediatr Nephrol 34:1087–1098
Selistre L, Rabilloud M, Cochat P, de Souza V, Iwaz J, Lemoine S et al (2016) Comparison of the Schwartz and CKD-EPI equations for estimating glomerular filtration rate in children, adolescents, and adults: a retrospective cross-sectional study. PLoS Med 13:e1001979
Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG et al (2007) Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol 18:2749–2757
White C, Akbari A, Hussain N, Dinh L, Filler G, Lepage N et al (2005) Estimating glomerular filtration rate in kidney transplantation: a comparison between serum creatinine and cystatin C-based methods. J Am Soc Nephrol 16:3763–3770
Pöge U, Gerhardt T, Stoffel-Wagner B, Palmedo H, Klehr HU, Sauerbruch T et al (2006) Cystatin C-based calculation of glomerular filtration rate in kidney transplant recipients. Kidney Int 70:204–210
Yang M, Xu G, Ling L, Niu J, Lu T, Du X et al (2017) Performance of the creatinine and cystatin C-based equations for estimation of GFR in Chinese patients with chronic kidney disease. Clin Exp Nephrol 21:236–246
Filler G, Torres-Canchala L, Sharma AP, González D, de Ferris ME, Restrepo JM (2023) What to do with kidney length and volumes in large individuals? Pediatr Nephrol 38:1395–1398
Filler G (2023) Editorial: there is still a need for kidney volume reference intervals in large children, adolescents, and young adults. Can J Kidney Health Dis 10:20543581231173295
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roussel, M., Bacchetta, J., Sellier-Leclerc, A.L. et al. Creatinine-based formulas are not ideal to estimate glomerular filtration rate in selected pediatric patients: data from a tertiary pediatric nephrology center. Pediatr Nephrol 39, 3023–3036 (2024). https://doi.org/10.1007/s00467-023-06275-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06275-4