Abstract
Background
With adult patients, the measurement of [TIMP-2]*[IGFBP7] can predict the risk of moderate to severe AKI within 12 h of testing. In pediatrics, however, the performance of [TIMP-2]*[IGFBP7] as a predictor of AKI was less studied and yet to be widely utilized in clinical practice. This study was conducted to validate the utility of [TIMP-2]*[IGFBP7] as an earlier biomarker for AKI prediction in Chinese infants and small children.
Methods
We measured urinary [TIMP-2]*[IGFBP7] using NEPHROCHECK® at eight perioperative time points in 230 patients undergoing complex cardiac surgery and evaluated the performance of [TIMP-2]*[IGFBP7] for predicting severe AKI within 72 h of surgery.
Results
A total of 50 (22%) of 230 developed AKI stages 2–3 within 72 h after CPB initiation. In the AKI stage 2–3 patients, two patterns of serum creatinine (SCr) elevations were observed. The patients with only a transient increase in SCr within 24 h (< 24 h, early AKI 2–3) did not experience a worse outcome than patients in AKI stage 0–1. AKI stage 2–3 patients with SCr elevation after 24 h (24–72 h, late AKI 2–3), as well as AKI dialysis patients (together designated severe AKI), did experience worse outcomes. Compared to AKI stages 0–1, significant elevations of [TIMP-2]*[IGFBP7] values were observed in severe AKI patients at hours T2, T4, T12, and T24 following CPB initiation. The AUC for predicting severe AKI with [TIMP-2]*[IGFBP7] at T2 (AUC = 0.76) and maximum T2/T24 (AUC = 0.80) are higher than other time points. The addition of the NEPHROCHECK® test to the postoperative parameters improved the risk assessment of severe AKI.
Conclusions
Multiple AKI phenotypes (early versus late AKI) were identified after pediatric complex cardiac surgery according to SCr-based AKI definition. Urinary [TIMP-2]*[IGFBP7] predicts late severe AKI (but not early AKI) as early as 2 h following CPB initiation.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) remains a disorder with high morbidity and mortality and occurs in up to 40 to 50% of infants and children undergoing cardiac surgery with cardiopulmonary bypass (CPB) for the correction of a congenital heart defect [1, 2]. AKI following pediatric CPB has been associated with adverse outcomes, including longer duration of mechanical ventilation, prolonged length of hospital stays, and increased mortality [2, 3].
Currently, diagnosis of AKI is primarily based on the KDIGO criteria (Kidney Disease Improving Global Outcomes) [4] and requires careful assessment of serum creatinine and urine output. Urine output is an inexpensive and easy test; however, no consensus on its monitoring in different centers or whether to use actual or ideal body weight has been reached so far [5]. SCr concentrations could be impacted by many confounding variables, such as muscle mass, diet, hydration status, drug usage, and does not differentiate structural kidney damage and functional hemodynamic triggers [6,7,8,9]. Therefore, identification of AKI by SCr may be problematic and offer no ability to predict the outcome or otherwise define more specific characteristics of the injury [10]. Multiple measurements of SCr permit a calculation of the duration of elevated SCr, therefore allowing the definition of different AKI classifications, such as transient or persistent AKI [11, 12], and providing more information to delineate AKI characteristics. However, despite the benefits of multiple SCr measurements, this approach is still unsuitable for the timely diagnosis of AKI since changes in serum creatinine and/or urine output may occur after the occurrence of actual kidney damage [13]. Access to novel biomarkers capable of filling those gaps could improve diagnostic precision of AKI and provide timely patient management through avoidance of nephrotoxic drugs and better hemodynamic monitoring, as recommended in the KDIGO guidelines.
Previous research has demonstrated that (used in combination) detection of an increase in the tissue inhibitor metalloproteinases-2 (TIMP-2) and of the insulin-like growth factor-binding protein 7 (IGFBP7) can signal a risk of AKI [14,15,16]. Both are kidney tubular cells, expressed soluble proteins thought to be involved in G1 cell-cycle arrest during the earliest phases of cellular stress and tubular injury caused by ischemic or inflammatory processes, which interrupt the integrity and division of cells with damaged DNA [17, 18]. The measurement of [TIMP-2]*[IGFBP7] can estimate the risk of moderate to severe AKI (stages 2–3) within 12 h for critically ill adult patients. For such patients, a 0.3 cut-off value is routinely used in clinical practice to initiate preventive and protective kidney measures [15]. In pediatrics, although several previous studies have provided evidence about the potential of [TIMP-2]*[IGFBP7] as an early biomarker for AKI after pediatric cardiac surgery [16, 19], the sample size in each study was small and kinetic characteristics of the biomarker still need to be defined including the biomarker baseline before the operation, elevation time of the biomarker level before AKI occurs, and the cut-off values for patient stratification.
In this study, we tried to validate the performance of [TIMP-2]*[IGFBP7] in Chinese infants and small children after complex cardiac surgery. Our specific aims are as follows: (1) describe the incidence of AKI according to the KDIGO AKI definition, (2) delineate the kinetics of [TIMP-2]*[IGFBP7] during the first 48 h following surgery, and (3) evaluate the diagnostic performance of [TIMP-2]*[IGFBP7] to predict AKI stages 2–3 defined by KDIGO criteria.
Material and methods
Patient population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board and the Ethics Committee of Shanghai Children’s Medical Center (SCMCIRB-K2019009). Written informed consent was obtained from the parent or guardian of any participating patient. All patients were aged > 28 days and < 3 years, and were undergoing complex congenital heart surgery according to ESC Guideline [20] (risk adjusted classification for congenital heart surgery [RACHS] score ≥ 2 [21]) with CPB between November 2019 and June 2020. Patients with severe pre-existing kidney insufficiency (SCr > 2 times age-adjusted normal range) or significant abnormalities of the kidneys or genitourinary tract were excluded.
Clinical data collection and preprocessing
All patients were followed from the time of enrollment, and those later found to violate the inclusion criteria were excluded from subsequent data analysis. Clinical data, including patient demographics, operative characteristics, postoperative characteristics, such as hourly urine output, and vasoactive inotrope scores (VIS), as available in the hospital record, were collected. The inotrope scores were calculated as described by Wernovsky [22]. Our adjusted VIS was calculated as follows: dopamine dose (μg/kg/min) + dobutamine dose (μg/kg/min) + (100 × epinephrine dose) (μg/kg/min) + (10 × milrinone dose) (μg/kg/min) + (100 × norepinephrine dose) (μg/kg/min).
Sample collection and biomarker measurements
Fresh urine samples were taken from each study participant at the following predetermined time points beginning just before CPB surgery: 0 h (T0, preoperatively); 2 h; 4 h; 6 h; 8 h; 12 h; 24 h; 48 h (T2–T48) following initiation of CPB. The urine samples were collected from the urinary catheter and centrifuged immediately after collection at 1000 g for 10 min at 4 °C to remove any sediment and stored at 2–8 °C until testing. All samples were tested within 20 h after being collected, and the remainder of all samples was aliquoted and stored at − 80 °C for further use. The markers TIMP-2 and IGFBP7 were measured in fresh urine samples with the NEPHROCHECK® test (Astute Medical, San Diego) following the package insert recommendations [15]. The ASTUTE140® Meter is a bench-top analyzer that converts a fluorescent signal obtained for each of the two biomarkers, TIMP-2 and IGFBP7, into a single numerical result called the AKIRISK™ Score. The osmolality of the urine samples was measured with an FM-8P osmometer (Shanghai Jinggong). Serum creatinine levels were measured using a commercial kit (Beckman Coulter, Brea, USA) at baseline (2–3 days prior to surgery), as well as 4 h, 8 h, 12 h, 24 h, 48 h, and 72 h after initiation of CPB. All patients received routine standard care during the study period, including modified ultrafiltration (MUF) to each patient immediately after CPB. Any indications of AKI within 72 h following surgery were staged 1, 2, or 3 (according to KDIGO guidelines [4]) based on SCr measurements obtained after each [TIMP-2]*[IGFBP7] time point.
Statistical methods
Continuous variables with normal distribution are described as the mean and standard deviation. Non-normal continuous variables are described as the median and inter-quantile range (IQR). Normality properties of continuous variables were tested by the Shapiro–Wilk test. All comparisons of continuous variables (e.g., age, weight, and CPB duration) among more than two groups of patients were performed using the Kruskal–Wallis test. Additionally, the Wilcoxon rank-sum was used for comparisons of [TIMP-2]*[IGFBP7] between the groups of AKI 0–1 and moderate-severe AKI group (stages 2–3). All comparisons of categorical variables were verified by chi-squared analysis.
A univariate logistic regression model was employed to evaluate the association between severe AKI occurrence and various clinical characteristics and [TIMP-2]*[IGFBP7] from T2 to T24. Independent prediction value of [TIMP-2]*[IGFBP7], weight, and CPB duration were studied by multivariate logistic regression. Automatic variable selection methods were not used for the multivariate logistic model. Weight and CPB duration were included in the multivariate logistic model according to domain knowledge and univariate logistic modeling results.
The predictive value of these variables for severe AKI occurrence was evaluated by receiver operating curve (ROC) and corresponding area under curve (AUC) analyses. ROC and AUC were calculated using the R package pROC [23]. The 95% confidence intervals (CI) of AUC were calculated according to DeLong’s method [24].
Statistical inferences were intended to be exploratory. The type I error rate was set to 0.05. No multiple testing adjustment was performed. All statistical analyses were performed using the R statistical environment version 3.6.1 (R Core Team, Vienna, Austria, 2019).
Results
Study cohort and patient characteristics
A total of 273 children planned for cardiac surgery with CPB were initially enrolled in this study. Figure 1 depicts the patient inclusion process. A total of 230 patients were included in the dataset at the end of the study. Patient characteristics of the study cohort are provided in Table 1. According to KDIGO guidelines, 117 (51%), 63 (27%), and 50 (22%, including 6 patients treated with peritoneal dialysis) patients were staged as AKI 0, AKI 1, and AKI 2–3, respectively. AKI 2–3 patients were generally younger in age and smaller in weight than AKI 0–1 patients (Table 1). AKI 2–3 patients also had higher maximal vasoactive inotrope scores (MVIS), longer duration of CPB, and cross clamp-time than AKI 0–1 patients. However, the length of hospital stay (and intensive care unit stay) was not significantly different between AKI 0–1 and AKI 2–3 patients. Two patients died during our study. One of these received peritoneal dialysis at 7 h after initiation of surgery and was staged as AKI 3 (as confirmed by SCr elevation). The other patient did not develop AKI within 72 h after surgery (no SCr elevation) and was therefore classified as AKI 0. Still, this patient eventually developed AKI stage 3 and died two weeks later.
Patterns of SCr elevation for AKI stage 2–3 patients
Figure 2A shows the dynamic profiles of SCr within the first 72 h following the initiation of CPB. Although the first routine test of SCr in the hospital is typically one day after cardiac surgery, this study added two additional SCr measurements before 24 h. From the median profile analysis of SCr, elevation rise in some AKI patients was observed as early as 4–6 h after CPB initiation, and some patients reached AKI stages 2–3 within 24 h as their SCr level elevated 2 to 3 times above the baseline level. A detailed analysis of AKI stage 2–3 patients revealed subtle patterns for SCr elevation (Figs. 2B, C). The median value of the two different patterns of SCr level is displayed in Fig. 2C. Briefly, two patterns of SCr elevation after CPB were identified in AKI stage 2–3 patients. In the first group of patients (orange line), SCr transiently and rapidly increased 2 to 3 times above the baseline level within the first 24 h (T2–T12), but then decreased to below to 2 times baseline after 24 h. Accordingly, we defined this group of patients as “early AKI 2–3”, reflecting the fact that SCr only transiently elevated within the first 24 h. In the second group of patients (green line), SCr levels progressively reached a level of 2 to 3 times the baseline at and/or after 24 h (T24–T72). Accordingly, this group of patients was defined as “late AKI 2–3”. Because SCr values may be influenced by dialysis at an early stage, patients who had received dialysis within the first 72 h were not included in this SCr pattern classification.
Profiles of serum creatinine concentrations in patients with no AKI and AKI within 72 h of surgery. A Creatinine profile for three AKI groups of AKI 0, AKI 1, and AKI 2–3. B Individual profile of creatinine for each early AKI 2–3 and late AKI 2–3 patients. C Median creatinine profile for early AKI 2–3 and late AKI 2–3 groups
The comparison of independent patient characteristics of “early AKI 2–3” and “late AKI 2–3” groups with the AKI 0–1 group shows clear differences (Table 2). Compared to AKI 0–1 patients, “early AKI 2–3” patients were younger in age and smaller in weight but did not have worse post-operative outcomes (longer ICU or hospital stays). In addition, the duration of CPB and cross-clamp time in the “early AKI 2–3” group were similar to those of the AKI 0–1 group. In contrast, patients from the “late AKI 2–3” group have longer CPB and cross-clamp time durations (P < 0.01) and higher MVIS (P < 0.01). The ICU and hospital lengths of stay for “late AKI 2–3” patients were also longer than for “early AKI 2–3” and AKI 0–1 patients (P < 0.05). AKI dialysis patients, who received peritoneal dialysis for more severe status, also had worse operative and post-operative characteristics, although with limited cases numbers. These results indicate that “late AKI 2–3” patients and AKI dialysis patients often experienced worse outcomes and need to be predicted as early as possible. We, therefore, designate both as “severe AKI” patients in our later analysis for NEPHROCHECK® evaluation.
Distribution and dynamic profile of [TIMP-2]*[IGFBP7]
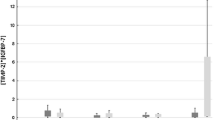
The overall distribution of [TIMP-2]*[IGFBP7] results are displayed by boxplot in Figs. 3A and B. Very high levels of [TIMP-2]*[IGFBP7] were observed for most of the patients before the initiation of CPB (T0), and no significant differences of [TIMP-2]*[IGFBP7] were observed at T0 between the AKI groups (Fig. 3A). Figure 3B shows [TIMP-2]*[IGFBP7] distributions at each time point from T2 to T48 after CPB initiation. Compared to the AKI 0–1 group, [TIMP-2]*[IGFBP7] in severe AKI patients (AKI 2–3 post 24 h and AKI dialysis patients) showed significantly higher expression at 2, 4, and 24 h after CPB initiation (T2, T4, T24, respectively), whereas there is no significant difference in the expression levels of [TIMP-2]*[IGFBP7] between the early AKI 2–3 and AKI 0–1 group.
Distribution and profile of [TIMP-2]*[IGFBP7] measurements. A Distribution of [TIMP-2]*[IGFBP7] at T0. B Distribution of [TIMP-2]*[IGFBP7] for three AKI groups. C Median profile of [TIMP-2]*[IGFBP7] for groups AKI 0–1 and severe AKI. D Profile of [TIMP-2]*[IGFBP7] measurement adjusted by osmolality. For adjustment, raw [TIMP-2]*[IGFBP7] value was divided by corresponding osmolality test value. ***, p < 0.001; **, p < 0.01; *, p < 0.05
Figure 3C shows the kinetic profile of [TIMP-2]*[IGFBP7] after CPB initiation. Compared to AKI 0–1, severe AKI shows two periods of [TIMP-2]*[IGFBP7] elevation. One period lies between T2 and T4, and the other between T12 and T24 after CPB initiation. Due to the significant volume of fluids administered to the patients during and after the surgery, the urine [TIMP-2]*[IGFBP7] levels might be influenced by the urine concentration. We, therefore, normalized the expression levels of [TIMP-2]*[IGFBP7] in urine by osmolality. The two peaks of [TIMP-2]*[IGFBP7] were more clearly displayed in the osmolality-adjusted profile of severe AKI patients (Fig. 3D and Graphical Abstract).
Predictive value of [TIMP-2]*[IGFBP7] for severe AKI
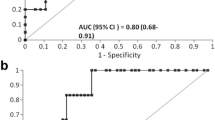
We further analyzed the predictive performances of [TIMP-2]*[IGFBP7] for severe AKI at single or combined time points (from T2 to T24). To evaluate the combined predictive value of [TIMP-2]*[IGFBP7] across multiple time points, a composite [TIMP-2]*[IGFBP7] score was derived as the maximum value between T2 and T4 (maximum T2/T4) and between T2 and T24 (maximum T2/T24). An independent predictive value of all [TIMP-2]*[IGFBP7] scores for the risk of severe AKI was evaluated by logistic regression. The best time points are shown in Table 3. Univariate logistic results show that patient weight, CPB duration, and [TIMP-2]*[IGFBP7] at T2, T24, maximumT2/T4, and maximumT2/T24 were all associated with severe AKI risk (Table 3, left columns). When combined with patient weight and CPB duration, T24, maximumT2/T4, and maximumT2/T24 showed significant association with severe AKI occurrence (Table 3, right columns). Figure 4A gives ROC values for the best univariate models. The corresponding AUC and number of available samples for each model are listed in the accompanying table. AUC for maximumT2/T24 is 0.80 (95% CI: 0.71–0.89), which is the highest AUC obtained among all evaluated time points. T2 (AUC = 0.76), T24 (AUC = 0.72), and maximumT2/T4 (AUC = 0.73) also show values predictive of severe AKI. Figure 4B shows the ROC curves and corresponding AUCs for a combined multivariate logistic model. A clinical model based only on weight and CPB duration indicated that an AUC of 0.71 is predictive of severe AKI. The addition of [TIMP-2]*[IGFBP7] at different time points further improves the performance of the two-parameter clinical model for prediction of severe AKI. Among them, maximumT2/T24 and T24, combined with the clinical model, had an increased AUC of 0.84 (0.70–0.94) and 0.81 (0.70–0.92), respectively. In addition, the clinical model combined with [TIMP-2]*[IGFBP7] at T2 or maximumT2/T4 achieved an AUC = 0.74 or 0.77, respectively, indicating an additive value of [TIMP-2]*[IGFBP7] at an early stage for prediction of severe AKI.
Performance of [TIMP-2]*[IGFBP7] for severe AKI risk prediction using Cox hazard model. A Receiver operating characteristic (ROC) curve for [TIMP-2]*[IGFBP7] measured at 4 time points with corresponding AUC and 95% CI. B ROC curve and corresponding AUC for Cox models of [TIMP-2]*[IGFBP7] at different times combined with weight and CPB duration (clinical model)
Discussion
Although predictive and prognostic values of TIMP-2 and IGFBP7 for AKI have been widely studied for adults in a wide range of clinical settings [25,26,27,28,29] (including adult cardiac surgery-associated acute kidney injury (CSA-AKI) [30,31,32,33,34,35]), its applicability to CSA-AKI in children remains to be validated [16, 36, 37]. In this observational study, we compared the kinetics of [TIMP-2]*[IGFBP7] to SCr-based AKI definition in a Chinese pediatric cohort. Different AKI phenotypes (early versus late AKI 2–3) were defined through multiple measurements of SCr. Patient outcomes indicate that only the late AKI 2–3 group should be classified as severe AKI patients. [TIMP-2]*[IGFBP7] could give an effective risk assessment on the severe AKI occurrence when used alone or in combination with other clinical parameters. This risk assessment could be given as early as 2–4 h after CPB initiation, suggesting that urine [TIMP-2]*[IGFBP7] might be used as an early biomarker for CSA-AKI.
In our cohort, because diuretic drugs are routinely given after cardiac surgery, that may impact the urine output of patients, AKI staging was evaluated using only SCr elevations. This may lead to underestimation of the number of AKI patients. In total, 113 (~ 49%) patients developed AKI. Of these, 50 (~ 22%) developed stage 2–3 AKI by the first 72 h following cardiac surgery. This AKI occurrence is mostly consistent with previous reports [2, 3, 38]. However, for the first time, two patterns of SCr elevation were observed in AKI stage 2–3 patients. Patients with transient early AKI stages 2–3 do not have worse outcomes than the AKI stage 0–1 patients. The late AKI 2–3 group and the AKI dialysis group are severe AKI populations (21 cases), which constitute 9% of total patients. Our results indicate that the current SCr-based AKI diagnosis (only assessed by peak changes in SCr) may not be efficient to classify the severity of AKI in children. From previous studies [39, 40] and ours, the “duration” of SCr elevation is also important for the severity grading of AKI and impacts patient outcomes. However, information on “duration” cannot be obtained without multiple measurements of SCr over several days and retrospective analysis of SCr changes. These observations were consistent with the understanding of limitations of SCr-based AKI diagnosis [32]. In our study, the transient rising of SCr is probably indicative of dysfunction rather than kidney injury or damage. The reason for this transient SCr elevation is unclear, but many confounding factors unrelated to kidney injury [41, 42], such as hydration status, may impact SCr concentrations and lead to early AKI diagnosis. This phenomenon occurred more frequently in younger patients of our cohort, indicating the confounder may have more impact on younger children. That may explain why in a similar study of an older pediatric population than ours, no SCr elevation was observed at 4 h after CPB initiation [8]. However, in a recent report studying AKI epidemiology of neonatal patients, a similar early AKI with little clinical significance was reported [10]. These findings suggested that it is necessary to develop new biomarkers to help understand different AKI pathophysiological conditions and define true kidney injury in small children.
From our results, [TIMP-2]*[IGFBP7] did not elevate in early AKI 2–3 but only predicted late AKI 2–3 patients, the true severe AKI group. This prediction could be given as early as 2 h after CPB initiation, indicating the biomarker is not only specific but also timely for severe AKI prediction. It was suggested different volume statuses could impact the urine concentration and subsequently affect the [TIMP-2]*[IGFBP7] values [43]. To exclude such impact by urine concentrations, for the first time on critically ill patients, we normalized the [TIMP-2]*[IGFBP7] values with urine osmolality and found the adjusted profile of urine [TIMP-2]*[IGFBP7] flattened in AKI 0–1 patient (Figs. 3C and D). However, the trend of increased [TIMP-2]*[IGFBP7] in severe AKI patients was not affected. From the median profile of urine [TIMP-2]*[IGFBP7] (especially the osmolality-adjusted one), two peaks of [TIMP-2]*[IGFBP7] levels were observed after the initiation of CPB. The first peak (T2–T4) is obtained during or immediately after the surgery. Ignoring the high baseline values, the kinetic profile of urine [TIMP-2]*[IGFBP7] observed in our study is very similar to the ones in adult studies [33, 44]. Mayer et al. found that [TIMP-2]*[IGFBP7] levels were significantly higher in patients with AKI 1 h after CPB and 24 h after weaning from CPB (P < 0.05). Cumming et al. also reported an increase of urine [TIMP-2]*[IGFBP7] immediately after CPB and a higher peak at day 1 post-surgery in severe AKI patients. The reason for these two peaks after cardiac surgery is not fully understood. We believe that the first peak reflects the kidney stress due to CPB during the surgery. That may also explain why, when combined with weight and CPB duration, the early time points after CPB initiation do not serve as an independent risk factor in the prediction of AKI (Table 3 T2). Conversely, the second peak may be caused by postoperative kidney stress after ICU admission. A combination of the two peaks of [TIMP-2]*[IGFBP7] (maximum T2/T24) could best predict severe AKI within 72 h after the surgery (when used alone (AUC = 0.80) or combined with the clinical model (AUC = 0.84).
In the current study, a very high baseline (T0) of [TIMP-2]*[IGFBP7] was observed in many SCMC patients. These unexpected results were not observed in previous studies. Accordingly, we explored possible explanations for the high T0 [TIMP-2]*[IGFBP7]. First, we found that the T0 levels have a weak correlation with the patient’s age and weight but not with the RACHS score (Supplementary Figs. 1A–C). In an additional experiment with 13 patients, we compared [TIMP-2]*[IGFBP7] at T0 to the day before the surgery (T00). Very low levels of [TIMP-2]*[IGFBP7]] were observed at T00, whereas, at T0 they were elevated. This was especially seen in the younger patients (Supplementary Fig. 1D). Although this comparison was only performed in limited cases (due to the practical difficulty to collect T00), we suspected that the strict 6 h fasting process (no IV solutions) before T0 may have caused the high values of [TIMP-2]*[IGFBP7] at T0, especially in the smaller age/lower weight patients. In Western countries, an additional IV solution is usually given to younger patients to avoid dehydration during the fasting process, and this may explain why such results were never observed in previous studies. Due to the high T0 values observed in our study, the real baseline on pediatric cardiac surgery patients is still unclear but is likely impacted by the patient care at each clinical practice. The previous reports on pediatric populations also show a significant variation on the basal [TIMP-2]*[IGFBP7] values [16, 19, 36]. Recently, a study involving neonates and infants undergoing cardiac surgery showed the baseline [TIMP-2]*[IGFBP7] increasing with patient age. Further studies on this point need to be performed in the future.
Besides the high baseline, there are several other important limitations to the current study. First, the results were generated from a relatively small sample size from a single center, especially the numbers of severe AKI. It will need to be validated in a larger cohort. Second, AKI staging was evaluated using only SCr elevations since urine output in our study can be confounded by the use of diuretics. Third, neonates were not included in the study. Because neonates are more vulnerable to AKI, it is very meaningful to investigate the performance of [TIMP-2]*[IGFBP7] for AKI prediction in this population in future studies.
Collectively, our results demonstrate that [TIMP-2]*[IGFBP7] may be a specific and timely biomarker to assess the risk of severe CSA-AKI in infants and pediatric patients. This assessment could cover both intraoperative and postoperative risks for AKI development and thus could provide clinicians opportunities for early protective interventions.
Data availability
All datasets underlying the manuscript results are available upon request to the corresponding author (XZM).
Abbreviations
- AKI:
-
Acute kidney injury
- CPB:
-
Cardiopulmonary bypass
- SCr:
-
Serum creatinine
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- TIMP-2:
-
Tissue inhibitor metalloproteinases-2
- IGFBP7:
-
Insulin-like growth factor-binding protein 7
- RACHS:
-
Risk adjusted classification for congenital heart surgery
- RACHS-1:
-
Risk adjustment for congenital heart surgery score version 1
- IQR:
-
Inter-quantile range
- ROC:
-
Receiver operating curve
- AUC:
-
Area under curve
- CI:
-
Confidence intervals
- MVIS:
-
Maximal vasoactive inotrope scores
- CSA-AKI:
-
Cardiac surgery-associated acute kidney injury
References
Aydin SI, Seiden HS, Blaufox AD, Parnell VA, Choudhury T, Punnoose A, Schneider J (2012) Acute kidney injury after surgery for congenital heart disease. Ann Thorac Surg 94:1589–1595
Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, Kim RW, Parikh CR, TRIBE-AKI Consortium (2011) Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med 39:1499
Morgan CJ, Zappitelli M, Robertson CM, Alton GY, Sauve RS, Joffe AR, Ross DB, Rebeyka IM, Western Canadian Complex Pediatric Therapies Follow-Up Group (2013) Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr 162:127.e121
Kellum J, Lameire N, Aspelin P, Barsoum R, Burdmann E, Goldstein S, Herzog C, Joannidis M, Kribben A, Levey A, MacLeod A, Mehta R, Murray P, Naicker S, Opal S, Schaefer F, Schetz M, Uchino S (2012) Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138
Schneider A, Ostermann M (2017) The AKI glossary. Intensive Care Med 43:893–897
Delanaye P, Mariat C, Cavalier E, Maillard N, Krzesinski JM, White CA (2011) Trimethoprim, creatinine and creatinine-based equations. Nephron Clin Pract 119:c187–c194
Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S (1983) Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr 37:478–494
Preiss DJ, Godber IM, Lamb EJ, Dalton RN, Gunn IR (2007) The influence of a cooked-meat meal on estimated glomerular filtration rate. Ann Clin Biochem 44:35–42
Liu KD, Thompson BT, Ancukiewicz M, Steingrub JS, Douglas IS, Matthay MA, Wright P, Peterson MW, Rock P, Hyzy RC et al (2011) Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med 39:2665–2671
Alten JA, Cooper DS, Blinder JJ, Selewski DT, Tabbutt S, Sasaki J, Gaies MG, Bertrandt RA, Smith AH, Reichle G et al (2021) Epidemiology of acute kidney injury after neonatal cardiac surgery: a report from the multicenter neonatal and pediatric heart and renal outcomes network. Crit Care Med 49:e941–e951
Maciel AT, Nassar AP Jr, Vitorio D (2016) Very transient cases of acute kidney injury in the early postoperative period after cardiac surgery: the relevance of more frequent serum creatinine assessment and concomitant urinary biochemistry evaluation. J Cardiothorac Vasc Anesth 30:56–63
Basu RK, Wong HR, Krawczeski CD, Wheeler DS, Manning PB, Chawla LS, Devarajan P, Goldstein SL (2014) Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 64:2753–2762
Waikar SS, Bonventre JV (2009) Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20:672–679
Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, DeMuth GE, Fitzgerald R, Gong MN, Graham DD, Gunnerson K et al (2014) Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 189:932–939
Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS et al (2013) Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17:R25
Meersch M, Schmidt C, Van Aken H, Rossaint J, Gorlich D, Stege D, Malec E, Januszewska K, Zarbock A (2014) Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLoS One 9:e110865
Wetz AJ, Richardt EM, Wand S, Kunze N, Schotola H, Quintel M, Brauer A, Moerer O (2015) Quantification of urinary TIMP-2 and IGFBP-7: an adequate diagnostic test to predict acute kidney injury after cardiac surgery? Crit Care 19:3
Boonstra J, Post JA (2004) Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 337:1–13
Gist KM, Goldstein SL, Wrona J, Alten JA, Basu RK, Cooper DS, Soranno DE, Duplantis J, Altmann C, Gao Z et al (2017) Kinetics of the cell cycle arrest biomarkers (TIMP-2*IGFBP-7) for prediction of acute kidney injury in infants after cardiac surgery. Pediatr Nephrol 32:1611–1619
Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, Lung B, Kluin J, Lang IM, Meijboom F et al (2021) 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J 42:563–645
Jenkins KJ (2004) Risk adjustment for congenital heart surgery: the RACHS-1 method. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 7:180–184
Wernovsky G, Wypij D, Jonas RA, Mayer JE Jr, Hanley FL, Hickey PR, Walsh AZ, Chang AC, Castaneda AR, Newburger JW et al (1995) Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 92:2226–2235
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Muller M (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12:77
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Gocze I, Koch M, Renner P, Zeman F, Graf BM, Dahlke MH, Nerlich M, Schlitt HJ, Kellum JA, Bein T (2015) Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS One 10:e0120863
Pilarczyk K, Edayadiyil-Dudasova M, Wendt D, Demircioglu E, Benedik J, Dohle DS, Jakob H, Dusse F (2015) Urinary [TIMP-2]*[IGFBP7] for early prediction of acute kidney injury after coronary artery bypass surgery. Ann Intensive Care 5:50
Beitland S, Waldum-Grevbo BE, Nakstad ER, Berg JP, Troseid AS, Brusletto BS, Brunborg C, Andersen GO, Sunde K (2016) Urine biomarkers give early prediction of acute kidney injury and outcome after out-of-hospital cardiac arrest. Crit Care 20:314
Guzzi LM, Bergler T, Binnall B, Engelman DT, Forni L, Germain MJ, Gluck E, Gocze I, Joannidis M, Koyner JL et al (2019) Clinical use of [TIMP-2]*[IGFBP7] biomarker testing to assess risk of acute kidney injury in critical care: guidance from an expert panel. Crit Care 23:225
Godi I, De Rosa S, Martino F, Bazzano S, Martin M, Boni E, Carta MR, Tamayo Diaz C, Mari G, Lorenzin A et al (2020) Urinary [TIMP-2] x [IGFBP7] and serum procalcitonin to predict and assess the risk for short-term outcomes in septic and non-septic critically ill patients. Ann Intensive Care 10:46
Tai Q, Yi H, Wei X, Xie W, Zeng O, Zheng D, Sun J, Wang G, Wang S, Liu G (2020) The accuracy of urinary TIMP-2 and IGFBP7 for the diagnosis of cardiac surgery-associated acute kidney injury: a systematic review and meta-analysis. J Intensive Care Med 35:1013–1025
Zaouter C, Potvin J, Bats ML, Beauvieux MC, Remy A, Ouattara A (2018) A combined approach for the early recognition of acute kidney injury after adult cardiac surgery. Anaesth Crit Care Pain Med 37:335–341
Vandenberghe W, De Loor J, Hoste EA (2017) Diagnosis of cardiac surgery-associated acute kidney injury from functional to damage biomarkers. Curr Opin Anaesthesiol 30:66–75
Cummings JJ, Shaw AD, Shi J, Lopez MG, O’Neal JB, Billings FT 4th (2019) Intraoperative prediction of cardiac surgery-associated acute kidney injury using urinary biomarkers of cell cycle arrest. J Thorac Cardiovasc Surg 157:1545-1553.e5
Su LJ, Li YM, Kellum JA, Peng ZY (2018) Predictive value of cell cycle arrest biomarkers for cardiac surgery-associated acute kidney injury: a meta-analysis. Br J Anaesth 121:350–357
Esmeijer K, Schoe A, Ruhaak LR, Hoogeveen EK, Soonawala D, Romijn F, Shirzada MR, van Dissel JT, Cobbaert CM, de Fijter JW (2021) The predictive value of TIMP-2 and IGFBP7 for kidney failure and 30-day mortality after elective cardiac surgery. Sci Rep 11:1071
Dong L, Ma Q, Bennett M, Devarajan P (2017) Urinary biomarkers of cell cycle arrest are delayed predictors of acute kidney injury after pediatric cardiopulmonary bypass. Pediatr Nephrol 32:2351–2360
Bojan M, Pieroni L, Semeraro M, Froissart M (2020) Cell-cycle arrest biomarkers: usefulness for cardiac surgery-related acute kidney injury in neonates and infants. Pediatr Crit Care Med 21:563–570
Blinder JJ, Goldstein SL, Lee VV, Baycroft A, Fraser CD, Nelson D, Jefferies JL (2012) Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg 143:368–374
Perinel S, Vincent F, Lautrette A, Dellamonica J, Mariat C, Zeni F, Cohen Y, Tardy B, Souweine B, Darmon M (2015) Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: results of a multicenter cohort study. Crit Care Med 43:e269-275
Brown JR, Kramer RS, Coca SG, Parikh CR (2010) Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg 90:1142–1148
Desanti De Oliveira B, Xu K, Shen TH, Callahan M, Kiryluk K, D’Agati VD, Tatonetti NP, Barasch J, Devarajan P (2019) Molecular nephrology: types of acute tubular injury. Nat Rev Nephrol 15:599–612
Makris K, Spanou L (2016) Acute kidney injury: diagnostic approaches and controversies. Clin Biochem Rev 37:153–175
Noto A, Cortegiani A, David A (2019) NephroCheck: should we consider urine osmolality? Crit Care 23:48
Mayer T, Bolliger D, Scholz M, Reuthebuch O, Gregor M, Meier P, Grapow M, Seeberger MD, Fassl J (2017) Urine biomarkers of tubular renal cell damage for the prediction of acute kidney injury after cardiac surgery-a pilot study. J Cardiothorac Vasc Anesth 31:2072–2079
Acknowledgements
Zhuoming Xu, Wei Wang, and Ji Liang are co-corresponding authors for this study. We would like to thank the patients and their parents for their support and participation in this study. This work was supported by the Shanghai Key Laboratory of Clinical Molecular Diagnostics for Pediatrics (20dz2260900).
Funding
This work was supported by the Shanghai Key Laboratory of Clinical Molecular Diagnostics for Pediatrics (20dz2260900).
Author information
Authors and Affiliations
Contributions
Y Tao, F Heskia, JH Huang, J Liang, W Wang, and ZM Xu designed the study. R Qin, Y Tao, MJ Zhang, LQ Chen, and F Wu collected patient data and samples. LQ Chen and F Wu performed NEPHROCHECK® tests. B Kang, Y Tao, F Heskia, HW Chen, J Liang, and ZM Xu analyzed the data. Y Tao, F Heskia, MJ Zhang, K Brengel-Pesce, X Mo, J Liang, W Wang, and ZM Xu discussed the results and performed additional analyses. The first draft of the manuscript was written by Y Tao, F Heskia, B Kang, LQ Chen, J Liang, and ZM Xu, and all authors commented on previous versions of the manuscript. ZM Xu, W Wang, and J Liang take responsibility for the content of the manuscript and the accuracy of the reported data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board and the Ethics Committee of Shanghai Children’s Medical Center (SCMCIRB-K2019009). Written informed consent was obtained from all participating patients and/or their parents. Clinical Trial Registry Number: NCT03963284.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tao, Y., Heskia, F., Zhang, M. et al. Evaluation of acute kidney injury by urinary tissue inhibitor metalloproteinases-2 and insulin-like growth factor-binding protein 7 after pediatric cardiac surgery. Pediatr Nephrol 37, 2743–2753 (2022). https://doi.org/10.1007/s00467-022-05477-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05477-6