Abstract
Background
Acute kidney injury (AKI) is associated with worse outcomes and increased morbidity and mortality in pediatric intensive care unit (PICU) patients. The renal angina index (RAI) has been proposed as an early prediction tool for AKI development.
Objectives
The objective was to evaluate outcomes of RAI-positive patients and to compare RAI performance with traditional AKI markers across different patient groups (medical/post-surgical). This was an observational retrospective study. All children admitted to a tertiary hospital PICU over a 3-year period were included. Electronic medical records were reviewed. Day 1 RAI was calculated, as was the presence and staging of day 3 AKI.
Results
A total of 593 patients were included; 56% were male, the mean age was 55 months, and 17% had a positive RAI. This was associated with day 3 AKI development and worse outcomes, such as greater need for kidney replacement therapy, longer duration of mechanical ventilation, vasoactive support and PICU stay, and higher mortality. For all-stage kidney injury, RAI presented a sensitivity of 87.5% and a specificity of 88.1%. Prediction of day 3 all-stage AKI by RAI had an AUC=0.878; its performance increased for severe AKI (AUC = 0.93). RAI was superior to serum creatinine increase and KDIGO AKI staging on day 1 in predicting severe AKI development. The performance remained high irrespective of the type of admission.
Conclusions
The RAI is a simple and inexpensive tool that can be used with medical and post-surgical PICU patients to predict AKI development and anticipate complications, allowing for the adoption of preventive measures.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) occurs in up to 33% of patients in pediatric intensive care units (PICUs) and is associated with worse outcomes and increased morbidity and mortality [1,2,3,4,5,6,7]. Delayed diagnosis is frequent, which may be due to the wide use of serum creatinine (sCr)—a “late” AKI marker [1, 8,9,10,11,12,13]. In 2010, Goldstein et al. introduced the concept of “renal angina,” reflecting the need for real-time markers that can predict AKI progression earlier and more accurately [9]. The renal angina index (RAI), proposed by Basu et al., was first used in 2014 [14]. As Ricci (2018) stated, “the idea is that a patient with a positive score should be managed, from a kidney standpoint, with a timing similar to that for an anginous patient before irreversible damage occurs” [15]. RAI is assessed 12 h after PICU admission and allows for a prediction of severe AKI 3 days later. The index is a product of the points assigned to risk and injury criteria [16], as shown in Table 1.
RAI was demonstrated, in its first validation studies, to be efficacious [14, 17, 18], with a good area under the curve and high negative predictive values. Since its creation, only a few studies validating RAI have been published, none of them in a European setting. A systematic review and meta-analysis from 2020 included 14 RAI studies, mainly regarding non-surgical patients, with more than half of them having fewer than 100 participants [19]. Despite recognizing some discussion and controversy, in this review, it was pointed out that RAI can potentially be a reliable tool for AKI prediction [19]. Therefore, it can play an important role in AKI risk detection and in the early anticipation of all available therapeutic options.
To our knowledge, there are no prior studies comparing RAI performance across different types of admissions (medical and post-surgical patients, divided by the type of surgery involved).
Objectives
The purpose of this paper is to provide further assessment of RAI as a valid predictor of AKI on PICU admission in a large European cohort, by evaluating outcomes of patients who are RAI positive and comparing the RAI performance with traditional AKI markers across different patient groups (medical/post-surgical). Our hypotheses are:
-
1.
Patients who are RAI positive on admission will have worse outcomes than those who are RAI negative.
-
2.
RAI will predict day 3 AKI better than sCr increase/KDIGO AKI staging on day 1.
-
3.
RAI will have good performance in all patient groups, irrespective of the underlying motive of admission.
Methods
Study design
Unicentric, observational study with retrospective enrollment. Sequential systematic sampling of all the patients admitted to a tertiary European PICU over a 3-year consecutive period (between 1 January 2017 and 31 December 2019).
Inclusion criteria
Age between 1 month and 17 years and 364 days, LOS equal to or longer than 72 h, sCr and fluid balance information (FO) in the first 12 h after admission, and sCr determination on day 3 of PICU hospitalization.
Exclusion criteria
LOS less than 72 h, history of stage 5 chronic kidney disease (or maintenance dialysis), kidney transplantation 90 days prior to admission, and missing data for RAI calculation (absence of sCr and FO at 12 h, sCr at day 3 or height for estimation of baseline sCr when not available).
Eligible patients’ electronic medical records were reviewed for variables regarding demographic and anthropometric information and disease, admission, and outcome characterization. The formula developed by Basu et al. [5, 14, 16], which is described in Table 1, was used to calculate the RAI. This was performed using the data available 12 h after patient admission. RAI ≥ 8 was considered positive. The baseline sCr was retrieved from the electronic medical record as the lowest registered in the previous 3 months. If the baseline sCr was unobtainable, it was calculated using the patient’s height and an estimated glomerular filtration rate of 120 mL/min per 1.73 m2 as previously described in the literature [7]. AKI on days 1 and 3 was defined according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria staging. KDIGO stages 2 and 3 were considered severe AKI [20] (Table 2). The primary outcome was the presence of all-stage AKI on day 3. Secondary outcomes were initiation of kidney replacement therapy (KRT), need for vasoactive support, duration of mechanical ventilation, administration of antibiotics, initiation of extracorporeal membrane oxygenation (ECMO), PICU LOS, and mortality (mortality was only counted if it occurred during the patient’s stay in the PICU).
For analysis purposes, data were anonymized, and each patient was given a unique identifier code. Continuous variables are reported as medians (interquartile range—IQR) and were compared using the Mann-Whitney test. Categorical variables are presented using frequencies and proportions and were compared by the x2 or Fisher’s exact test. The area under the curve (AUC), as presented on the ROC curve, was calculated for comparison of the performance of different AKI predictors in our sample (and in different subgroups) and for comparison with other studies. Statistical analysis was performed using IBM® Statistical Package for the Social Sciences (SPSS Statistics®) Version 26. A p value < 0.05 was considered significant. This study was approved by the Ethics Committee of Centro Hospitalar e Universitário São João.
Results
Patient characteristics
A total of 929 patients were admitted during the study period; 593 (64%) were included, and 265 were excluded due to LOS less than 72 h, 68 due to missing data, and 3 due to a history of stage 5 chronic kidney disease.
The mean patient age was 55 months (IQR 11–151) [4.6 years (0.9–12.6)], and 56% of patients were male. A total of 20 patients (3.4%) needed KRT, and the overall mortality was 2.7%.
-
1.
Patients who are RAI positive on admission have worse outcomes than those who are RAI negative
Patients were gathered into two groups (Table 3) according to the RAI score (negative vs. positive) on day 1 of the PICU stay. In total, 17% had an RAI ≥ 8.
A positive RAI on day 1 was associated with a higher incidence of diseases related to the cardiovascular system (12.9% vs. 4.1%, p < 0.0001) and sepsis (8.9% vs. 2.4%, p = 0.001). Surgical patients more often had a negative RAI (54.3% vs. 43.6%, p = 0.046). No other diagnosis groups presented statistically significant differences. An RAI score ≥ 8 was associated with the development of all-stage AKI (35% vs. 1%, p < 0.0001). It was also predictive of worse outcomes, including increases in the use of KRT (14% vs. 1%, p < 0.0001), the duration of mechanical ventilation (8 vs. 5 days, p < 0.0001), vasoactive support (6 vs. 4 days, p = 0.042), PICU LOS (14 vs. 8 days, p < 0.0001), and mortality (8% vs. 2%, p < 0.0001).
The characteristics of patients who developed AKI on day 3 compared with those who did not are displayed in Table 4. AKI on D3 was associated with diagnosis related to the cardiovascular system, shock, and sepsis. It was also associated with increases in KRT use, antibiotic use, duration of mechanical ventilation, LOS, and mortality. Three percent of the patients without AKI on day 1, 10% of patients with day 1 KDIGO 1, 52% of the patients with day 1 KDIGO 2, and 78% of the patients with day 1 KDIGO 3 developed all-stage AKI on day 3. Severe AKI on day 1 (but not KDIGO 1) was associated with AKI on day 3.
-
2.
RAI is a valid tool and predicts day 3 AKI better than sCr increase/KDIGO AKI staging on day 1
For all-stage kidney injury (KDIGO AKI 1–3), the RAI sensitivity was 87.5%, the specificity was 88.1%, the positive predictive value (PPV) was 34.7%, and the negative predictive value (NPV) was 99%. The likelihood ratio was 7.33.
For severe AKI (KDIGO AKI 2–3), RAI had a sensitivity of 100%, a specificity of 97%, a PPV of 60%, and an NPV of 100%.
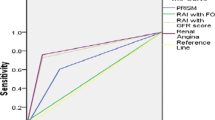
Prediction of all-stage day 3 AKI by RAI (AUC) was superior to the prediction by sCr increase or KDIGO on day 1 (Fig. 1; Table 5). The performance was even better when predicting severe D3 AKI (KDIGO 2 and 3) (Fig. 2; Table 5).
-
3.
RAI has a good performance for predicting severe AKI, irrespective of the underlying motive of admission
A subanalysis dividing medical and post-surgical patients (and dividing the post-surgical patients by the type of surgery involved) displayed a high performance (AUC), irrespective of the type of admission/surgery involved (Table 5). RAI performance was surpassed by the absolute increase in sCr in medical patients in the prediction of all-stage AKI (but not in severe AKI, where it maintained the best performance).
Discussion
Our results demonstrate, in a large sample, that (1) patients who have a positive RAI have worse outcomes than those who are RAI negative; (2) RAI has a better performance than traditional markers in identifying patients at high risk for AKI by conjugating baseline kidney function markers with clinical and laboratory data; (3) RAI has a high performance, irrespective of the underlying type of admission.
-
1.
RAI performance as an AKI predictor and comparison with traditional AKI markers
The overall AUC for RAI was 0.878 in our study. Previous studies reported AUCs for RAIs between 0.73 and 0.9 [21,22,23]. Moreover, our results also show that RAI is an even better predictor for severe AKI, as demonstrated by the AUC increase to 0.93 and its superior AUCs to those of sCr or KDIGO in all subgroups of patients, irrespective of the underlying type of admission (medical/post-surgical). In our cohort, RAI presented a better sensitivity, specificity, NPV, and PPV than previously described [22, 23]. We should emphasize that none of the patients who were RAI negative developed severe AKI on day 3, which confirms the high specificity of this diagnostic tool.
The absolute sCr increase on day 1 showed a better performance than KDIGO on day 1. This finding is in line with recent studies advocating that systems based on the absolute increases in sCr provide a promising alternative to the KDIGO system for characterizing AKI severity [24]. Other authors also pointed out the limitations of staging systems in providing accurate pictures [25].
-
2.
RAI limitations
One of the limitations of RAI is the need for a baseline sCr value for its calculation. In our cohort, when baseline sCr was unobtainable, we estimated it using a height-based method [7]. Other methods, such as age-based methods [26], also have other important limitations; the authors of these methods mention that they only used them when it was not possible to obtain a close “to the date” baseline sCr or to use height to estimate this parameter [26]. This need for estimation likely impacts the results of the different studies.
Another important limitation of RAI is that, even considering the findings published in different studies, the patients with the highest RAI scores developed severe AKI only in 41–67% of cases (35% all-stage AKI and 21% severe AKI in our study). This reinforces the fact that clinicians should be cautious of its interpretation, especially when it implies treatment decisions [6, 8, 14, 17].
-
3.
Novel urinary/plasma AKI biomarkers and their utilization with RAI
Numerous clinical investigations have also evaluated novel urinary and plasma biomarkers that have the potential ability to detect kidney insult earlier, adding more information about pathophysiology and prognosis, such as urinary neutrophil gelatinase-associated lipocalin (uNGAL), kidney injury molecule-1 (KIM-1), urine interleukin-18 (uIL-18), and liver-type fatty acid-binding protein (L-FABP) [1, 4, 10, 18]. There is growing evidence that they do not have an optimal performance when used as single markers but that when used in conjunction with a positive RAI, they can increase the performance in detecting patients at high risk of AKI. Specifically, Menon et al. (2016) concluded that the addition of uNGAL to the RAI model increased the AUC from 0.80 to 0.97 [18]. In our study, RAI’s overall AUC, without adding uNGAL, was globally higher than in Menon et al.’s study (ranging from 0.878 (all-stage AKI) to 0.930 (severe AKI)). It is possible that adding this biomarker to the model would also increase AUC in our sample, especially for the groups of patients/conditions where AUC was lower (medical patients and post-cardiac surgery patients, Table 5). Further trials are needed to ensure the transition from laboratory to clinical practice [1, 4, 6, 8, 9, 11, 14, 18], including studies aiming at identifying patients/conditions for which the addition of a biomarker to RAI would be of greater benefit.
-
4.
Patient outcomes and comparison to previous studies
In our study, some outcomes are parallel to those in previous reports. In the AWARE study, AKI developed in 26.9% of patients admitted to the PICU, and severe AKI developed in 11.6% [27]. In our series, these values were lower, at 12.5% and 5.7%, respectively. The mortality rates in the AWARE cohort were 11% in patients with AKI and 2.5% in patients without AKI, similar to our results (15% and 2%, respectively). Previous works demonstrated that severe AKI was associated with increased time of mechanical ventilation, use of ECMO, use of KRT, and mortality [21, 27, 28]. Our results are similar except for the use of ECMO; in our series, this treatment was used mainly in respiratory (n = 4) patients, post surgically (n = 2) and only in one case with cardiovascular diagnosis.
The medical conditions associated with AKI development in our study included cardiovascular diseases, sepsis, shock, and endocrine disease, similar to a previous report, with the exception of endocrine diseases, which should be considered cautiously due to their low representation [21, 27, 29].
-
5.
Study limitations
This is a unicentric, retrospective study. Therefore, not all of the data were recorded previously with the specific purpose of the study, limiting some interpretations of the results (such as the need for estimation of baseline sCr in many patients). Our results might be biased since approximately half of our patients were surgical patients, limiting comparisons with other studies with higher proportions of medical patients with conditions known to predispose them to AKI development. However, our subanalysis by medical/surgical groups and by different surgical procedures allows for a clearer picture of RAI performance in the different scenarios. Another limitation was the impossibility of having in-time patient severity scores performed in our PICU.
Conclusion
In our study, RAI proved to be a simple and inexpensive tool to predict severe AKI development in medical and post-surgical PICU patients, with good sensitivity and specificity and with better performance than traditional single markers—irrespective of the type of admissions involved. RAI-positive patients experienced worse outcomes—emphasizing that early AKI detection can be particularly beneficial for those patients.
Therefore, the systematic use of RAI on admission can be of added value on PICUs, allowing for an early implementation of preventive measures and improving patient prognosis. Preventive measures are currently the mainstream preference for non-dialytic AKI management. This includes aggressive sepsis treatment, provision of adequate nutrition, adequate fluid balance targets, and avoidance of kidney toxic drugs and intravenous iodinated contrast.
Further studies must be conducted to compare outcomes after implementation of RAI in PICUs and to optimize early AKI detection scores such as RAI.
Availability of data and material
The data that support the findings of this study are available from Centro Hospitalar e Universitário de São João, with restricted access due to patient privacy. The authors had access to these data after a reasonable request and authorization from the referred institution.
Code availability
Not applicable.
Abbreviations
- AKI:
-
Acute kidney injury
- KDIGO:
-
Kidney Disease Improving Global Outcomes criteria staging
- LOS:
-
Length of stay
- PICU:
-
Pediatric intensive care unit
- KRT:
-
Kidney replacement therapy
References
Fragasso T, Ricci Z, Goldstein SL (2018) Pediatric acute kidney injury. Contrib Nephrol 193:113–126. https://doi.org/10.1159/000484968
Kari JA, Alhasan KA, Shalaby MA, Khathlan N, Safdar OY, Al Rezgan SA, El Desoky S, Albanna AS (2018) Outcome of pediatric acute kidney injury: a multicenter prospective cohort study. Pediatr Nephrol 33:335–340. https://doi.org/10.1007/s00467-017-3786-1
Symons JM, Chua AN, Somers MJ, Baum MA, Bunchman TE, Benfield MR, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Hackbarth R, Alexander SR, Mahan J, McBryde KD, Goldstein SL (2007) Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol 2:732–738. https://doi.org/10.2215/CJN.03200906
McGalliard RJ, McWilliam SJ, Maguire S, Jones CA, Jennings RJ, Siner S, Newland P, Peak M, Chesters C, Jeffers G, Broughton C, McColl L, Lane S, Paulus S, Cunliffe NA, Baines P, Carrol ED (2020) Identifying critically ill children at high risk of acute kidney injury and renal replacement therapy. PLoS One 15:e0240360. https://doi.org/10.1371/journal.pone.0240360
Basu RK, Chawla LS, Wheeler DS, Goldstein SL (2012) Renal angina: an emerging paradigm to identify children at risk for acute kidney injury. Pediatr Nephrol 27:1067–1078. https://doi.org/10.1007/s00467-011-2024-5
Lameire N, Van Biesen W, Vanholder R (2017) Epidemiology of acute kidney injury in children worldwide, including developing countries. Pediatr Nephrol 32:1301–1314. https://doi.org/10.1007/s00467-016-3433-2
Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL (2008) Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol 3:948–954. https://doi.org/10.2215/CJN.05431207
McMahon BA, Koyner JL (2016) Risk stratification for acute kidney injury: are biomarkers enough? Adv Chronic Kidney Dis 23:167–178. https://doi.org/10.1053/j.ackd.2016.03.001
Goldstein SL, Chawla LS (2010) Renal angina. Clin J Am Soc Nephrol 5:943–949. https://doi.org/10.2215/CJN.07201009
Basu RK, Kaddourah A, Terrell T, Mottes T, Arnold P, Jacobs J, Andringa J, Goldstein SL, Prospective Pediatric AKI Research Group (ppAKI) (2015) Assessment of Worldwide Acute Kidney Injury, Renal Angina and Epidemiology in critically ill children (AWARE): study protocol for a prospective observational study. BMC Nephrol 16:24. https://doi.org/10.1186/s12882-015-0016-6
Goldstein SL (2018) The renal angina index to predict acute kidney injury: are adults just large children? Kidney Int Rep 3:516–518. https://doi.org/10.1016/j.ekir.2018.03.004
Waikar SS, Bonventre JV (2009) Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20:672–679. https://doi.org/10.1681/ASN.2008070669
Doi K, Yuen PS, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, Star RA (2009) Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol 20:1217–1221. https://doi.org/10.1681/ASN.2008060617
Basu RK, Zappitelli M, Brunner L, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL (2014) Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int 85:659–667. https://doi.org/10.1038/ki.2013.349
Ricci Z (2018) A renal angina index to overcome the silence of the kidneys. Lancet Child Adolesc Health 2:83–84. https://doi.org/10.1016/S2352-4642(17)30183-9
Basu RK, Kaddourah A, Goldstein SL, AWARE Study Investigators (2018) Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: a multicentre, multinational, prospective observational study. Lancet Child Adolesc Health 2:112–120. https://doi.org/10.1016/S2352-4642(17)30181-5
Basu RK, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL (2014) Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol 9:654–662. https://doi.org/10.2215/CJN.09720913
Menon S, Goldstein SL, Mottes T, Fei L, Kaddourah A, Terrell T, Arnold P, Bennett MR, Basu RK (2016) Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol Dial Transplant 31:586–594. https://doi.org/10.1093/ndt/gfv457
Abbasi A, Mehdipour Rabori P, Farajollahi R, Mohammed Ali K, Ataei N, Yousefifard M, Hosseini M (2020) Discriminatory precision of renal angina index in predicting acute kidney injury in children; a systematic review and meta-analysis. Arch Acad Emerg Med 8:e39
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2:1–138. https://doi.org/10.1038/kisup.2012.7
Huang L, Shi T, Quan W, Li W, Zhang L, Liu X, Huang S, Li Y, Li X (2020) Assessment of early renal angina index for prediction of subsequent severe acute kidney injury during septic shock in children. BMC Nephrol 21:358. https://doi.org/10.1186/s12882-020-02023-0
Gawadia J, Mishra K, Kumar M, Saikia D (2019) Prediction of severe acute kidney injury using renal angina index in a pediatric intensive care unit. Indian Pediatr 56:647–652. https://doi.org/10.1007/s13312-019-1587-2
Sethi SK, Raghunathan V, Shah S, Dhaliwal M, Jha P, Kumar M, Paluri S, Bansal S, Mhanna MJ, Raina R (2018) Fluid overload and renal angina index at admission are associated with worse outcomes in critically ill children. Front Pediatr 6:118. https://doi.org/10.3389/fped.2018.00118
Wang HE, Jain G, Glassock RJ, Warnock DG (2013) Comparison of absolute serum creatinine changes versus Kidney Disease: Improving Global Outcomes consensus definitions for characterizing stages of acute kidney injury. Nephrol Dial Transplant 28:1447–1454. https://doi.org/10.1093/ndt/gfs533
Barasch J, Zager R, Bonventre JV (2017) Acute kidney injury: a problem of definition. Lancet 389:779–781. https://doi.org/10.1016/S0140-6736(17)30543-3
Roy JP, Johnson C, Towne B, Menke F, Kiger S, Young W, Basu R, Chima R, Fei L, Krallman K, Goldstein SL (2019) Use of height-independent baseline creatinine imputation method with renal angina index. Pediatr Nephrol 34:1777–1784. https://doi.org/10.1007/s00467-019-04294-8
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, AWARE Investigators (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376:11–20. https://doi.org/10.1056/NEJMoa1611391
Kaur R, Dhooria GS, Pooni PA, Bhat D, Bhargava S, Kakkar S, Arora K, Bansal N (2018) Utilization of the renal angina index in PICU of a developing country for prediction of subsequent severe acute kidney injury. Pediatr Nephrol 33:2185–2191. https://doi.org/10.1007/s00467-018-4001-8
Raman S, Tai CW, Le Marsney R, Schibler A, Gibbons K, Schlapbach LJ (2020) Prediction of acute kidney injury on admission to pediatric intensive care. Pediatr Crit Care Med 21:811–819. https://doi.org/10.1097/PCC.0000000000002411
Author information
Authors and Affiliations
Contributions
Conceptualization: Francisco Ribeiro-Mourão, Ana Carvalho Vaz, André Azevedo, Helena Pinto, Marta João Silva, Joana Jardim, Augusto Ribeiro. Methodology: Francisco Ribeiro-Mourão, Joana Jardim, Helena Pinto, Marta João Silva. Formal analysis and investigation: Francisco Ribeiro-Mourão, Ana Carvalho Vaz, André Azevedo, Joana Jardim. Writing - original draft preparation: Francisco Ribeiro-Mourão, Ana Carvalho Vaz, André Azevedo. Writing - review and editing: Francisco Ribeiro-Mourão, Ana Carvalho Vaz, André Azevedo, Helena Pinto, Marta João Silva, Joana Jardim, Augusto Ribeiro. Supervision: Joana Jardim, Augusto Ribeiro. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Approval was obtained from the ethics committee of Centro Hospitalar e Universitário São João. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
Waived by ethics committee.
Consent for publication
Waived by ethics committee
Competing interests
The authors declare no competing interests.
Additional information
Prior presentations
There are no prior presentations of this work as abstract or poster.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PPTX 155 kb).
Rights and permissions
About this article
Cite this article
Ribeiro-Mourão, F., Vaz, A.C., Azevedo, A. et al. Assessment of the renal angina index for the prediction of acute kidney injury in patients admitted to a European pediatric intensive care unit. Pediatr Nephrol 36, 3993–4001 (2021). https://doi.org/10.1007/s00467-021-05116-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05116-6