Abstract
Background
The effectiveness of rhGH on growth and final height (FH) was determined in children with CKD and kidney failure using data linkage from two national databases.
Methods
Data on Australian children with CKD and kidney failure treated with rhGH were obtained by linking ANZDATA and OzGrow registries. The CKD cohort included children treated with rhGH prior to kidney replacement therapy (KRT). The KRT cohort consisted of children with kidney failure, some received rhGH, and some were untreated. Height standard deviation scores (Ht-SDS) were calculated with final height defined as last height recorded in girls > 16 years of age and boys > 17 years of age.
Results
In the CKD group, there were 214 children treated with rhGH prior to KRT. In the KRT group, there were 1,032 children, 202 (19%) treated with rhGH and 830 (81%) untreated. Growth significantly improved in the rhGH-treated CKD group (ΔHt-SDS = +0.80 [+0.68 to +0.92]; p < 0.001) and the rhGH-treated KRT group (ΔHt-SDS = +0.38 [+0.27 to +0.50]; p < 0.001). Within the KRT cohort, final height was available for 423 patients (41%), of which 137 (32%) had been treated with rhGH. The rhGH-treated group demonstrated marginally better catch-up growth (ΔHt-SDS = +0.05 [−0.18 to 0.29]) compared to the non-rhGH-treated group (ΔHt-SDS = −0.03 [−0.16 to 0.10]; p = 0.49).
Conclusions
This large linkage study confirms rhGH is effective in improving height in children with CKD pre-KRT. However, rhGH appears to have a variable impact on growth once children have commenced KRT resulting in a marginal impact on final height.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Linear growth impairment remains a common challenge for children with kidney failure [1,2,3,4,5,6,7,8,9,10,11] and can be associated with significant physical and psychological consequences. Short adult height has been associated with significant impairment in social and work domains in patients with chronic kidney disease (CKD), including poorer educational achievement, a lower level of employment and a lower chance of being married [6-8, 12-14.] The aetiology of growth failure is complex with variable contributions from chronic kidney disease–mineral and bone disorder (CKD-MBD), uraemia, acidosis, anaemia, inflammation and suboptimal nutrition [3,4,5,6,7,8,9,10,11,12, 15, 16].
Despite correction of underlying metabolic abnormalities along with optimization of nutrition and dialysis, 35–50% of children with kidney failure still become adults with short stature [1, 3, 5, 6, 8, 9]. Even after successful kidney transplantation, catch-up growth is not guaranteed and is modified by age at transplantation, graft function and steroid exposure [8, 11, 15].
Growth hormone resistance is another significant contributor to growth failure in children with kidney failure. Since the introduction of recombinant human growth hormone (rhGH) in the early 1990s, many studies reported increased growth velocity and improved height following rhGH treatment leading to widespread use in children with kidney failure [1, 2, 4, 9, 15]. However, the quality of evidence to justify use of rhGH in this population appears relatively weak, with a previous Cochrane review finding only 16 RCTs that were collectively described as being of either poor quality or poorly reported [2]. Many studies had low numbers and did not include all enrolled participants in the analysis, suggesting that findings may be confounded by selection bias [2].
In Australia, rhGH prescribing has been strictly regulated by the government, which included a period (1987–2015) wherein information pertaining to rhGH use in all treated children was recorded in a comprehensive national database (OzGrow). Additionally, information on all children and adults who commenced kidney replacement therapy (KRT) (including dialysis and transplantation) in Australia and New Zealand has been captured in the Australian and New Zealand Dialysis and Transplant Registry (ANZDATA) since 1977 [17]. By linking data recorded in these two comprehensive databases, we were able to capture data on all children who received KRT within Australia treated with growth hormone for the indication of chronic kidney disease or kidney failure from 1989 to 2015. Using this complete dataset, we aimed to explore the effect of rhGH on growth in children with CKD, both pre- and post-KRT, and its effect on their final height.
Methods
Study population
The study population included two cohorts. The first cohort (CKD group) consisted of children prior to the commencement of KRT who were treated with rhGH for at least 6 months and had information recorded in the OzGrow database. Growth hormone treatment was approved for use in Australia as part of the national Pharmaceutical Benefits Scheme (PBS) and was available for children with CKD (eGFR < 30ml/min/1.73m2) during the period of this study for boys < 12 years of age and girls < 10 years of age with height < 25th centile and growth velocity < 25th centile [18]. As a requirement for applying to commence rhGH treatment, informed consent was obtained from the patient’s parent or guardian for specific information to be supplied to the National rhGH Treatment Program and transferred to the OzGrow database.
The second cohort (KRT group) consisted of children and adolescents less than 18 years of age who received KRT (defined by ANZDATA as > 6 months of dialysis or a kidney transplant) in Australia between 1 October 1987 and 30 December 2015. The same restriction as listed above was applied to use of rhGH during KRT, and this period was selected as concurrent data on rhGH treatment for all children within Australia with kidney failure and was recorded in OzGrow.
Data collection and linkage
Data were obtained from ANZDATA and OzGrow. ANZDATA collects data on all patients receiving chronic KRT (> 6 months) in Australia and New Zealand with extensive demographic data collection at the time of commencement of KRT and then annual data submission thereafter. Collected data includes information on KRT (treatment characteristics during haemodialysis, peritoneal dialysis and transplantation, growth (height and weight) and comorbidities). There is no time limit to follow-up. ANZDATA collects data in accordance with the Australian Commonwealth Privacy Act and associated state legislation governing health data collection, and individual opt-in patient consent is not required for the registry data. The reported clinical and research activities are consistent with the Principles of the Declaration of Istanbul, outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. The methods of data collection and validation are described in detail on the ANZDATA registry website [17].
Data from ANZDATA were linked to rhGH usage information recorded in the Australian OzGrow database. OzGrow collected data on all Australian patients receiving rhGH from 1 October 1987 to 30 December 2015 under the National rhGH Treatment Program. OzGrow data included height measurements and dosing regimens only, with no other information pertaining to cause or course of CKD. Height-SDS (Z-score) was calculated from the LMS parameters (L (Box-Cox), M (median) and S (coefficient of variation)) by comparing the child’s measure with the median size for that age and dividing the result by the standard deviation using growth charts for infants and older children developed by the National Centre for Health Statistics [19]. Inclusion of the L parameter in the calculation (z = (measure/M) L−1/(L/S)) takes any skew in the growth reference into account [19]. Data from ANZDATA was linked to OzGrow by matching patient initials and date of birth performed by ANZDATA Registry staff independently of the investigators and then supplied to the investigators as deidentified patient data.
Statistical analysis
Continuous data were described using mean and standard deviation (SD) for normally distributed data and median and interquartile range (IQR) for non-normally distributed data. Categorical data were described with percentages. Predictors of final height were determined by multiple linear regression analysis following univariate analysis. Data were analysed using Stata v13 for Windows (StataCorp, College Station, Texas) with p values < 0.05 considered statistically significant.
Ethical approval
Ethical approval for this study was obtained from the Human Research Ethics Committee at Children’s Health Queensland Hospital and Health Service: HREC/18/QRCH/44 and SSA/18/QRCH/5.
Results
CKD cohort
During the 28-year period (1987–2015), 369 patients commenced rhGH for growth failure according to Australian PBS criteria. Of these, 214 were treated with rhGH for at least 6 months pre-KRT, and this group formed the CKD cohort. In this cohort, the mean age at the start of rhGH was 8.3 years (range 11 months to 17.5 years) with a male predominance (70%). The median treatment time on rhGH was 32 months (IQR 16–55). There was significant catch-up growth in those children with CKD treated with rhGH, from a mean height-SDS −2.55 (95% CI, −2.42 to −2.68) at the start of treatment to −1.75 (−1.60 to −1.89) at the end of treatment (p < 0.001) (Fig. 1).
KRT cohort
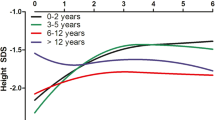
Over the same period, 1,087 patients less than 18 years of age commenced KRT in Australia. Fifty-five children (5%) had either no initial height recorded at the time of entry to KRT or only a single height recorded after commencing KRT and were excluded, leaving 1,032 children as the final cohort of patients in this group. KRT included periods of both on dialysis and living with a kidney transplant, with approximately 55% of rhGH-untreated and 75% of rhGH-treated patients spending more than 50% time with a transplant, respectively (Fig. 2). In this cohort, 202 received treatment with rhGH at some time post-commencement of KRT (19%). Only 27% of children who had Ht-SDS < 1 at the time of entry to KRT subsequently received rhGH at some stage during subsequent course of KRT, suggesting that some children who may have benefited from rhGH treatment did not receive this therapy. Baseline clinical characteristics of the KRT cohort according to rhGH use are summarized in Table 1. For the entire cohort, mean age at commencement of KRT was 9.8 years (range 0.7–16.8 years), with a slight male predominance (60%). The largest classes of kidney failure were glomerulopathies (22%) and congenital anomalies of the kidneys and urinary tract (CAKUT) (including renal hypoplasia/dysplasia) (20%), followed by posterior urethral valves (12%) and reflux nephropathy (11%). Metabolic conditions that are associated with short stature (oxalosis and cystinosis) contributed to 2.88% of our cohort. Over the study period, 958 patients received 1,228 transplants (76% one transplant, 21% two transplants, 3% three transplants and 2 patients received four transplants). Immunosuppression regimens for the majority of patients consisted of a calcineurin inhibitor with an anti-metabolite; pre-1998 treatment was with cyclosporin/azathioprine, followed by cyclosporin/mycophenolate (from 1998) and then predominantly tacrolimus/mycophenolate from 2007 onwards. Most children received initial and long-term prednisolone treatment (91% and 94% of transplanted patients were being treated with prednisolone at 1- and 5-year post-transplants, respectively).
Children who were older at commencement of KRT (10.5 years) were less likely to receive rhGH, compared to those who were younger when entering KRT (mean 6.5 years, p < 0.001). While the children who had received rhGH during CKD had demonstrated catch-up growth, these children remained significantly shorter at entry to KRT, with a mean height-SDS −1.86 (SD 1.16) compared to those children who had not received rhGH pre-KRT (mean height-SDS −1.19, SD 1.86; p < 0.001). As a group, all children who started rhGH during KRT or had been treated with rhGH during CKD and continued into KRT were significantly shorter at the time of commencement of KRT (mean height-SDS −2.05, SD 1.63) compared to those who were not treated with rhGH during KRT (mean height-SDS −1.11, SD 1.78; p < 0.001).
The median time to start rhGH treatment in those not on rhGH at the time of entry to KRT was 15 months (range 0–140), with mean dose 21 IU/m2/wk (SD 5) and median treatment time 26 months (range 1–186). As with the CKD group, those treated with rhGH during KRT demonstrated significant catch-up growth while on treatment with rhGH with mean height-SDS at commencement of rhGH of −2.40 (SD 1.14) and height-SDS at end of treatment of −2.07 (SD 1.32) (p < 0.001).
Final height was available for 423 children in the KRT group (41% of KRT cohort) of which 137 (32%) had received rhGH treatment during KRT. The final height of those children treated with rhGH (median height-SDS −1.69, IQR −2.92 to −1.09) was significantly shorter than those who did not receive rhGH (median height-SDS −1.17, IQR −2.14 to −0.40; p < 0.001) (Fig. 3). Overall, there was minimal catch-up growth for the whole cohort who reached final height, with mean change in height-SDS (delta height-SDS = final height-SDS minus height-SDS at commencement of KRT) of −0.01 (SD 1.21). Children who started KRT at a younger age and those who spent a greater time with a transplant demonstrated better catch-up growth (p = 0.05 and p < 0.01, respectively), (Fig. 4). Response to rhGH as measured by change in height-SDS varied widely, particularly in younger children (Fig. 5). Overall, compared to the untreated group, the rhGH-treated group demonstrated only marginally better catch-up growth from commencement of KRT to final height with a change in Ht-SDS +0.05 (−0.18 to 0.29) compared to the non-rhGH-treated group (change in Ht-SDS = −0.03, −0.16 to 0.10; p = 0.46). At the time of final height, 309 (73%) were being treated with a transplant, of which 307 (99%) were on prednisolone.
Predictors of final height
Multivariable analysis of potential predictors of final height was performed including significant variables from the univariable analysis. The most significant predictors of final height were the height at commencement of KRT and younger age at commencement of KRT, with female sex and class of primary kidney disease less strongly predictive (Table 2). An R2 value of 0.51 was calculated indicating the model did not account for all variables.
Discussion
In this large 28-year retrospective cohort study, we confirmed findings from previous studies that reported improved growth in children with CKD treated with growth hormone. While this effect is maintained for some children on KRT with an overall improvement in height during rhGH treatment during KRT, the individual response appears variable, and rhGH-treated children’s final height remained significantly shorter than their peers. Children treated with growth hormone during KRT demonstrated only marginal catch-up growth compared to non-treated children. However, these groups were not matched on clinical characteristics (such as starting height, disease, duration of CKD and others) which limits interpretation of this finding. We were able to limit selection bias in studying the effects of rhGH in a complete cohort of children with kidney failure by linking the OzGrow and ANZDATA Registries. This allowed us to analyse concurrent data on rhGH use and access extended follow-up data to examine long-term outcomes such as final height.
Recent ESPN working group guidelines reported an increase in final adult height of 7.2cm (ΔHt-SDS = 1.1–1.9) after 2–5 years of rhGH therapy, with a recommended rhGH dosage of 28–30 IU/m2/week [1]. An overall improvement in Ht-SDS +0.05 in our study equates to 0.375 cm of growth over 3.5 years of treatment [20]. Of note in this study, the mean time-averaged dose of rhGH given to our cohort (21 IU/m2/week) was ~30% less than the current recommended dose in CKD. Throughout the period of our study, initial prescribing instructions in relation to rhGH required prescribers to start at low dose (13.5 IU/m2/week) and then escalate their dosing regimen titrating to effect (maximum dose of 28.5 IU/m2/week) (Australian Department of Health and Ageing Guidelines for the Pharmaceutical Benefit Scheme Growth Hormone Program 2007, 2013; available on request). Further, during the period of treatment for each child, there were relatively frequent or relatively infrequent changes to the dose of growth hormone which meant that analysis of different doses within each individual child was felt unlikely to be informative. This prescribing practice resulted in lower average rhGH doses reported in this cohort and is likely to have had a negative impact on the effectiveness of rhGH. This finding highlights the need for constant reassessment of dose, with an increase in dose considered for less responsive children. Furthermore, the lesser improvements in overall height seen in our cohort may have also been due to differences in treatment duration, steroid exposure, age at commencement of therapy and dialytic clearance in those not yet transplanted. Greater improvements in final height may have a significant psychological and social impact on an affected child, which needs consideration on prescription.
In keeping with previous studies of growth hormone in CKD, the CKD group in our study had a significant improvement in height-SDS when treated with rhGH. Catch-up growth in this group was strongly associated with age at commencement of therapy, with younger age predictive of a greater response to rhGH. A recent Cochrane review [2] of 16 RCTs concluded that 1 year of rhGH therapy (in CKD and post-KRT cohorts) compared to placebo resulted in a mean increase in Ht-SDS of 0.82, which is consistent with the improvement in Ht-SDS of 0.80 seen in children with CKD in our study. The same review [2] also noted that the duration of studies was too short to determine the effect of rhGH on final height.
In this study, although children who started KRT at a younger age and those who spent a greater time with a transplant demonstrated better catch-up growth, there was no substantial change in height-SDS from the start of KRT to final height in either the rhGH-treated or untreated groups. A previous study reported that catch-up growth for children treated with rhGH continued over an extended treatment period with cumulative increase in Ht-SDS of 1.1–1.9 within 5–6 years at a dose of 28–30 IU/m2/week (n = 103) [8]. Our cohort was treated for a mean of 3.4 years at a dose of 21 IU/m2/week which may account for the reduced effectiveness of rhGH in our cohort. Importantly, the KRT time course included periods of both dialysis and transplantation which may account for lack of catch-up growth with significant catch-up growth rare in children on dialysis [8]. However, most children spent over 50% of KRT with a transplant and approximately 15% of children in both groups spent all of KRT with a transplant. Other factors such as genetic potential, disease class and transplant function may also have contributed to this difference [11].
A recent large study of final height of children with kidney failure during childhood found a median final height-SDS of −1.33 in those reaching adulthood in 2006–2011 [8], which was almost identical to our cohort with 50% achieving a final height above the 9th percentile (equivalent to a median final height-SDS = −1.35). In keeping with other studies, younger age at commencement of KRT and aetiology of kidney failure were also significant predictors of final height with male gender a weak predictor factor for poorer growth [4, 8, 9, 15]. In contrast to other studies [1, 3], the use of rhGH was not predictive of catch-up growth in our cohort.
Interestingly, growth appeared to slow following commencement of KRT in both rhGH-treated and untreated children (data not shown), with subsequent improvement over time, perhaps reflecting the fact that most children (73%) underwent a period of dialysis prior to transplantation. An attenuated response to rhGH in children has been reported in children on dialysis regardless of modality and is thought to be due to a higher degree of growth hormone insensitivity in kidney failure [1, 8]. This is in keeping with studies that suggest that growth in dialysis-treated children can be difficult to maintain and significant changes in stature post-transplant are rare [6]. Although rhGH-treated children in our study did show catch-up growth, this was not consistent across the group, and variability in growth following commencement of KRT was also seen in children who did not receive rhGH.
Our transplant cohort showed significant improvement in growth with rhGH treatment although growth appeared to plateau following cessation of rhGH therapy and final height was not significantly different compared to children who did not receive rhGH. While there was a similar final height in these two groups, there was slightly better catch-up growth as evidenced by the change in height-SDS. It appears likely that other factors aside from the relative GH resistance found in CKD may influence the response to rhGH post-transplant, including genetic height potential, underlying aetiology of disease, transplant function and potentially dose and compliance with rhGH therapy [21].
Strengths of this study include the comprehensive nature of the data which is inclusive of all children who have received KRT and who were prescribed rhGH in Australia. Unfortunately, ANZDATA only captures data on children who have commenced KRT, so we have no information on children with CKD who were not treated with rhGH prior to KRT. Additionally, important social outcomes are not measured within ANZDATA, and we were unable to compare our cohort with other studies that have shown improved quality of life in children with CKD after treatment with rhGH [13, 14]. Additional limitations of our study include lack of data on adherence, pubertal status at the time of treatment with rhGH and mid parental height (as a potential marker of genetic growth potential).
In conclusion, growth in children with kidney failure is the result of a complex interplay of many factors, including a relative resistance to endogenous growth hormone. For children with CKD, this study confirms that rhGH improves growth velocity, albeit modestly. Similarly, the use of rhGH in children on KRT is effective in achieving catch-up growth in some children. However, there is significant inter-person variability in response, and taken as a group, the response is not significantly different than that of children who didn’t receive rhGH. Our cohort received a significantly lower dose of rhGH than is currently recommended. This factor is likely to have contributed to the decreased effectiveness of rhGH in our study. Further, 99% of our KRT cohort were prescribed steroids post-transplant which is likely to have further attenuated the rhGH response. Due to the retrospective nature of this study, it is not possible to determine if children who received rhGH would have demonstrated even poorer catch-up growth in the absence of rhGH treatment. More research is needed to determine the factors that predict a good response to rhGH to allow for more targeted therapy. In addition, steroid-sparing protocols post-transplant to allow for a greater improved final height in these children need to be considered. In the interim, in the absence of a robust conducted randomized controlled trial and given the known benefits of improved growth for the psychological health of affected children, it appears reasonable to at least trial rhGH in children on KRT who are growing poorly although consideration should be given to ceasing in those who don’t respond within a reasonable time frame.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Abbreviations
- CKD:
-
Chronic kidney disease
- ANZDATA:
-
Australian and New Zealand Dialysis and Transplant Registry
- rhGH:
-
Recombinant human growth hormone
- KRT:
-
Kidney replacement therapy
References
Drube J, Wan M, Bonthuis M, Wuhl E, Bachetta J, Santos F, Grenda R, Edefonti A, Harambat J, Shroff R, Tonshoff B, Haffner D (2019) Clinical practice recommendations for growth hormone treatment in children with chronic kidney disease. Nat Rev Nephrol 15:577–589
Hodson E, Willis N, Craig J (2012) Growth hormone for children with chronic kidney disease. Cochrane Database Syst Rev 2:CD003264. https://doi.org/10.1002/14651858.CD003264.pub3
Hartung E, Furth S (2013) Growth in children on renal replacement therapy: a shrinking problem? Pediatr Nephrol 28:1905–1908. https://doi.org/10.1007/s00467-013-2545-1
Gil S, Vaiani E, Guercio G, Ciaccio M, Turconi A, Delgado N, Rivarola M, Belgorosky A (2012) Effectiveness of rhGH treatment on final height of renal transplant recipients in childhood. Pediatr Nephrol 27:1005–1009. https://doi.org/10.1007/s00467-011-2090-8
Seikaly M, Salhab N, Gipson D, Yiu V, Stablien D (2006) Stature in children with chronic kidney disease: analysis of NAPRTCS database. Pediatr Nephrol 21:793–799. https://doi.org/10.1007/s00467-006-0040-7
Carvalho de Camargo M, Henriques C, Vieira S, Komi S, Ribeiro Leao E, Nogueira P (2014) Growth of children with end-stage renal disease undergoing daily hemodialysis. Pediatr Nephrol 39:439–444. https://doi.org/10.1007/s00467-013-2676-4
Harambat J, van Stralen K, Kim J, Tizard E (2012) Epidemiology of chronic kidney disease in children. Pediatr Nephrol 27:363–373. https://doi.org/10.1007/s00467-011-1939-1
Harambat J, Bonthuis M, van Stralen K, Tizard E (2014) Adult height in patients with Advanced CKD requiring renal replacement therapy in childhood. Clin J Am Soc Nephrol 9:92–99. https://doi.org/10.2215/CJN.00890113
Rees L (2016) Growth hormone therapy in children with CKD after more than two decades of practice. Pediatr Nephrol 31:1421–1435. https://doi.org/10.1007/s00467-015-3179-2
Akchurin O, Kogon A, Kumar J, Sethna C, Hammad H, Christos P, Mahan J, Greenbaum L, Woroniecki R (2017) Approach to growth hormone therapy in children with chronic kidney disease varies across North America: the Midwest Pediatric Nephrology Consortium report. BMC Nephrol 18:181. https://doi.org/10.1186/s12882-017-0599-1
Franke D, Thomas L, Steffens R, Pavicic L, Gellermann J, Froede K, Querfeld U, Haffner D, Zivicnjak M (2015) Patterns of growth after kidney transplantation among children with ESRD. Clin J Am Soc Nephrol 10:127–134. https://doi.org/10.2215/CJN.02180314
Rodig N, McDermott K, Schneider M, Hotchkiss H, Yadin O, Seikaly M, Furth S, Warady B (2014) Growth in children with chronic kidney disease: a report from the chronic kidney disease in children study. Pediatr Nephrol 29:1987–1995. https://doi.org/10.1007/s00467-014-2812-9
Al-Uzri A, Matheson M, Gipson D, Mendley S, Hooper S, Yadin O, Rozansky D, Moxey-Mims M, Furth S, Warady B, Gerson A (2013) The impact of short stature on health-related quality of life in children with chronic kidney disease. J Pediatr 163:736–741. https://doi.org/10.1016/j.jpeds.2013.03.016
Francis A, Didsbury M, van Zwieten A, Chen K, James L, Kim S, Howard K, Williams G, Treidel O, McTaggart S, Walker A, Mackie F, Kara T, Nassar N, Teixeiro-Pinto A, Tong A, Johnson D, Craig J, Wong W (2019) Quality of life of children and adolescents with chronic kidney disease: a cross-sectional study. Arch Dis Child 104:134–140. https://doi.org/10.1136/archdischild-2018-314934
Haffner D, Schaeffer F, Nissel R, Wuhl E, Tonshoff B, Mehls O (2000) Effect of growth hormone treatment on the adult height of children with chronic renal failure. N Engl J Med 343:923–930. https://doi.org/10.1056/NEJM200009283431304
Klare B, Montoya C, Fischer D (2012) Normal adult height after steroid-withdrawal within 6 months of pediatric kidney transplantation: a 20 years single center experience. Transpl Int 25:276–282. https://doi.org/10.1111/j.1432277.2011.01400.x
ANZDATA (2019) Australia and New Zealand Dialysis and Transplant Registry. https://www.anzdata.org.au/anzdata. Accessed April 2019
Australian Government Department of Health (2017) Pharmaceutical benefits scheme (PBS). https://www.pbs.gov.au/pbs/home. Accessed 5 July 2019
Centers for Disease Control and Prevention (2009) National Center for Health Statistics. Percentile Data Files with LMS values. https://www.cdc.gov/growthcharts/percentile_data_files.htm. Accessed 21 July 2019
Deodati A, Cianfarani S (2011) Impact of growth hormone therapy on adult height of children with idiopathic short stature: a systematic review. BMJ 342:c7157. https://doi.org/10.1136/bmj.c7157
Savage M, Bang P (2012) The variability of responses to growth hormone therapy in children with short stature. Indian J Endocrinol Metab 16(Supp 2):178–184. https://doi.org/10.4103/2230-8210.104034
Acknowledgements
The authors gratefully acknowledge the substantial contributions of the entire Australia and New Zealand nephrology community (physicians, surgeons, database managers, nurses and patients) in providing information for and maintaining the ANZDATA Registry database. We also gratefully acknowledge the Australasian Pediatric Endocrine Group Subcommittee OzGrow for their assistance in collating and providing data on rhGH use.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Melanie Aldridge and Steven McTaggart. The first draft of the manuscript was written by Melanie Aldridge and Steven McTaggart, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval for this study was obtained from the Human Research Ethics Committee at Children’s Health Queensland Hospital and Health Service: HREC/18/QRCH/44 and SSA/18/QRCH/5.
Consent to participate
ANZDATA collects data in accordance with the Australian Commonwealth Privacy Act and associated state legislation governing health data collection, and individual opt-in patient consent is not required for the registry data.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PPTX 84 kb)
Rights and permissions
About this article
Cite this article
Aldridge, M.K., Trnka, P., Francis, A. et al. Effectiveness of growth hormone on growth and final height in paediatric chronic kidney disease. Pediatr Nephrol 37, 651–658 (2022). https://doi.org/10.1007/s00467-021-05259-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05259-6