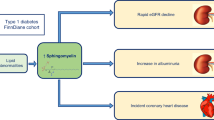
Abstract
Background
This study evaluated urinary sphingolipids as a marker of diabetic kidney disease (DKD) in adolescents and young adults with youth-onset type 1 and type 2 diabetes.
Methods
A comprehensive panel of urinary sphingolipids, including sphingomyelin (SM), glucosylceramide (GC), ceramide (Cer), and lactosylceramide (LC) species, was performed in patients with youth-onset diabetes from the SEARCH for Diabetes in Youth cohort. Sphingolipid levels, normalized to urine creatinine, were compared in 57 adolescents and young adults with type 1 diabetes, 59 with type 2 diabetes, and 44 healthy controls. The association of sphingolipids with albumin-to-creatinine (ACR) ratio and estimated glomerular filtration rate (eGFR) was evaluated.
Results
The median age (interquartile range [IQR]) of participants was 23.1 years (20.9, 24.9) and the median duration of diabetes was 9.3 (8.5, 10.2) years. Urinary sphingolipid concentrations in patients with and without DKD (ACR ≥ 30 mg/g) were significantly elevated compared to healthy controls. There were no significant differences in sphingolipid levels between participants with type 1 and type 2 diabetes. In multivariable analysis, many sphingolipid species were positively correlated with ACR. Most significant associations were evident for the following species: C18 SM, C24:1 SM, C24:1 GC, and C24:1 Cer (all p < 0.001). Sphingolipid levels were not associated with eGFR. However, several interaction terms (diabetes type*sphingolipid) were significant, indicating diabetes type may modify the association of sphingolipids with eGFR.
Conclusion
Urinary sphingolipids are elevated in adolescents and young adults with youth-onset diabetes and correlate with ACR. Urinary sphingolipids may therefore represent an early biomarker of DKD.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a leading cause of kidney disease, accounting for approximately 45% of cases of kidney failure in the United States [1]. The management of diabetic kidney disease (DKD) remains challenging, as sensitive and specific markers of disease progression are lacking. While albuminuria has traditionally been clinically used to identify patients at risk for disease progression, it has significant limitations. Up to 50% of patients with diabetes and albuminuria can regress to normoalbuminuria [2], and 29–41% of patients with diabetes may experience a decline of kidney function despite having normal urinary albumin excretion [3,4,5]. Therefore, a pressing need for the development of more sensitive and specific biomarkers of disease progression in DKD has been widely recognized [6].
Sphingolipids, a class of lipids involved in intracellular signaling and cellular metabolism, have been identified as a potential mediator of DKD in both type 1 and type 2 diabetes mellitus. Urinary sphingolipids may therefore represent a novel early biomarker of kidney injury and disease progression in DKD. Dysregulation of sphingolipid metabolism occurs in diabetes, resulting in increased de novo synthesis of sphingolipids in kidney tissue [7, 8]. Subsequent accumulation of sphingolipid species has been shown to mediate early changes of DKD including glomerular hypertrophy and extracellular matrix productions [9, 10]. Sphingolipids have also been identified as mediators of particular comorbidities exclusively associated with type 2 diabetes including insulin resistance, inflammation, and hepatic steatosis [11,12,13]. Furthermore, obesity-related fatty acid dysregulation and inflammation are potential causes of increased sphingolipid production [14]. Sphingolipids may therefore play a more important role in the pathophysiology of DKD in type 2 diabetes.
In the current study, we investigated urinary sphingolipid profiles in a sub-cohort of participants with youth-onset diabetes in the SEARCH for Diabetes in Youth (SEARCH) study. We aimed to 1) compare urinary sphingolipid profiles in youth-onset type 1 and type 2 diabetes, and 2) investigate the association of urinary sphingolipids with traditional markers of DKD. We hypothesized that urinary sphingolipids would be more elevated and associated with albuminuria in adolescents and young adults with youth-onset type 2 diabetes mellitus.
Methods
Study cohort and design
This is a cross-sectional study that compares urinary sphingolipids in a sub-cohort of adolescents and young adults with youth-onset type 1 and type 2 diabetes in the SEARCH study. SEARCH is a multicenter observational cohort study of youth with diabetes mellitus, and the methods of the SEARCH study have been previously reported [15, 16]. Briefly, youths less than 20 years of age who received a new diagnosis of non-gestational diabetes were identified from six recruitment centers from 2000–2010. A subset of participants at least 10 years of age and with 4 years diabetes duration were recruited for an outcome visit between 2011 and 2015. From this cohort, urine samples from 57 participants with type 1 diabetes and 59 with type 2 diabetes were randomly selected with serum creatinine and cystatin C values available for estimation of glomerular filtration rate (eGFR). Clinical characteristics of study participants and laboratory data were provided by the SEARCH data coordinating center, including age, race, diabetes type and duration, medications, body mass index (BMI), hemoglobin A1C levels, lipid profiles, serum creatinine and cystatin C, and urinary albumin and creatinine. As trends in diabetes incidence vary by race, race was determined via self-report by the parent or participant, depending on age. eGFR was estimated using the average of the recently derived cystatin C and creatinine U25 equation, as this provides a more accurate and precise measurement of GFR compared to single-marker estimates [17]. DKD was defined as a urinary albumin-to-creatinine ratio (ACR) ≥ 30 mg/g. To provide a comparison group of healthy youth without diabetes, 43 healthy control samples were selected from a local biorepository at Cincinnati Children’s Hospital Medical Center (CCHMC). This cohort was frequency matched according to age and sex to the study cohort. The study was approved by the local Institutional Review Board.
Sphingolipid analysis
A comprehensive panel of urinary sphingolipids was performed that included the following sphingolipid species: ceramide (Cer) species (C16, C18, C20, C24, C24:1); glucosylceramide (GS) species (C16, C18, C20, C22, C24, C24:1, C26); lactosylceramide (LC) species (C16, C18, C20, C24, C24:1, C26); and sphingomyelin (SM) species (C16, C18, C20, C22, C24:1, C24). Individual species of the sphingolipid panel were selected based on previous studies demonstrating these species were elevated in obesity and diabetes [10, 18]. Testing was performed at the CCHMC Clinical Mass Spectroscopy Laboratory (Director, Kenneth Setchell, PhD), as previously reported [18]. Individual sphingolipid species were detected and quantified in urine specimens by liquid chromatography electrospray ionization tandem mass spectrometry (LC–ESI–MS/MS) using a Waters Xevo TQ-S triple-quadrupole mass spectrometer interfaced with Acquity UPLC system (Waters, Milford, MA). Urine was extracted twice with the Folch procedure. Optimized parameters were determined with individual standard compounds. Sphingolipids were separated by an Acquity UPLC CSH C18 column and quantified under positive ion mode. Reference standards of all the sphingolipid species quantified were obtained from Avanti Lipids (Alabaster, Alabama) and used to construct calibration curves. For quantification, the precursor and product ion pairs for multiple reaction monitoring were selected by MS/MS spectra of standards or calculated theoretically if the standard for a given sphingolipid was not available. Quantification of sphingolipid species with various fatty acid chain lengths was performed using the curve of each sphingolipid species with the closest number of chain length.
Statistical analysis
Descriptive statistics were summarized and comparisons between the groups made using chi-squared testing for categorical variables and Kruskal–Wallis testing for continuous variables. Comparisons were made within each individual sphingolipid species, as well as for aggregate levels for each category. Linear regression modeling was used to investigate the association of urinary sphingolipids levels with ACR and eGFR. Multivariable analysis was then performed to assess the independent associations while controlling for clinically relevant covariates, including age, BMI, diabetes duration, serum triglyceride level, hypertension, HbA1c, sex, and diabetes type. Interactions between diabetes type and sphingolipid levels were investigated to determine if the relationship between urinary sphingolipids and outcomes was modified by diabetes type. Log transformation was used as appropriate to fulfill the assumptions of linear regression modeling. All analyses were performed with SAS statistical software, version 9.4.
Results
Demographic and clinical characteristics
The study cohort included 116 adolescents and young adults with youth-onset diabetes: 57 with type 1 diabetes and 59 with type 2 diabetes. The median age (IQR) of participants was 23.1 years (20.9, 24.9) and the median duration of diabetes was 9.3 (8.5, 10.2) years. Overall, 53% were females, 38.8% were Black, 37% were white, 21.6% were Hispanic, and 2.6% were Asian American/Pacific Islander. Demographic characteristics of the control subjects were similar to study subjects: mean age 23 years (21, 25), and 49% female.
Comparison of clinical and demographic characteristics between participants with type 1 and type 2 diabetes are presented in Table 1. There were no differences in sex or race between participants with type 1 and type 2 diabetes. There were no differences in indices of glycemic control or diabetes duration between those with type 1 and type 2 diabetes, though participants with type 2 diabetes were slightly older. Those with type 2 diabetes also had an increased prevalence of cardiovascular risk factors, including higher BMI, triglyceride levels, blood pressure, and lower HDL cholesterol levels. eGFR and ACR were similar in participants with type 1 and type 2 diabetes.
Total (Table 2) and individual levels (Fig. 1) of different subspecies of SM, Cer, GC, and LC were markedly higher in females compared to males among children with diabetes. There was no significant difference among sphingolipid classes according to race/ethnicity. BMI was not associated with total levels of any sphingolipid class. Increase in age was associated with a weak but statistically significant decrease in total LC (r = -0.21, p = 0.027) and total SM levels (r = -0.29, p = 0.002). There was a significant correlation of HbA1c with total Cer (r = 0.27), GC (r = 0.24), and LC (r = 0.27) levels (all p < 0.01). Sphingolipid levels did not correlate with LDL and HDL cholesterol, or serum triglycerides.
Among control subjects, total levels of LC were higher in females (4.9 vs. 3.2 nmol/gm, p = 0.002), but other sphingolipid subspecies did not reach statistical significance.
Urinary sphingolipid profiles
All sphingolipid species were significantly higher in adolescents and young adults with type 1 and type 2 diabetes compared to healthy controls (Fig. 2). The most significant differences were seen for all C16 species (C16SM, C16Cer, C16GC, and C16LC), C24:1Cer and C24:1LC, and C24Cer, C24GC, and C24LC. Results remained similar when stratified by sex, though some species did not reach statistical significance (Supplemental Fig. 1). There were no differences in any sphingolipid species between participants with type 1 and type 2 diabetes. Therefore, subsequent analyses were performed using a single dataset combining type 1 and type 2 diabetes.
Comparison of sphingolipid levels in adolescent and young adults with DKD (ACR ≥ 30), those without DKD, and healthy controls is shown in Fig. 3. In general, sphingolipids were higher in those with DKD compared to those without, and those without DKD had elevated levels compared to healthy controls. This pattern was most prominent and significant for C16SM, C24:1SM, C24Cer, C24:1Cer, C22 GC, C24 GC, and C24:1 GC.
Association of urinary sphingolipid species with ACR and eGFR
In adjusted analyses, all SM species as well as many Cer and GC species were significantly associated with ACR (Fig. 4). Lactosylceramide species were not associated with ACR. The most significant associations were seen for C18SM, C24:1 SM, C24:1Cer, and C24:1 GC species (all p < 0.001), where there was more than a twofold increase in ACR per IQR increase in sphingolipid levels. To investigate if the association of sphingolipids with ACR varied according to diabetes type, interaction terms were tested for each species (diabetes type × sphingolipid species). All interaction terms were not significant.
There was no association of any sphingolipid species with eGFR in adjusted analyses (Supplemental Fig. 2). However, several interaction terms were significant, indicating the association of urinary sphingolipids with eGFR varied according to diabetic type (Supplemental Fig. 3). Interaction terms were most significant for C24:1 Cer, C24:1 GC, C16 LC, C18 LC, C20 LC, and C24:1 LC. For these sphingolipid species, there was a significant positive association of species C20 LC and C24 LC with eGFR in type 1 diabetes. However, in type 2 diabetes, negative associations were observed between eGFR and the following sphingolipid species: C24:1 Cer, C24:1 GC, C18 LC, and C24:1 LC.
Discussion
We demonstrated that urinary sphingolipids are significantly elevated in adolescents and young adults with youth-onset diabetes compared to healthy controls. Many individual urinary sphingolipid species were associated with increased ACR, indicating these species may represent early biomarkers of kidney injury. Sphingolipid profiles were similar in both type 1 and type 2 diabetes, indicating that diabetes per se, rather than obesity-related metabolic abnormalities such as insulin resistance, inflammation, and fatty acid dysregulation, is primarily responsible for increased urinary sphingolipid levels.
Sphingolipid dysregulation has been implicated in the pathogenesis of a variety of kidney diseases, including lupus nephritis, Fabry’s disease, and diabetes [19]. Though the exact mechanism of sphingolipid-mediated kidney injury has yet to be elucidated, it may occur via the disruptive effects of sphingolipid accumulation on podocyte integrity or mitochondrial function [20, 21]. As dysregulated lipid metabolism has been implicated in the pathogenesis of diabetes, particular attention has been given to the role of sphingolipids in DKD [22]. Previous studies have demonstrated that increased plasma sphingolipid species correlate with albuminuria in type 1 and type 2 diabetes [23,24,25]. Urinary levels of ceramide were investigated by Morita et al. in adults with type 2 diabetes mellitus, and they found elevated levels in those with more advanced DKD [26]. Our study similarly showed that sphingolipid species were elevated in adolescents and young adults with DKD, and many species demonstrated strong correlation with albuminuria. Together, these finding indicate sphingolipids may represent a useful biomarker of DKD.
Despite increasing evidence linking altered sphingolipid metabolism to DKD, the clinical application of these biomarkers for disease progression has not been established. In our cohort, urinary sphingolipids were elevated even among subjects without albuminuria, suggesting these may represent a more sensitive marker of early kidney injury. Increased production of sphingolipids in kidney tissue may cause elevation in urinary levels in the absence of more global injury to the glomerular basement membrane and subsequent albuminuria. Therefore, urinary sphingolipids may identify kidney injury in those patients with DKD but with normal albumin excretion. However, we were unable to assess the long-term risk of kidney disease progression among those adolescents with elevated urinary sphingolipid levels, and longitudinal studies are therefore needed to evaluate the prognostic value of urinary sphingolipids in DKD. It is possible that increased urinary sphingolipids may be a result of early kidney injury but not causally related. Intracellular levels of all sphingolipid species may increase in proportion to diabetic severity, with subsequent release into the urine in proportion to the extent of diabetic injury from other processes. Future studies are needed to assess whether urinary sphingolipids are more predictive than urinary albumin in assessing the risk of progression of kidney disease.
Considering the well-established associations between obesity, inflammation, and sphingolipid synthesis [14, 27, 28], the finding of similar urinary sphingolipid profiles in type 1 and type 2 diabetes was not in agreement with our hypothesis and was somewhat surprising. Hyperglycemia as the main cause of increased urinary sphingolipids, rather than metabolic dysfunction, was further supported by the following findings: 1) significant correlations existed between HbA1c and urinary Cer, GC, and LC species, and 2) no correlation was seen between sphingolipid levels and BMI or serum triglycerides. A possible explanation is that hyperglycemia induces metabolic pathways that increase production of intermediates needed for sphingolipid production. For example, reduced nicotinamide adenine dinucleotide phosphate (NADPH) can be generated from glucose shunting to the pentose phosphate pathway, and this is a required cofactor for both fatty acid and dihydrosphingosine synthesis [9, 29]. Aerobic glycolysis also can generate acetyl CoA and serine, both of which are substrates for de novo sphingolipid synthesis [30]. Thus, increased sphingolipid production in response to hyperglycemia may represent a common pathophysiologic pathway of DKD in both type 1 and type 2 diabetes.
An interesting finding of this study was the interaction between diabetes type and eGFR for several sphingolipid species, most notably C24:1 Cer, C24:1 GC, and several LC species. Specifically, these urinary sphingolipids tended to be associated with an increase in eGFR in type 1 diabetes, while they were associated with a decrease in eGFR among those with type 2 diabetes. This might indicate that urinary sphingolipids are associated with hyperfiltration in type 1 diabetes, which is presumed to be the earliest manifestation of kidney injury [31]. Conversely, urinary sphingolipids may associate with more advanced kidney injury (i.e., a decline in eGFR) in type 2 diabetes. It may also be that obesity-associated hyperfiltration, which occurs primarily in type 2 diabetes, is a confounding factor that modifies the relationship between urinary sphingolipids and eGFR between those with type 1 and type 2 diabetes.
To our knowledge, this is the first study to report sex-related differences in urinary sphingolipids, with females consistently demonstrating higher levels across all individual species. Other studies have shown elevations of serum sphingolipids levels in females, and similarly hypothesized this may be attributed to sex-related differences in metabolism-related genes [32, 33]. Considering sex-related differences in control subjects were much less apparent (only LC species were significantly higher), this may indicate that sphingolipid production in response to hyperglycemia is more pronounced in females. Evidence suggests women with diabetes may be at higher risk for progression of kidney disease [34, 35], and females with youth-onset diabetes have increased prevalence of hyperfiltration and proteinuria [36,37,38]. Therefore, increased urinary sphingolipid production in females with diabetes may explain these sex-related differences in DKD. Future studies with long-term follow-up are needed to further investigate the interplay between sex, urinary sphingolipid levels, and risk for progression of DKD.
This study has many strengths. Participants were selected from a large, multicenter, representative population of adolescents and young adults with diabetes [16]. Our cohort included only patients with both a serum creatinine and cystatin C value, thereby enabling us to estimate GFR using the U25 estimating equations, which have been validated in both children and young adults [17]. However, several limitations deserve mention. First, the observational nature of the study prevents any inference regarding the causative effect of urinary sphingolipids in the pathogenesis of DKD, though a growing body of evidence suggests the pathogenic role of glomerular sphingolipid accumulation in kidney disease [30]. Second, ACRs were obtained using a single spot urine, rather than repeated measurements that are recommended to diagnose persistent albuminuria due to the day-to-day variation that can occur in urine albumin excretion [39]. Finally, although we investigated a comprehensive panel of urinary sphingolipids, it is possible a downstream product of sphingolipids may be responsible for mediating DKD. For example, GM3 ganglioside, a higher order glycosphingolipid, antagonizes insulin resistance and is increased in the kidney tissue of rats with type 1 diabetes [40, 41].
In conclusion, we demonstrated that urinary sphingolipids are similarly elevated in both type 1 and type 2 diabetes. This indicates that hyperglycemia, rather than obesity associated-metabolic dysfunction, is responsible for increased urinary sphingolipid levels. Several urinary sphingolipids were independently associated with albuminuria and were increased even in those with normal urinary albumin excretion. Urinary sphingolipids may therefore represent an early biomarker of DKD.
Funding and assistance
Grant Support (SEARCH 4)
The SEARCH for Diabetes in Youth Cohort Study (1R01DK127208-01, 1UC4DK108173) is funded by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases and supported by the Centers for Disease Control and Prevention.
The Population Based Registry of Diabetes in Youth Study (1U18DP006131, U18DP006133, U18DP006134, U18DP006136, U18DP006138, and U18DP006139) is funded by the Centers for Disease Control and Prevention (DP-15–002) and supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Grant Support (SEARCH 1, 2, 3)
SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05–069, and DP-10–001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases.
Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Cincinnati's Children's Hospital Medical Center (U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708), Seattle Children's Hospital (U58/CCU019235-4, U01 DP000244, and U18DP002710-01] and Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200–2010-35171).
Data availability
Data from the SEARCH for Diabetes in Youth that were used in this study are available for request at the NIDDK Central Repository (NIDDK-CR) website, Resources for Research (R4R), https://repository.niddk.nih.gov/.
References
Saran R, Robinson B, Abbott KC et al (2019) US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 73:A7–A8
Macisaac RJ, Jerums G (2011) Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens 20:246–257
Caramori ML, Fioretto P, Mauer M (2003) Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes 52:1036–1040
Dwyer JP, Parving HH, Hunsicker LG, Ravid M, Remuzzi G, Lewis J (2012) Renal dysfunction in the presence of normoalbuminuria in type 2 diabetes: Results from the demand study. Cardiorenal Med 2:1–10
MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G (2004) Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 27:195–200
Lee CH, Lam KS (2015) Biomarkers of progression in diabetic nephropathy: The past, present and future. J Diabetes Investig 6:247–249
Sas KM, Nair V, Byan J et al (2015) Targeted lipidomic and transcriptomic analysis identifies dysregulated renal ceramide metabolism in a mouse model of diabetic kidney disease. J Proteomics Bioinform Suppl 14:002
Liu G, Han F, Yang Y et al (2011) Evaluatin of sphingolipid metabolism in renal cortex of rats with streptoztocin-induced diabetes and the effects of rapamycin. Nephrol Dial Transplant 26:1493–1502
Zador IZ, Deshmukh GD, Kunkel R, Johnson K, Radin NS, Shayman JA (1993) A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes mellitus. J Clin Invest 91:797–803
Subathra M, Korrapati M, Howell LA et al (2015) Kidney glycosphingolipids are elevated early in diabetic nephropathy and mediate hypertrophy of mesangial cells. Am J Physiol Renal Physiol 309:F204–F215
Bijl N, Sokolovic M, Vrins C et al (2009) Modulation of glycosphingolipid metabolism significantly improves hepatic insulin sensitivity and reverses hepatic steatosis in mice. Hepatology 50:1431–1441
Boon J, Hoy AJ, Stark R et al (2013) Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 62:401–410
Griess K, Rieck M, Muller N et al (2023) Sphingolipid subtypes differentially control proinsulin processing and systemic glucose homeostasis. Nat Cell Biol 25:20–29
Aburasayn H, Al Batran R, Ussher JR (2016) Targeting ceramide metabolism in obesity. Am J Physiol Endocrinol Metab 311:E423-435
SEARCH Study Group (2004) SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 25:458–471
Hamman RF, Bell RA, Dabelea D et al (2014) The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care 37:3336–3344
Pierce CB, Munoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ (2021) Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int 99:948–956
Davis S, Nehus E, Inge T, Zhang W, Setchell K, Mitsnefes M (2018) Effect of bariatric surgery on urinary sphingolipids in adolescents with severe obesity. Surg Obes Relat Dis 14:446–451
Mallela S, Merscher S, Fornoni A (2022) Implications of sphingolipid metabolites in kidnes diseases. Int J Mol Sci 23:4244
Li G, Kidd J, Gehr T, Li P (2021) Podocyte sphingolipid signaling in nephrotic syndrome. Cell Phsiol Biochem 55(Suppl 4):13–34
Roszczyc-Owsiejczuk K, Zabielski P (2021) Sphingolipids as a culprit of mitochondrial dysfunction in insulin resistance and type 2 diabetes. Front Endcrionol 12:635175
Hammand S, Lopes-Virella M (2023) Circulating sphingolipids in insulin resistance, diabetes, and associated complications. Int J Mol Sci 24:14015
Lui J, Ghosh S, Kovalik J et al (2016) Profiling of plasma metabolites suggests altered mitochondrial fuel usage and remodeling of sphingolipid metabolism in individuals with type 2 diabetes and kidney disease. Kidney Int Rep 2:470–480
Barlovic D, Harjustsalo V, Sandhom N, Forsblom C; FinnDiane Study Group (2020) Sphingomyelin and progression of renal and coronary heart disease in individuals with type 1 diabetes. Diabetologia 63:1847–1856
Makinen V, Tynkkynen T, Soininen P et al (2012) Sphingomyelin is associated with kidney disease in type 1 diabetes (The FinnDiane Study). Metabolomics 8:369–375
Morita Y, Kuran M, Sakai E et al (2020) Analysis of urinary sphingolipids usiing liquid chromatography-tandem mass spectrometyr in diabetic nephropathy. J Diabetes Invest 11:441–449
Bikman BT, Summers SA (2011) Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest 121:4222–4230
Russo SB, Ross JS, Cowart LA (2013) Spingolipids in obesity, type 2 diabetes, and metabolic disease. Handb Exp Pharmacol 216:373–401
Stathem M, Marimuthu S, O’Neal J et al (2015) Glucose availability and glycolytic metabolism dictate glycosphingolipid levels. J Cell Biochem 116:67–80
Shayman JA (2018) Targeting Glucosylceramide Synthesis in the Treatment of Rare and Common Renal Disease. Semin Nephrol 38:183–192
Tonneijck L, Muskiet MH, Smits MM et al (2017) Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J Am Soc Nephrol 28:1023–1039
Lau CE, Siskos AP, Maitre L et al (2018) Determinants of the urinary and serum metabolome in children from six European populations. BMC Med 16:202
Mittelstrass K, Ried JS, Yu Z et al (2011) Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet 7:e1002215
Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, Group US (2006) Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 55:1832–1839
Yu MK, Lyles CR, Bent-Shaw LA, Young BA, Authors P (2012) Risk factor, age and sex differences in chronic kidney disease prevalence in a diabetic cohort: the pathways study. Am J Nephrol 36:245–251
Bjornstad P, Cherney DZ (2018) Renal hyperfiltration in adolescents with type 2 diabetes: physiology, sex differences, and implications for diabetic kidney disease. Curr Diab Rep 18:22
Bjornstad P, Nehus E, El Ghormli L et al (2018) Insulin sensitivity and diabetic kidney disease in children and adolescents with type 2 diabetes: An observational Analysis of data from the today clinical trial. Am J Kidney Dis 71:65–74
Lovshin JA, Skrtic M, Bjornstad P et al (2018) Hyperfiltration, urinary albumin excretion, and ambulatory blood pressure in adolescents with Type 1 diabetes mellitus. Am J Physiol Renal Physiol 314:F667–F674
Silverstein J, Klingensmith G, Copeland K et al (2005) Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care 28:186–212
Tagami S, Inokuchi Ji J, Kabayama K et al (2002) Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem 277:3085–3092
Yamashita T, Hashiramoto A, Haluzik M et al (2003) Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci U S A 100:3445–3449
Acknowledgements
Personal thanks The SEARCH for Diabetes in Youth Study is indebted to the many youths and their families, and their health care providers, whose participation made this study possible.
SEARCH 3/ 4
The authors wish to acknowledge the involvement of the Kaiser Permanente Southern California’s Marilyn Owsley Clinical Research Center (funded by Kaiser Foundation Health Plan and supported in part by the Southern California Permanente Medical Group); the South Carolina Clinical & Translational Research Institute, at the Medical University of South Carolina; NIH/National Center for Advancing Translational Sciences (NCATS) grant number UL1 TR000062 and UL1 Tr001450; Seattle Children's Hospital and the University of Washington, NIH/NCATS grant number UL1 TR00423; University of Colorado Pediatric Clinical and Translational Research Center, NIH/NCATS grant Number UL1 TR000154; the Barbara Davis Center at the University of Colorado at Denver (DERC NIH grant number P30 DK57516); the University of Cincinnati, NIH/NCATS grant number UL1 TR000077 and UL1 TR001425; and the Children with Medical Handicaps program managed by the Ohio Department of Health. This study includes data provided by the Ohio Department of Health, which should not be considered an endorsement of this study or its conclusions. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
Author information
Authors and Affiliations
Contributions
EN wrote the manuscript and performed the analysis. NS, SM, and MM contributed to the discussion and reviewed/edited the manuscript. KDRS and WZ developed and validated the urinary sphingolipid analyses and performed laboratory analyses and data interpretation. EN is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior presentation This data was presented in abstract form at the American Society of Nephrology meeting, 2020 (virtual only).
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nehus, E.J., Sheanon, N.M., Zhang, W. et al. Urinary sphingolipids in adolescents and young adults with youth-onset diabetes. Pediatr Nephrol 39, 1875–1883 (2024). https://doi.org/10.1007/s00467-023-06257-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06257-6