Abstract
Background
Optimal steroid treatment at onset of idiopathic nephrotic syndrome is still debated. The aim of this study was to analyze the clinical outcome at 24 months of follow-up in patients admitted to our unit for the first episode of steroid-sensitive nephrotic syndrome comparing two different steroid regimens.
Methods
We collected data on patients treated from 1992 to 2007 with prednisone according to the International Study on Kidney Diseases in Children 8-week regimen and since 2008 according to the Arbeitsgemeinschaft fur Padiatrische Nephrologie 12-week regimen. The primary outcome was to evaluate cumulative prednisone dosage at 12 and 24 months of follow-up in the two groups. As secondary outcomes, we considered mean relapse rate per patient; number of children without relapses at 6, 12, and 24 months; and number of patients who developed frequent relapses and steroid-dependent disease.
Results
Data were collected on 127 patients. Sixty-one subjects received the 8-week regimen and 66 the 12-week regimen. The mean cumulative prednisone dose at 12 and 24 months was not different, and the rate of patients without relapses was lower at 6 and 12 months in patients treated with the 8-week course, while no difference was observed at 24 months.
Conclusions
Despite the limitations of a retrospective study with limited follow-up, our data indicate that switching treatment from a shorter to a longer scheme did not improve the clinical outcome at 24 months of observation.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic nephrotic syndrome (INS) is the most common primary glomerular disease in young children, with a peak age at onset around 3 years [1]. Incidence is 1.5–16.9 cases/100,000 children/year [2]. Overall, nearly 80% of children presenting with INS are steroid-sensitive (SSNS). Of these, the vast majority will experience relapses and 50–70% will develop frequently relapsing nephrotic syndrome (FRNS) or steroid-dependent nephrotic syndrome (SDNS) [3,4,5]. Few prognostic factors have been identified to date [6]. Patients that respond more rapidly to the initial steroid treatment tend to have a less severe disease course [5, 7,8,9].
The initial steroid regimen for treatment of INS, developed in the 1970s by the International Study on Kidney Diseases in Children (ISKDC), consisted of a 4-week course of prednisone (PDN) at a daily dose of 60 mg/m2, followed by 4 weeks at 40 mg/m2 on alternate days [9]. Since then, several other regimens have been proposed, all using longer courses of PDN with or without progressive tapering, with the goal of consolidating remission and potentially improving subsequent disease course. Among these, the German “Arbeitsgemeinschaft fur Padiatrische Nephrologie” (APN) proposed extending the 8-week ISKDC course of PDN to 12 weeks (6 weeks of daily PDN + 6 weeks on alternate days) [10]. For years, the optimal regimen has been a source of intense debate in the pediatric nephrology community. Recently, three randomized controlled studies have shown no clear benefit in extending the initial course of PDN beyond 12 weeks, ending this long-lasting debate [4, 11, 12].
At the Bambino Gesù Children’s Hospital in 2008, we changed the protocol for initial treatment of INS from the ISKDC 8-week course (8 W) to the APN 12-week course (12 W). After 12 years, we retrospectively analyzed our data. The purpose of this study was to compare the two different steroid regimens in a real-world setting, analyzing the clinical outcome at 24 months in 127 children with nephrotic syndrome at onset.
Methods
We reviewed data from medical records of all patients presenting with a first episode of SSNS admitted to the Nephrology Unit of Bambino Gesù Children’s Hospital between January 1992 and April 2016. Inclusion criteria included the following: (1) diagnosis of SSNS, (2) at least 2-year follow-up, and (3) age at onset < 18 years. To avoid selection biases, we excluded all patients who did not present at our institution at disease onset. Other exclusion criteria included patient loss at follow-up and the presence of significant comorbidities.
Considering the retrospective nature of the analysis, the current study did not require the approval of the local ethics committee according to current legislation, but a notification was sent.
Data were retrospectively analyzed in line with personal data protection policies.
The following variables were collected: age; sex; height; weight; blood pressure; time to the first relapse; number of relapses at 6, 12, and 24 months; cumulative dose of prednisone at 12 and 24 months; mean prednisone dose prescribed after relapses; and prescription of steroid-sparing agents. Nephrotic syndrome, remission, and relapse were defined as previously described [5, 9, 13].
The first episode was treated between 1992 and 2007 according to the ISKDC protocol (PDN 60 mg/m2/day for 4 weeks, followed by 40 mg/m2/48 h for 4 additional weeks), hereafter defined as the 8-week regimen (8 W). Since 2008, all patients had been treated at disease onset with the APN protocol (PDN 60 mg/m2/day for 6 weeks, followed by 40 mg/m2/48 h for 6 additional weeks) [10], hereafter defined as the 12-week regimen (12 W).
Following the first episode, upon disease relapse, treatment regimens for relapses have evolved over time. Before 2008, most patients were treated with schedules that included progressive tapering of the PDN dose. Thereafter, most patients were treated upon relapse with PDN 60 mg/m2/day until 3–5 days after remission, followed by 40 mg/m2/48 h for 4 weeks. In order to harmonize results for the analysis, we calculated the cumulative PDN dose that patients received for each relapse. When patients developed FRNS or SDNS, the decision to prescribe maintenance therapy with low-dose PDN or to start a steroid-sparing agent, usually cyclosporine A or mycophenolate mofetil (MMF), was individualized and was made by the referring nephrologist of each patient. In general, low-dose maintenance PDN therapy was attempted before prescribing other immunosuppressive agents.
The primary outcome of the study was the cumulative PDN dose (mg/m2) at 12 and 24 months. Secondary outcomes included the mean time to first relapse; the relapse rate at 6, 12, and 24 months of follow-up; the number of patients with first relapse at 6, 12, and 24 months; the number of patients who developed FRNS or SDNS; and the number of patients requiring low-dose maintenance PDN therapy or other steroid-sparing agents.
Statistical analysis was performed with IBM SPSS Statistics 21.0.0.2 software (Segrate, Italy) and OriginPro 2018 software (Northampton, MA). All tests were two-sided and considered statistically significant for p values < 0.05. Categorical data are reported as counts and percentages. Continuous normal data are expressed as mean ± standard deviation. Continuous data that did not follow a normal distribution are expressed as median value and interquartile range. The normality of data was tested with the Kolmogorov–Smirnov test. Survival data were analyzed using the Kaplan–Meier method and compared using the log rank test.
Comparisons between groups were performed with t-test, Mann–Whitney U test, or with the χ2 test with Yates’ correction as appropriate. All analyses were restricted to the first 2 years of disease.
Results
One hundred and twenty-seven patients were included in the study. Of these, 61 patients received the 8 W PDN course and 66 the 12 W PDN course. Patient characteristics at disease onset were similar in the two cohorts (Table 1). Median time to remission combining both cohorts was 8 days [6,7,8,9,10]. We observed a small but significant difference between the two groups, with a shorter time to remission in the 8 W group (p = 0.024) (Table 1).
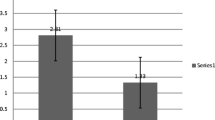
The cumulative PDN dose was significantly lower at 6 months in patients treated with the 8 W course (3425 ± 1166 mg/m2 vs. 3905 ± 897 mg/m2; p = 0.010). At 12 and 24 months, we no longer observed significant differences (Table 2; Fig. 1a).
The median time to first relapse was estimated by Kaplan–Meier survival curve and was 4.6 months [3.8–5.7 — 95% C.I.] in the 8 W group and 7.9 months [5.4–9.0 — 95% C.I.] in the 12 W group (Fig. 1b). The overall relapse-free survival was significantly better in the group treated with the 12 W protocol (p = 0.002 log rank test). This corresponded to a significantly lower relapse rate at 6 and at 12 months in patients treated with more PDN at onset. However, at 24 months, the relapse rate per patient and the overall ratio of patients with sustained remission were no longer significantly different between the two groups (Table 1). Likewise, the proportion of patients classified as FRNS or SDNS at the end of follow-up was not significantly influenced by the length of induction regimen at onset (Table 1).
The median cumulative dose of PDN used to treat subsequent relapses was similar in the 8 W and 12 W cohorts (p = 0.796) (Table 2). At 24 months, more patients had started low-dose maintenance therapy or had been treated with levamisole in the 8 W group (Table 2). The median dose of PDN prescribed for maintenance therapy was similar between groups (Table 1). No differences were observed in the prescription of cyclophosphamide, cyclosporine A, or MMF between the two groups.
The mean number of admissions during the first 2 years of disease was 1.2 ± 0.8 vs. 1.1 ± 0.5 (p = 0.288) in the 8 W and 12 W cohorts, respectively. Patients treated after 2008 (i.e., 12 W regimen) had on average shorter duration of hospital stay (5.3 ± 2.2 days vs. 7.2 ± 4.1 days, p = 0.001). During the study period, however, the criteria for being admitted to the hospital have become more stringent.
Younger patients at disease onset are known to have a more difficult course of SSNS. We therefore performed a sub-analysis, including only patients aged ≤ 3.5 years at disease onset. This sub-analysis confirmed a difference in cumulative PDN dose at 6 and 12 months, which in this sub-group was lower in the 8 W group. No difference between the two regimens was shown in terms of relapse rate, even at 6 and 12 months (Supplementary Tables 2 and 3).
Kidney biopsy was performed only in five patients, including three patients in the 8 W group and two patients in the 12 W group. All had minimal change disease.
Discussion
The major finding of this study is that switching the initial steroid regimen from 8 to 12 W does not change the clinical outcome at 24 months of follow-up. Our results confirm a recent meta-analysis which assessed data from two studies performed on a total of 171 children of European descent comparing the 8 W and the 12 W regimen and showed that time to first relapse was shorter in the 8 W regimen, but overall evolution at last follow-up toward FRNS or SDNS was comparable in the 2 arms [14]. These data are very relevant in clinical practice, because they suggest that steroid treatment at onset has little impact on the long-term outcome of SSNS.
The optimal strategy for treatment of the first episode at the onset of nephrotic syndrome in children has been much debated [15]. The first standardized protocol, a 4-week PDN course at a dose of 60 mg/m2/day, followed by 4 weeks of treatment at a dose of 40 mg/m2 on alternate days, was proposed at the end of the 1970s by the ISKDC [9] and was the reference scheme around the world for decades. In 1993, the APN proposed to prolong the daily and alternate-day ISKDC schedules from 4 to 6 weeks, based on trial results showing better rates of sustained remission at 2 years without substantial increase in side effects [10]. Thereafter, many centers including ours extended their protocol for onset of nephrotic syndrome from a total of 8 to a total of 12 weeks. In the same years, alternative schemes using extended PDN courses were proposed, with or without tapering of the PDN dose [2]. More recently, three high-quality randomized controlled studies (RCTs) have compared different induction schemes [4, 11, 12]. These studies have tested the benefits of extending PDN therapy from 3 to 6 months [11], from 2 to 6 months [12], and from 8 to 16 weeks [4]. All of them conclusively demonstrate that treatment of the first episode should not exceed a total of 12 weeks. However, the question of whether a total scheme of 12 weeks is preferable in terms of long-term benefit than a total scheme of 8 weeks remains open [16]. In addition, the recent 2020 Cochrane Database Systematic Review suggests that additional trials testing PDN schemes at onset of SSNS should not be performed [17].
High-quality RCTs are very important to develop guidelines and recommendations. Nonetheless, everyday life results may diverge from those obtained in a carefully monitored clinical setting, where patients are selected and followed using stringent criteria and protocols. Our study is based on a retrospective comparison of the application of the 8-week ISKDC and the 12-week APN protocols in a real-world setting. To avoid selection bias, we enrolled all consecutive patients who presented at our unit at disease onset since 1992, excluding secondary referrals, which may represent more severe cases. At 6 and 12 months, we observed in the 12-week protocol a higher cumulative PDN dose and a lower relapse rate compared to the 8-week protocol. Subsequently, patients treated with less PDN at disease onset relapsed earlier and so, even if the initial PDN exposure is lower, they receive additional PDN more rapidly. For this reason, over time, the difference in cumulative PDN dose was lost (Fig. 1a). At 24 months, we did not observe a clear advantage of one of the two PDN protocols in terms of relapse-free survival, severity of the nephrotic syndrome, or cumulative dose of PDN. Relapse-free survival curves showed a slight advantage in favor of a longer protocol (Fig. 1b). However, the relapse rate at 24 months was no longer significantly different between the two groups.
The other differences included a significant increase in the frequency of levamisole or a borderline difference in the frequency of low-dose maintenance PDN therapy in children receiving the 8 W regimen. These may be related to earlier relapses occurring in children treated with a shorter protocol, which could have prompted physicians to initiate alternative therapies more frequently. In recent years, we have increasingly used MMF to spare steroids. However, MMF is not easily available in Italy in liquid form and is rarely prescribed to small children. For this reason, only 4 patients received MMF during the first 2 years of their disease.
Small children are more likely to experience severe courses of SSNS [1, 11, 18,19,20]. In theory, this could justify treating younger patients with more intense therapy at disease onset. However, even when restricting the analysis to children younger than 3.5 years, we observed no substantial benefits in extending the initial PDN course from 8 to 12 weeks.
This study has limitations that are related to its retrospective nature. Data were collected over nearly 24 years. During this period, attitudes in treating SSNS have evolved, which may have influenced our results. Despite these limitations, this retrospective analysis confirms in a clinical setting that differences in PDN schemes used to treat the initial episode of SSNS have little impact on the long-term outcome of the disease [21]. However, using one of the other regimens modifies the short-term risk of relapse, influencing the proportion of patients that are classified as FRNS or SDNS at 6 and 12 months.
Abbreviations
- INS:
-
Idiopathic nephrotic syndrome
- SSNS:
-
Steroid-sensitive nephrotic syndrome
- FRNS:
-
Frequently relapsing nephrotic syndrome
- SDNS:
-
Steroid-dependent nephrotic syndrome
- ISKDC:
-
International Study on Kidney Diseases in Children
- PDN:
-
Prednisone
- APN:
-
Arbeitsgemeinschaft fur Padiatrische Nephrologie
- 8W:
-
8 Weeks
- 12W:
-
12 Weeks
- MMF:
-
Mycophenolate mofetil
- RCTs:
-
Randomized controlled studies
References
Dossier C, Delbet JD, Boyer O, Daoud P, Mesples B, Pellegrino B, See H, Benoist G, Chace A, Larakeb A, Hogan J, Deschênes G (2019) Five-year outcome of children with idiopathic nephrotic syndrome: the NEPHROVIR population-based cohort study. Pediatr Nephrol 34:671–678. https://doi.org/10.1007/s00467-018-4149-2
Noone DG, Iijima K, Parekh R (2018) Idiopathic nephrotic syndrome in children. Lancet 392:61–74. https://doi.org/10.1016/S0140-6736(18)31608-8
Koskimies O, Vilska J, Rapola J, Hallman N (1982) Long-term outcome of primary nephrotic syndrome. Arch Dis Child 57:544–548. https://doi.org/10.1136/adc.57.7.544
Webb NJA, Woolley RL, Lambe T, Frew E, Brettell EA, Barsoum EN, Trompeter RS, Cummins C, Deeks JJ, Wheatley K, Ives NJ; PREDNOS Collaborative Group (2019) Long term tapering versus standard prednisolone treatment for first episode of childhood nephrotic syndrome: phase III randomised controlled trial and economic evaluation. BMJ 365:l1800. https://doi.org/10.1136/bmj.l1800
Vivarelli M, Massella L, Ruggiero B, Emma F (2017) Minimal change disease. Clin J Am Soc Nephrol 12:332–345. https://doi.org/10.2215/CJN.05000516
Carter SA, Mistry S, Fitzpatrick J, Banh T, Hebert D, Langlois V, Pearl RJ, Chanchlani R, Licht CPB, Radhakrishnan S, Brooke J, Reddon J, Levin L, Aitken-Menezes K, Noone D, Parekh RS (2020) Prediction of short- and long-term outcomes in childhood nephrotic syndrome. Kidney Int Rep 5:426–434. https://doi.org/10.1016/j.ekir.2019.12.015
Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr (1997) Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8:769–776. https://doi.org/10.1681/ASN.V85769
Fakhouri F, Bocquet N, Taupin P, Presne C, Gagnadoux MF, Landais P, Lesavre P, Chauveau D, Knebelmann B, Broyer M, Grünfeld JP, Niaudet P (2003) Steroid-sensitive nephrotic syndrome: from childhood to adulthood. Am J Kidney Dis 41:550–557. https://doi.org/10.1053/ajkd.2003.50116
(1981) Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int 20:765–771. https://doi.org/10.1038/ki.1981.209
Ehrich JH, Brodehl J (1993) Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft fur Padiatrische Nephrologie. Eur J Pediatr 152:357–361. https://doi.org/10.1007/BF01956754
Sinha A, Saha A, Kumar M, Sharma S, Afzal K, Mehta A, Kalaivani M, Hari P, Bagga A (2015) Extending initial prednisolone treatment in a randomized control trial from 3 to 6 months did not significantly influence the course of illness in children with steroid-sensitive nephrotic syndrome. Kidney Int 87:214–224. https://doi.org/10.1038/ki.2014.240
Yoshikawa N, Nakanishi K, Sako M, Oba MS, Mori R, Ota E, Ishikura K, Hataya H, Honda M, Ito S, Shima Y, Kaito H, Nozu K, Nakamura H, Igarashi T, Ohashi Y, Iijima K; Japanese Study Group of Kidney Disease in Children (2015) A multicenter randomized trial indicates initial prednisolone treatment for childhood nephrotic syndrome for two months is not inferior to six-month treatment. Kidney Int 87:225–232. https://doi.org/10.1038/ki.2014.260
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group (2012) KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2:139–274
Schijvens AM, Teeninga N, Dorresteijn EM, Teerenstra S, Webb NJ, Schreuder MF (2021) Steroid treatment for the first episode of childhood nephrotic syndrome: comparison of the 8 and 12 weeks regimen using an individual patient data meta-analysis. Eur J Pediatr 180:2849–2859. https://doi.org/10.1007/s00431-021-04035-w
Williams AE, Gbadegesin RA (2021) Steroid regimen for children with nephrotic syndrome relapse. Clin J Am Soc Nephrol 16:179–181. https://doi.org/10.2215/CJN.19201220
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group (2021) KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 100:S1–S276. https://doi.org/10.1016/j.kint.2021.05.021
Hahn D, Samuel SM, Willis NS, Craig JC, Hodson EM (2020) Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev 2020:CD001533. https://doi.org/10.1002/14651858.CD001533.pub6
Hiraoka M, Tsukahara H, Matsubara K, Tsurusawa M, Takeda N, Haruki S, Hayashi S, Ohta K, Momoi T, Ohshima Y, Suganuma N, Mayumi M; West Japan Cooperative Study Group of Kidney Disease in Children (2003) A randomized study of two long course prednisolone regimens for nephrotic syndrome in children. Am J Kidney Dis 41:1155–1162. https://doi.org/10.1016/s0272-6386(03)00346-9
Andersen RF, Thrane N, Noergaard K, Rytter L, Jespersen B, Rittig S (2010) Early age at debut is a predictor of steroid-dependent and frequent relapsing nephrotic syndrome. Pediatr Nephrol 25:1299–1304. https://doi.org/10.1007/s00467-010-1537-7
Sato M, Ishikura K, Ando T, Kikunaga K, Terano C, Hamada R, Ishimori S, Hamasaki Y, Araki Y, Gotoh Y, Nakanishi K, Nakazato H, Matsuyama T, Iijima K, Yoshikawa N, Ito S, Honda M (2019) Prognosis and acute complications at the first onset of idiopathic nephrotic syndrome in children: a nationwide survey in Japan (JP-SHINE study). Nephrol Dial Transplant 36:475–481. https://doi.org/10.1093/ndt/gfz185.gfz185
Hoyer PF (2015) New lessons from randomized trials in steroid-sensitive nephrotic syndrome: clear evidence against long steroid therapy. Kidney Int 87:17–19. https://doi.org/10.1038/ki.2014.354
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lucchetti, L., Gatto, A., Gianviti, A. et al. Treatment of idiopathic nephrotic syndrome at onset: a comparison between 8- and 12-week regimens in everyday clinical practice. Pediatr Nephrol 38, 2101–2106 (2023). https://doi.org/10.1007/s00467-022-05824-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05824-7