Abstract
Background
Several novel biomarkers that predict acute kidney injury (AKI) have recently been proposed. We have evaluated the sequential patterns of biomarker elevation after pediatric cardiopulmonary bypass (CPB) and determined their diagnostic accuracy.
Methods
We measured the ability of neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), liver type fatty-acid binding protein (L-FABP), kidney injury molecule-1 (KIM-1), tissue inhibitor of metalloproteinase-2 (TIMP-2), and insulin-like growth factor binding protein 7 (IGFBP7), to predict AKI (≥50% increase in serum creatinine from baseline). Areas under the receiver-operator characteristic curves (AUCs) were calculated for each biomarker and for various biomarker combinations at multiple time points after CPB.
Results
Of 150 patients examined, AKI had developed in 50 patients by 24 h after CPB, with an elevated NGAL concentration first noted at 2 h post-CPB, increases in IL-18, L-FABP, and the product of TIMP-2 and IGFBP7 first noted at 6 h, and an elevated KIM-1 level noted at 12 h. At each time point, urine NGAL remained the marker with the highest predictive ability (AUC > 0.9). The addition of any other biomarker did not increase the predictive accuracy of NGAL alone at 2 and 6 h. At 12 h, when compared to NGAL alone, the combination of NGAL, IL-18, and TIMP2 improved the AUC for AKI prediction (from 0.938 to 0.973).
Conclusions
While urine NGAL remains a superior stand-alone test at the 2 and 6 h time points after pediatric CPB, a panel of carefully selected biomarkers may prove optimal at later time points.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) occurs in 40–50% of infants and children undergoing cardiac surgery with cardiopulmonary bypass (CPB) for correction of congenital heart defects [1]. Improvements in surgical techniques have expanded the complexity of congenital heart lesions that are correctable, but with an associated increase in the incidence of AKI [2]. More importantly, AKI following pediatric CPB has been independently associated with adverse outcomes, including longer duration of ventilation, prolonged length of hospitalization, and increased mortality [3, 4].The significant short- and long-term implications of AKI underscore the importance of making an early diagnosis in the post-operative period. This has hitherto not been possible, due to the reliance on serum creatinine (SCr), a delayed and unreliable measure of AKI.
During the past decade, preclinical studies [5, 6] have identified a number of urinary biomarkers of AKI that have now been validated in normal children [7] as well as in the pediatric CPB setting. These include neutrophil gelatinase-associated lipocalin (NGAL) [8,9,10,11], interleukin-18 (IL-18) [12], kidney injury molecule-1 (KIM-1) [13], and liver type-fatty acid binding protein (L-FABP) [14]. Most recently, two markers of cell cycle arrest, namely tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7), were shown to represent novel urinary biomarkers of AKI in critically ill adults [15]. In a preliminary study of children undergoing CPB, the product of urinary TIMP-2 and IGFBP7 levels was increased in those that developed AKI [16].
Additional larger studies are required in the pediatric CPB population to further extend the potential role for these cell cycle arrest markers as predictors of AKI. The aims of this study were to evaluate the sequential patterns of elevation of the cell cycle arrest biomarkers in comparison to the other better established AKI biomarkers after pediatric CPB, and to determine their accuracy in predicting AKI (individually and in combinations).
Materials and methods
Patient population
This single-center case–control study was approved by the Institutional Review Board of Cincinnati Children’s Hospital Medical Center. All patients aged <18 years undergoing cardiac surgery with CPB between January 2004 and July 2007 were approached for study inclusion. Patients with pre-existing chronic kidney disease (SCr > 2-fold the age-adjusted normal range) were excluded. Written informed consent from the legal guardian and assent from the patient, when appropriate, were obtained prior to enrollment. Past nephrotoxin use (such as angiotensin-converting enzyme inhibitors or contrast agents) was not an exclusion factor as long as the baseline Scr concentration was normal for age and the baseline level of the assayed biomarkers was normal. All patients received intra-operative steroids according to our standard protocol and 80–100% of maintenance fluid requirements during the post-operative 24 h to obviate prerenal azotemia. Urine samples for biomarker measurement were obtained prior to and at 2, 6, 12, and 24 h after initiation of CPB, and stored in aliquots at −80 °C. We have recently demonstrated that urinary biomarkers are very stable when stored in this fashion for prolonged periods of time [17]. The SCr was routinely measured at baseline (prior to surgery), immediately after CPB, and at least daily during the post-operative period. The primary outcome was AKI development within 72 h of surgery, defined and staged (Stage 1, 2, and 3) according to Kidney Disease: Improving Global Outcomes (KDIGO) criteria [18] based on SCr measurements. We defined all AKI as a ≥50% increase in SCr from baseline, and severe AKI as a ≥ 100% increase in SCr from baseline. Complexity of surgery was determined according to the Risk Adjustment for Congenital Heart Surgery Score version 1 (RACHS-1) scoring system [19].
Biomarker measurements
Laboratory investigators were blinded to clinical outcomes, and all biomarker measurements were performed simultaneously (within a 3-month period). The urine NGAL enzyme-linked immunosorbent assay (ELISA) was performed using a commercially available assay (NGAL ELISA Kit 036; Bioporto, Grusbakken, Denmark) that specifically detects human NGAL. The intra-assay coefficient of variation (CV) is 2.1% and inter-assay variation is 9.1%. Urine IL-18 (Kit 7625) and L-FABP (Kit Z-001) were measured using commercially available ELISA kits (Medical & Biological Laboratories Co., Nagoya, Japan and CMIC Co., Tokyo, Japan, respectively) per manufacturer’s instructions, with inter- and intra-assay CVs of 7.3 and 6.1% for IL18 and 7.5 and 10.9% for LFABP, respectively. The urine KIM-1 ELISA consisted of commercially available reagents (Duoset DY1750; R & D Systems, Inc., Minneapolis, MN), with intra- and inter-assay CVs for KIM-1 of 2 and 7.8%, respectively. Urine TIMP-2 was measured using a commercially available ELISA (Kit DTM200; R & D Systems, Inc.) per manufacturer’s instructions, with intra- and inter-assay CVs for TIMP-2 of 5.3 and 8.6%, respectively. The IGFBP7 assay was constructed using commercially available reagents (Duoset DY1334; R & D Systems, Inc.), with intra- and inter-assay CVs of 4.6 and 9.8%, respectively. All CVs were calculated and validated in our laboratory with actual patient samples.
Statistical methods
The analysis subset included patients for whom measurements of all six biomarkers (NGAL, IL-18, KIM-1, L-FABP, TIMP-2, IGFBP7) were available at baseline, and at 2, 6, 12, and 24 h after initiation of CPB (n = 150). Patient demographics and baseline clinical and biomarker measurements were compared between patients who did not develop AKI (No AKI group) and patients who did develop AKI in any form (All AKI group), using the nonparametric Wilcoxon rank sum test for continuous variables and the chi-square or Fisher exact test for categorical variables, as appropriate. Pearson correlation coefficients were calculated to assess for correlations between urinary biomarkers, demographics, and clinical characteristics at baseline, and for correlations among the various urinary biomarkers at each time point examined. At each time point, the mean biomarker measurements were compared between No AKI and All AKI patients, and between those with mild AKI (AKI Stage 1) and those with severe AKI (AKI Stage 2 or 3). Receiver-operator characteristic (ROC) curves were generated for each biomarker at each time point. The areas under the curve (AUC) were compared between biomarkers and between various biomarker combinations using the nonparametric approaches developed by DeLong et al. [20]. Statistical analysis was performed using SAS version 9.2 (SAS Institute, Cary, NC). P values of <0.05 were considered to be statistically significant.
Results
Patient demographics
Of the 391 patients initially enrolled [11], complete sets of urine samples for the measurement of all six chosen biomarkers (NGAL, IL-18, KIM-1, L-FABP, TIMP-2, IGFBP7) were available for 150 patients, whose characteristics are shown in Table 1. AKI developed in 50 of the 150 patients by 24 h after the CPB, with 25 displaying severe AKI (KDIGO Stage 2 or 3) (Fig. 1a). As previously described [11], patients who developed AKI had lower baseline SCr, were younger, and endured longer CPB times and longer lengths of hospital stay. Baseline urinary biomarker concentrations were similar in the AKI and non-AKI groups, with the exception of a small but significant increase in baseline L-FABP in the AKI group. There were no differences in baseline biomarker concentrations with respect to age, gender, or ethnicity [Electronic Supplementary Material (ESM) Table 1]. Only weak correlations between baseline biomarker concentrations and CBP time or length of stay were detected (ESM Table 1). There were no deaths, and none of the subjects in this cohort required dialysis.
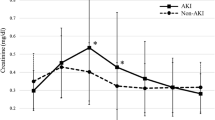
Temporal relationship of serum creatinine (SCr) and urinary biomarkers after pediatric cardiopulmonary bypass. Time points denote hours since initiation of cardiopulmonary bypass (CPB). No AKI patients who did not develop acute kidney injury (AKI) after CPB, All AKI all patients who developed AKI as defined by a ≥50% increase in SCr from baseline, Severe AKI subgroup of patients who developed AKI as defined by a ≥100% increase in SCr from baseline. NGAL Neutrophil gelatinase-associated lipocalin, IL-18 interleukin 18, KIM-1 kidney injury molecule 1, L-FABP liver fatty acid binding protein, TIMP-2 tissue inhibitor of metalloproteinases 2, IGFBP7 insulin-like growth factor binding protein 7. Mean values for each marker are shown; the corresponding 95% confidence intervals are shown in Table 2. Asterisk (*) denotes significant differences at p < 0.05 between the No AKI group and the All AKI group, hashtag (#) denotes significant differences at p < 0.05 between the All AKI group and AKI patients with severe AKI
Sequential biomarker patterns
The concentrations of all six urinary biomarkers (NGAL, IL-18, KIM-1, L-FABP, TIMP-2, IGFBP7) at baseline and at 2, 6, 12, and 24 h after initiation of CPB are summarized in ESM Table 2. The temporal trends in All AKI and non-AKI patients for each marker are illustrated in Fig. 1b–h). The level of each of the six urinary biomarkers rose significantly at some time point after CPB initiation in AKI patients, and four of the six markers (NGAL, IL-18, KIM-1, L-FABP) remained significantly elevated until the 24 h time point. Significant differences between AKI and non-AKI patients were first noted at 2 h for NGAL, at 6 h for IL-18, L-FABP, TIMP-2, and the product of TIMP-2 and IGFBP7, and at 12 h for KIM-1 and IGFBP7 (Fig. 2). This temporal pattern of biomarker elevation was similar in both the All AKI group and the severe AKI group. However, each of these differences was significantly magnified in patients with severe AKI in comparison to those in the All AKI group, indicating a severity-dependent response of all measured biomarkers. The correlation among all urinary biomarkers measured at different time points is shown in ESM Table 3. All biomarkers were significantly correlated to each other at later time points (12 and 24 h post-CPB), with weaker correlations noted at 6 h post-CPB.
Urinary biomarker [NGAL (ng/ml), IL-18 (pg/ml), KIM-1 (pg/dl), L-FABP (ng/ml), TIMP-2 • IGFBP7 (the product of TIMP-2 and ILGFBP7 divided by 10)] patterns in children with all forms of AKI (≥50% increase in SCr from baseline) or with severe AKI (≥100% increase in SCr from baseline) after CPB. Time points denote hours since CPB initiation. Mean values for each marker are shown. The corresponding 95% confidence intervals are shown in Table 2
Diagnostic accuracy of biomarkers to predict AKI
The progression of AUCs for each biomarker for prediction of all forms of AKI or of severe AKI at each time point is illustrated in Figs. 3 and 4, respectively. At 2 h following CPB initiation, urine NGAL was the only predictive biomarker of AKI, with an AUC of >0.9. At all subsequent time points, NGAL remained the marker with the highest predictive ability. At the 6 h time point, IL-18 and L-FABP were also significant predictors of AKI, with AUCs approaching 0.8. At 12 h, all biomarkers performed well, including KIM-1 and the product of TIMP-2 and IGFBP7. At 24 h, the [TIMP-2]•[IGFBP7] product was no longer predictive of AKI, in contrast with the other measured biomarkers.
We next determined if biomarker combinations at any of the time points examined could enhance the predictive performance of NGAL alone. The results are summarized in Table 2 and detailed in ESM Table 4. The addition of any other biomarker in any permutation or combination did not significantly increase the predictive accuracy of NGAL at the 2 h and 6 h time points. At the 12 and 24 h time points, the addition of biomarkers significantly improved the performance of NGAL. As illustrative examples, at 12 h post-CPB, the combination of NGAL and IL-18 or the combination of NGAL, IL-18, and TIMP-2 both significantly improved the AUC for AKI prediction when compared to NGAL alone (AUC increase from 93.8% for NGAL alone to 97.3% for the combination). There was no significant improvement obtained by adding more than three biomarkers.
Discussion
The pathogenesis of AKI after pediatric CPB is complex and multifactorial, and based largely on animal models. Primary mechanisms include ischemia, ischemia–reperfusion injury, mechanical blood trauma, oxidative stress, and activation of cell death and inflammatory cascades [1, 21]. Given the myriad pathways involved, it is important to systematically study several urinary biomarkers as predictors of AKI at various time points after this temporally defined renal injury. Our results confirm that all six urinary biomarkers examined (NGAL, IL-18, KIM-1, L-FABP, TIMP-2, and IGFBP7) are predictors of AKI after CPB, but at different time points that likely reflect the evolving pathophysiology. We found that urine NGAL was the only predictive biomarker at 2 h post-CPB, and remained the most predictive biomarker at all time points examined. At 6 h, both IL-18 and L-FABP also emerged as significant predictors of AKI. At the 12 h time point, all six biomarkers performed well. All six biomarkers could therefore individually provide an essential window of opportunity for application of early therapeutic interventions, prior to the elevation in SCr. While the performance of NGAL at early time points could not be surpassed by the addition of any other biomarkers, strategically chosen combinations did improve the predictive ability at later time points. For example, at 12 h post-CPB, the addition of IL-18 and TIMP-2 to NGAL significantly increased the AUC for AKI prediction from 93.8 to 97.3%. This may be attributed to the fact that urinary NGAL is derived primarily from the distal nephron, whereas urinary IL-18 and TIMP-2 originate from the proximal tubule, such that the combination may reflect the collective response of the entire nephron to AKI.
All six biomarkers studied exhibit strong biologic plausibility and represent key players in the causal mechanisms of experimental AKI. These proteins originate from the kidney and are present at very low concentrations in the uninjured state (as reflected by their low urinary concentrations in the pre-operative samples). The most extensively studied biomarker in AKI is NGAL. In animal models of early AKI, NGAL is one of the most highly induced proteins in the kidney [22,23,24], where it exerts a strong pro-proliferative anti-apoptotic action that enhances recovery [25]. The resultant secretion of NGAL into the urine comprises the major fraction of urinary NGAL protein. Well over 300 publications have now reported on NGAL in human AKI, and meta-analyses of its diagnostic utility have now appeared [26, 27]. The diagnostic accuracy of NGAL for the prediction of AKI has remained high, particularly in the pediatric CPB setting [1]. Collectively, the data to date on nearly 3000 children undergoing CPB and nearly 900 AKI events provide strong evidence for the utility of early NGAL measurements to predict AKI and its severity after pediatric cardiac surgery, with an AUC in the range of 0.8–0.9 [1], similar to the findings in this study. In children who develop an increase in SCr post-CPB, NGAL reliably discriminates between transient pre-renal azotemia and true intrinsic AKI with structural damage with 100% specificity [28]. Urine NGAL measured at 2, 6, 12, and 24 h post-CPB consistently improve the diagnostic accuracy for AKI prediction over a clinical model alone [11]. Early NGAL measurements also provide predictions of adverse outcomes in children with AKI post-CPB, including length of hospital stay, duration of mechanical ventilation, dialysis requirement, and mortality [9, 29]. The widespread availability of standardized clinical analytical platforms has further contributed to the emergence of NGAL as a stand-alone structural AKI biomarker [30, 31].
Interleukin-18 is a proximal tubular pro-inflammatory cytokine that is detected in the urine following ischemic AKI in animal models [32]. A multicenter study has confirmed the ability of urine IL-18 levels to moderately predict AKI during the first 6–12 h post-CPB time window in children, with AUCs in the range 0.72–0.82 [29], in agreement with the findings of our current study. Urinary IL-18 also correlated with adverse outcomes, including length of hospital stay and duration of mechanical ventilation [29]. L-FABP is induced in the proximal tubule early after AKI in animal models and has antioxidant properties that might protect the kidney from further injury [33]. Pediatric single-center studies have indicated the utility of urine L-FABP levels obtained 6 h post-CPB to moderately predict AKI, with AUCs in the range 0.75–0.78 [11, 14]. However, in a prospective multicenter study of children undergoing CPB, postoperative L-FABP levels were not associated with AKI [34]. KIM-1 is one of the most highly induced proteins in the proximal tubule after experimental AKI, where it mediates phagocytosis of damaged cells and thereby limits injury [35]. A proteolytically processed extra-cellular domain of KIM-1 can be detected in the urine of patients with AKI. Single-center studies in the pediatric CPB setting have yielded conflicting results, with two indicating moderate accuracy for predicting AKI at 12 h (AUC 0.7–0.8) [11, 13] and others indicating a poorer predictive performance [34, 36]. Standardized clinical assays for the measurement of IL-18, L-FABP, and KIM-1 are currently not available.
TIMP-2 and IGFBP7, both markers of cell cycle arrest, are upregulated in the proximal tubule after kidney injury in order to limit proliferation of damaged cells [15]. Several studies in critically ill adult populations have now established a cut-off of 2.0 for urinary [TIMP-2]•[IGFBP7]/1000 to have a high specificity (95%) but only moderate positive predictive value (49%) for AKI prediction [37]. The [TIMP-2]•[IGFBP7] product can be rapidly measured in the urine using a central laboratory clinical test system that has been approved by the Federal Drug Administration (FDA) for use in conjunction with clinical evaluation in patients 21 years or older who currently have—or have had within the past 24 h—acute cardiovascular and or respiratory compromise and are intensive care unit patients as an aid in the risk assessment for moderate or severe AKI within 12 h of patient assessment. However, the FDA has cautioned that the test results should be evaluated with other clinical and laboratory test information and are not to be used as a stand-alone test. Recent expert opinion papers have recommended that clinicians understand the limitations of this novel test and have also emphasized the need for additional studies [38, 39]. Furthermore, the central laboratory system has not been approved by the FDA for patients below the age of 21 years, and appropriate cut-offs for AKI prediction in the pediatric population have not been determined.
Pediatric studies using the [TIMP-2]•[IGFBP7] product to predict AKI are limited. In a small study of 46 hospitalized children with established AKI, an elevated urinary [TIMP-2]•[IGFBP7] product was shown to have prognostic value for the prediction of 30-day mortality [AUC 0.79; 95% confidence interval (CI) 0.61–0/97] and need for renal replacement therapy (AUC 0.67; 95% CI 0.5–0.84) [40]. Only two published studies have evaluated the utility of the [TIMP-2]•[IGFBP7] product in children undergoing CPB. In a case–control study of 51 children undergoing CPB, the urinary [TIMP-2]•[IGFBP7] product (measured using the clinical laboratory platform) was increased in the 12 subjects who developed AKI, with an AUC of 0.85 (95% CI 0.72–0.94) at 4 h post-CPB for predicting AKI [16]. The corresponding AUC for urinary NGAL was reported to be comparably good at 0.87 (95% CI 0.74–0.95). In a study of infants undergoing CBP, the urinary [TIMP-2]•[IGFBP7] product was significantly elevated in 31 subjects who developed AKI, but only at 12 h after CPB initiation [41]. In our larger study reported herein, the urinary [TIMP-2]•[IGFBP7] product (measured using a standardized ELISA kit) was increased in the 50 subjects who developed AKI, but the AUC for predicting AKI at the 6 h time point was slightly poorer (0.7). The AUC at the 12 h time point was substantially improved to 0.83. In comparison, the corresponding 12 h AUC for NGAL was superior at 0.94. Additional studies in larger multicenter populations are required to further assess the role of cell cycle arrest biomarkers in pediatric AKI.
Our study has several strengths. First, we used a rigorous protocol to collect urinary samples at several well-defined time points in the post-operative period, which allowed for the precise determination of the sequential rise in each biomarker after CPB. Second, we enrolled a relatively large, homogeneous cohort in whom the most likely etiology for the AKI would be ischemia–reperfusion injury after CPB. Third, this is the first study to report on all six of the most promising biomarkers for the prediction of AKI after pediatric CPB.
This study does have important limitations. First, we recognize the single-center recruitment with the clinical approach to patients specific to our center, which may not be fully applicable to other centers with alternative protocols. The results will need to be validated at the multicenter level. Second, the biomarker measurements were done in samples following prolonged storage—and not in real time as would be done in clinical practice. Third, the definition of AKI was based on a rise in SCr, a delayed and imprecise marker of AKI, rendering it very likely that patients with milder forms of structural kidney injury were misclassified to the control “No AKI” group. Fourth, we did not assess biomarkers to predict severe sustained AKI (i.e. AKI Stage 2 or 3 that persisted for 72 h), arguable the more clinically meaningful form of AKI, due to the small sample size in our cohort. Fifth, we did not include urine output as one of the criteria to define AKI, since urine output in this cohort can be confounded by the use of diuretics. Sixth, we did not assess biomarkers for prediction of adverse outcomes, since the primary goal of this study was to determine the sequential patterns of elevation of the biomarkers after pediatric CPB, and to determine their accuracy in predicting AKI (individually and in combinations).
In summary, this study demonstrates the sequential elevation of six predictive AKI biomarkers after pediatric CPB. The predictive ability of each biomarker is dependent on the specific time point when tested. Urine NGAL was the only predictive biomarker at 2 h post-CPB and remained the most predictive biomarker at all time points examined. A recent meta-analysis of 13 studies in children who developed AKI from all causes revealed a high diagnostic accuracy of urine NGAL for AKI prediction, with a combined AUC of 0.94, indicating the potential utility of NGAL beyond the CPB cohort studied herein [42]. However, although standardized laboratory platforms for NGAL measurement are available and launched globally, this test has not yet been approved by the FDA, and concrete recommendations with definitive cut-offs for routine clinical use cannot be made at the present time. Future studies that successfully utilize NGAL measurements as entry criteria and/or surrogate end-points for AKI therapeutic trials will further enhance the clinical utility of injury biomarkers.
References
Jefferies JL, Devarajan P (2016) Early detection of acute kidney injury after pediatric cardiac surgery. Prog Pediatr Cardiol 41:9–16
Pederson K (2012) Acute kidney injury in children undergoing surgery for congenital heart disease. Eur J Pediatr Surg 22:426–433
Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, Kim RW, Parikh CR, TRIBE-AKI Consortium (2011) Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med 39:1493–1499
Morgan CJ, Zappitelli M, CMT R, Alton GY, Sauve RS, Joffe AR, Ross DB, Rebeyka IM, Western Canadian Complex Pediatric Therapies Follow-Up Group (2013) Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr 162:120–127
Devarajan P (2015) Genomic and proteomic characterization of acute kidney injury. Nephron 131:85–91
Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P (2003) Differential gene expression following early renal ischemia–reperfusion. Kidney Int 63:1714–1724
Bennett MR, Nehus E, Haffner C, Ma Q, Devarajan P (2015) Pediatric reference ranges for acute kidney injury biomarkers. Pediatr Nephrol 30:677–685
Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P (2005) Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365:1231–1238
Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P (2008) Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 3:665–673
Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P (2011) Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr 158:1009–1015
Krawczeski CD, Goldstein SL, Woo JG, Wang Y, Piyaphanee N, Ma Q, Bennett M, Devarajan P (2011) Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol 58:2301–2309
Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL (2006) Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int 70:199–203
Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV (2009) Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int 76:863–869
Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, Noiri E, Devarajan P (2008) Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int 73:4654–4672
Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmelé T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA (2013) Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Critical Care 17:R25. doi: 10.1186/cc12503
Meersch M, Schmidt C, Van Aken H, Rossaint J, Görlich D, Stege D, Malec E, Januszewska K, Zarbock A (2014) Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLoS One 9:e110865
Schuh MP, Nehus E, Ma Q, Haffner C, Bennett M, Krawczeski CD, Devarajan P (2016) Long-term stability of urinary biomarkers of acute kidney injury in children. Am J Kidney Dis 67(1):56–61
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) KDIGO clinical practice guidelines for acute kidney injury. Section 2: AKI definition. Kidney Int. Suppl 2:19–36
Jenkins KJ (2004) Risk adjustment for congenital heart surgery: the RACHS-1 method. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 7:180–184
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Devarajan P (2006) Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17:1503–1520
Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P (2003) Identification of neutrophil gelatinase-associated lipocalin as a novel urinary biomarker for ischemic injury. J Am Soc Nephrol 4:2534–2543
Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P (2004) Neutrophil Gelatinase-associated Lipocalin (NGAL): a novel urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol 24:307–315
Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D'Agati V, Lin CS, Barasch J (2011) The NGAL reporter mouse detects the response of the kidney to injury in real time. Nat Med 17:216–222
Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P (2004) Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 15:3073–3082
Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A (2009) Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 54:1012–1024
Haase-Fielitz A, Haase M, Devarajan P (2014) Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem 51:335–351
Basu RK, Wong HR, Krawczeski CD, Wheeler DS, Manning PB, Chawla LS, Devarajan P, Goldstein SL (2014) Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 64:2753–2762
Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, Kim RW, Koyner JL, Coca SG, Edelstein CL, Shlipak MG, Garg AX, Krawczeski CD, TRIBE-AKI Consortium (2011) Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol 22:1737–1747
Devarajan P (2011) Biomarkers for the early detection of acute kidney Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr 23:194–200
Devarajan P (2010) Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med 4:265–280
Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL (2002) Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest 110:1083–1091
Kamijo-Ikemori A, Sugaya T, Obama A, Hiroi J, Miura H, Watanabe M, Kumai T, Ohtani-Kaneko R, Hirata K, Kimura K (2006) Liver-type fatty acid-binding protein attenuates renal injury induced by unilateral ureteral obstruction. Am J Pathol 169:1107–1117
Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Coca SG, TRIBE-AKI Consortium (2013) Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol 8:1079–1088
Yang L, Brooks CR, Xiao S, Sabbisetti V, Yeung MY, Hsiao LL, Ichimura T, Kuchroo V, Bonventre JV (2015) KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest 125:1620–1636
Peco-Antić A, Ivanišević I, Vulićević I, Kotur-Stevuljević J, Ilić S, Ivanišević J, Miljković M, Kocev N (2013) Biomarkers of acute kidney injury in pediatric cardiac surgery. Clin Biochem 46:1244–1251
Hoste EA, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD, Feldkamp T, Uettwiller-Geiger DL, McCarthy P, Shi J, Walker MG, Kellum JA, Investigators S (2014) Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant 29:2054–2061
Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M, Humphreys BD, Koyner JL, Liu KD, Mour G, Nolin TD, Bihorac A, American Society of Nephrology Acute Kidney Injury Advisory Group (2016) Clinical use of the urine biomarker [TIMP-2]×[IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis 68:19–28
Lameire N, Vanmassenhove J, Van Biesen W, Vanholder R (2016) The cell cycle biomarkers: promising research, but do not oversell them. Clin Kidney J 9:353–358
Westhoff JH, Tönshoff B, Waldherr S, Pöschl J, Teufel U, Westhoff TH, Fichtner A (2015) Urinary tissue inhibitor of metalloproteinase-2 (TIMP-2) • insulin-like growth factor-binding protein 7 (IGFBP7) predicts adverse outcome in Pediatric acute kidney injury. PLoS One 10:e0143628
Gist KM, Goldstein SL, Wrona J, Alten JA, Basu RK, Cooper DS, Soranno DE, Duplantis J, Altmann C, Gao Z, Faubel S (2017) Kinetics of the cell cycle arrest biomarkers (TIMP-2*IGFBP-7) for prediction of acute kidney injury in infants after cardiac surgery. Pediatr Nephrol. doi:10.1007/s00467-017-3655-y
Filho LT, Grande AJ, Colonetti T, Della ESP, da Rosa MI (2017) Accuracy of neutrophil gelatinase-associated lipocalin for acute kidney injury diagnosis in children: systematic review and meta-analysis. Pediatr Nephrol. doi:10.1007/s00467-017-3704-6
Acknowledgments
PD has received funding from the National Institutes of Health (grant number P50 DK096418). We are grateful to Dr. Jun Ying for assistance with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This single-center case–control study was approved by the Institutional Review Board of Cincinnati Children’s Hospital Medical Center. Written informed consent from the legal guardian, and assent from the patient when appropriate, were obtained prior to enrollment.
Disclosures
P.D. is a co-inventor on patents (7,776,824 and 7,977,110) related to NGAL as a biomarker of kidney injury, and declares licensing agreements with BioPorto Diagnostics and Abbott Diagnostics. All other authors have no conflicts of interest to report.
Electronic supplementary material
ESM 1
(DOCX 42 kb)
Rights and permissions
About this article
Cite this article
Dong, L., Ma, Q., Bennett, M. et al. Urinary biomarkers of cell cycle arrest are delayed predictors of acute kidney injury after pediatric cardiopulmonary bypass. Pediatr Nephrol 32, 2351–2360 (2017). https://doi.org/10.1007/s00467-017-3748-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3748-7