Abstract
Background
Oral cyclophosphamide (CYP) is an important therapeutic agent in treatment of steroid-sensitive nephrotic syndrome having a steroid-dependent (SD) or frequent relapsing (FR) course. This retrospective observational study aimed to determine response to oral CYP and factors associated with positive response in these patients.
Methods
We studied 100 children (male; 75) with FR (19%) and SD (81%) NS treated with CYP in the Pediatric Nephrology clinic. Responders were defined as children in whom steroids were stopped for at least 6 months following CYP and factors affecting response were analysed. Relapse-free survival was estimated by Kaplan–Meier method.
Results
Median age at onset of NS was 3 years (IQR 2–5.2). Median age at CYP was 5.7 years (IQR 3.7–7.9). Fifty percent of patients were in the responder group at 6 months of CYP. Relapse-free survival post CYP therapy was 31% at 1 year, 11% at 2 years. Factors predicting good response were age at onset of NS > 3 years with 61.2% response at 6 months (p = 0.028) and older age at CYP initiation (> 5 years) with 61% response (p = 0.008). Multivariate regression analysis showed age at start of CYP > 5 years was an independent factor for good response (p = 0.044, OR = 2.903, CI −1.03 to 8.18).
Conclusions
Judicious selection of patients, especially with age of onset of NS more than 3 years and initiation of CYP after age of 5 years, can predict better response in this group of patents.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nephrotic syndrome (NS) is an important chronic disease in children characterized by massive proteinuria, hypoalbuminemia, hyperlipidemia and edema. The majority of patients with NS show remission of proteinuria following treatment with corticosteroids, and are therefore called ‘steroid sensitive’ (SS), but among them a substantial proportion have a relapsing course or become steroid-dependent (SD) [1]. In these patients, side effects of steroids can occur, including hypertension, cataracts, short stature, systemic infections, obesity and psychological disturbances. Hence, to reduce adverse effects of steroids, several non-steroid immunosuppressive agents are used for treatment. One such drug is cyclophosphamide (CYP) [2,3,4,5,6,7,8,9].
Cyclophosphamide is an old alkylating drug, which exerts immunosuppressive activity by causing cytotoxic effects on proliferating lymphocytes [10]. It is still commonly used as a steroid-sparing agent in frequent relapsing (FR)/SDNS in developing countries and is known to reduce frequency of relapses and achieve long-term remission [11].
We undertook this study to ascertain the efficacy of oral CYP and predictors of response in 100 Indian children with SSNS.
Methods
Study design and population
We performed a retrospective single-centre cohort study of 100 children between 3 months and 18 years of age with FR/SDNS. Patients were followed up in the Pediatric Nephrology clinic in the Department of Pediatrics, Dayanand Medical College and Hospital, Ludhiana, Punjab, India. They were treated with oral CYP during the period from 2007 to 2019. The recommended dose was 2–2.5 mg/kg/day of oral CYP over 10–12 weeks (cumulative dose of < 168 mg/kg). Therapy was initiated after remission with oral prednisolone (2 mg/kg/day). Prednisolone was co-administered at a dose of 1.5 mg/kg on alternate days for 4 weeks, followed by 1 mg/kg for the next 4 weeks and tapered and stopped over the next 2–3 months. Patients with congenital NS (age at onset < 3 months), steroid-resistant nephrotic syndrome and follow-up period less than 12 months following CYP and patients who could not complete the intended course were excluded from the study. Approval for the study was obtained from the Institutional Ethics Committee. Our treatment protocol was in accordance with the Indian Society of Pediatric Nephrology guidelines on nephrotic syndrome (2008) [12], where levamisole could be a satisfactory initial choice for patients with frequent relapses or steroid dependence followed by use of CYP or MMF. Although the parents were offered all therapeutic options and all available medications are approved in the country, treatment by CYP was preferred by many parents over other steroid-sparing agents because of the lower cost and shorter duration of therapy.
Data collection
Detailed demographic data, like age, gender, education status, age of onset of NS, hypertension at onset, gross hematuria and drugs (steroids or steroid-sparing agents) received before starting CYP, were retrieved from the Pediatric Nephrology clinic patient file record. Indication to start CYP, the number of relapses and steroid-dependent dose (mg/kg/day) prior to starting CYP was recorded. Age at start of CYP and duration of illness before treatment were also recorded.
Side effects like leucopenia, minor infections, major infections (those requiring hospitalization), haemorrhagic cystitis and alopecia encountered during the course of CYP were noted. Complete blood counts were monitored in patients every 2 weeks. If total leucocyte count decreased to less than 4000 cells/mm3 or patients suffered any infection requiring hospitalization, the drug was temporarily stopped and restarted when counts increased to ≥ 4000 cells/mm3. Oral mesna was not used for the prevention of acrolein-induced hemorrhagic cystitis.
Urine dipstick albumin was monitored regularly on patient diary. Kidney function tests were done annually in all patients. Kidney biopsy was not performed in these patients. Parameters, like time to first relapse, duration for which steroids were stopped, number of relapses and dependent dose post CYP therapy, were documented.
Definitions
Definitions and treatment guidelines for NS published by the Indian Society of Pediatric Nephrology group were used for the study [12].
Based upon the status post CYP, patients were classified as either of the following:
-
(A)
Responder – Those in whom steroids were stopped for a period of at least 6 months following treatment with CYP.
-
(B)
Non-responder – Those in whom either steroids were not stopped or stopped for less than 6 months. Patients were categorized as sustained remission if there was no relapse and off steroids for a period of at least 2 years. We compared the factors affecting response to oral CYP between responders and non-responders.
Statistical methods
Data are presented as mean ± SD (standard deviation) or median and interquartile range (IQR) for continuous variables and percentages for categorical variables. Comparison of quantitative variables was done using Student t-test and Mann–Whitney U test for independent samples for parametric and non-parametric data respectively. For comparing categorical data, Chi-square (χ2) test was performed. Kaplan–Meier analysis was performed to estimate the relapse-free survival since the start of CYP, with the end point as time of first relapse. The ROC curve is used to identify the best cut-off of the age of onset of NS, age at start of CYP treatment and steroid-dependent dose before CYP. Cox proportional hazards analysis was used to examine the effect of various factors on steroid-free survival. All statistical calculations were done using SPSS version 21. A p value less than 0.05 was considered significant.
Results
Population characteristics
Of approximately 550 cases of FR/SDNS patients attending the Pediatric Nephrology clinic, one hundred patients (75 boys and 25 girls) received oral CYP during the study period. The patients’ characteristics are shown in Table 1. Median age at the onset of NS was 3 years (IQR 2–5.2). Median duration of follow-up after CYP was 3.9 (IQR 2.2–7.1) with minimum follow-up period of 12 months. Median age at CYP was 5.7 years (IQR 3.7–7.9).
Of the 100 children, 81% were diagnosed with SDNS and 19% were diagnosed with FRNS. The median steroid dependence dose was 1.2 mg/kg/day (IQR 0.8–1.4) in SDNS patients. Prior to initiating CYP, 59% received steroids alone, 38% patients received levamisole and 3% received mycophenolate mofetil. CYP was administered at a median cumulative dose of 154 mg/kg (IQR 149–160).
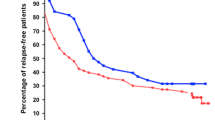
Median time to first relapse post CYP was 4 months (IQR 2.25–8), with 7 months (IQR 2–15) in FRNS vs. 4 months (IQR 3–8) in SDNS (p = 0.318). Using the Kaplan–Meier curve, relapse-free survival time post CYP therapy was 50% (95% CI 40–60) at 6 months, 31% (95% CI 22–40) at 1 year and 11% (95% CI 5–17) at 2 years (sustained remission) (Fig. 1). Post CYP therapy, the median-dependent dose of steroid reduced from 1.2 (IQR 0.8–1.5) to 0.8 (IQR 0.42–1) mg/kg/day (p < 0.001).
At initiation of CYP, 41% patients had features of steroid toxicity, with hypertension (11%), short stature (11%), overweight (10%) and obesity (7%). Sub capsular cataract was seen in 2% patients.
Factors affecting response to oral CYP
Median age at CYP in responders was 7 years (IQR 4.9–8.1) compared to non-responders 4.6 years (IQR 3.35–7.27) (p = 0.129). Median age of onset of NS in the responder group was 4.2 years (IQR 2.4–5.6) compared to non-responders 2.7 years (IQR 2–5.07) (p = 0.11). Median duration of illness before CYP in the responder group was 1.9 years (IQR 1.5–3.3) compared to non-responders 1.8 years (IQR 1.33–2.98) (p = 0.375).
There was a significant difference in remission rate in different age groups at 1-year post CYP (< 5 years: 0% patients; 5–10 years: 29.5%; 11–18 years: 39.1%) (p = 0.05), although this effect was not sustained at 2-year post CYP (< 5 years: 0% patients; 5–10 years: 13.6%; 11–18 years: 10.9%) (p = 0.461). Time to first relapse in different age groups did not differ significantly (< 5 years: 4 months (IQR 3–5.25); 5–10 years: 4 months (IQR 2–6.75); 11–18 years: 5 months (IQR 2.75–14.25), p = 0.196).
Logistic regression analysis
In univariate analysis, the age at onset of NS influenced CYP response, with 39.2% response with onset < 3 years compared to 61.2% response with onset > 3 years (ROC curve cut-off for age of onset at 3 years had 61.2% sensitivity and 60.8% specificity, p = 0.028). Gender did not have a significant effect on response (p = 0.817). Young age at CYP initiation was significantly associated with a lower response rate (34.1% for age ≤ 5 years; 61% for age > 5 years, p = 0.008) (Fig. 1). ROC curve cut-off of age at start of CYP > 5 years predicted moderately good response at 6 months with 72% sensitivity and 54% specificity. The positive predictive value was 61.02% (CI 52.5–68.9%) and negative predictive value was 65.9% (CI 53.6–76.3%). Area under the curve was 0.645 (p = 0.012) (Fig. 2).
CYP responsiveness was slightly better in FRNS (68.4%) at 6 months compared to SDNS (45.7%), but was not statistically significant (p = 0.074). Duration of NS prior to initiating CYP did not affect the response to CYP therapy (p = 0.423). Children with steroid-dependent dose > 1 mg/kg/day had only 37% responders at 6 months compared to 50% responders with dependent dose of ≤ 1 mg/kg/day (ROC curve cut-off for steroid-dependent dose 1 mg/kg/day had 70% sensitivity and 43% specificity) (p = 0.270).
Based on the results of the univariate analysis, a multivariate logistic regression analysis was performed, which showed children with age at start of CYP > 5 years were significantly associated with good response to oral CYP at 6 months (p = 0.044, OR = 2.903, CI −1.03 to 8.18). The correlation of age of onset of NS was non-significant (p = 0.902) (Tables 2 and 3).
There was a significant difference in cumulative dosage received based on body surface area (BSA) in different age groups (< 5 years: 3619.3 mg/m2 (IQR 3575.44–3740.73); 5–10 years: 4067.03 mg/m2 (IQR 3877.46–4307.93); 11–18 years: 4669.35 mg/m2 (IQR 4423.83–5268.22), p = 0.0001), although not with milligramme per kilogramme-based dosages.
However, there was no difference in cumulative dose (mg/m2) of CYP in the responder group, 4226.86 mg/m2 (IQR 3857.23–4774.18) compared to the non-responder group, 4366.83 mg/m2 (IQR 4094.61–4705.44) (p = 0.268). The area under the curve calculated for cumulative dose based on surface area and response to CYP did not show significance (AUC: 0.543, 95% CI 0.428–0.658, p = 0.461).
We also studied the influence of pre-treatment steroid-sparing medications on the study results. Of the 38% patients who received levamisole (with poor response) prior to CYP, 19 patients (50%) responded compared to response in 30 patients (50.8%) who received only steroids (59%) (p = 0.935).
Cox proportional hazard analysis was done to examine the effects of factors on steroid-free survival. Of the factors studied, i.e. age of onset of NS, duration of NS before CYP, age at CYP treatment and type of NS (FR/SD), age at start of CYP > 5 years was the only parameter that had an influence on steroid-free survival post CYP (p = 0.036) (RR: 1.879; 95% CI 1.043–3.383).
At 1-year post CYP, of the 100 children with FR/SDNS, 31% were completely off all medications, 21% were infrequent relapsers, 5% were FRNS and 43% were still SDNS. Thus, 52% of patients improved and had a milder course after CYP and did not require alternative treatment. The FR/SDNS patients (48%) were treated with levamisole (17%), calcineurin inhibitors (14%), mycophenolate mofetil (13%) and low-dose alternate day steroids alone (4%).
Adverse effects of CYP treatment were observed in 25 patients (leukopenia (TLC ≤ 4000 cells/mm3) in 23% patients, haemorrhagic cystitis and urinary tract infection in 1% each). Leukopenia was clinically insignificant and was reversible after a few weeks of withholding CYP. All patients finally completed their full cumulative dosage of therapy. None of the patients had nail pigmentation or liver dysfunction. Six patients required hospitalization within 1 year of stopping CYP: pneumonia (2 cases), spontaneous bacterial peritonitis (2 cases) and urinary tract infection (2 cases). All children had normal kidney function (eGFR using Schwartz formula) at the time of last follow-up.
There was improvement in the height at 1-year post CYP, with median change in z-score in the responder group (+0.74) compared to the non-responder group (−0.15) (p = 0.074). There was no improvement seen in weight parameter (p = 0.182). There was a statistically significant positive effect on blood pressure, with 2% of children needing antihypertensives in the responder group compared to the non-responder group (14%) at 1-year post CYP (p = 0.002).
Discussion
This study was undertaken to investigate response to oral CYP therapy in 100 Indian children with FR/SDNS. Fifty percent of children were responders with relapse-free survival at 6 months, while 31% and 11% children had relapse-free survival after 1-year and 2-year post CYP, respectively. Age at initiation of CYP treatment, i.e. more than 5 years of age, was the significant factor in predicting good response.
Cammas et al. showed better relapse-free survival of 65%, 44%, 27% and 13% after 6 months, 1 year, 2 years and 5 years, respectively. Sustained remission > 2 years was associated with age at treatment > 5 years and cumulative dose of CYP > 170 mg/kg (p = 0.02) [1]. In our study, we did not exceed a cumulative dose ≥ 168 mg/kg as per the ISPN guidelines, in order to prevent side effects. There is a positive correlation between cumulative dose and gonadal toxicity, with a risk of definitive sterility over 200 mg/kg. Thus, the maximum dose currently recommended is 168 mg/kg corresponding to a 12-week course of 2 mg/kg/day [13, 14].
That CYP is more effective in older children could be explained on the basis of the pharmacokinetic pathway of CYP. In children, a higher drug clearance is observed compared with adults [15]. Calculations using body weight may result in drug underexposure in younger children [16].
A better outcome in older children may also be related to mild severity in children with a later onset compared to early onset [13]. Children with early-onset NS may be more sensitive for triggers for first manifestation and for relapses. These children are also exposed to more frequent viral infections predisposing to further relapses. Moreover, sporadic cases of genetic forms of NS could be present in this early age group, further affecting response.
Genetic factors might play a role, with HLA-DR 7 predicting the response to alkylating agents in SSNS. The open question remains as to whether younger age points to a genetic factor that is more relevant if onset of SSNS occurs earlier in life. Vester et al. showed significant correlation of cumulative dosage per BSA of more or less than 5040 mg/m2 (45% vs. 11%, p < 0.01) with sustained remission. Therefore, children may need a BSA-adjusted dose to achieve a comparable effect [17].
In our study, although there was a significant difference in cumulative dosage based on BSA in different age groups, this difference did not influence responsiveness to CYP. This observed effect might be very much influenced by the fact that steroid titration was done over months and not abruptly after 4 weeks of alternate-day steroids, as is suggested by recent studies [18]. Glutathione-S-transferase (GST) plays an important role in inactivation of drugs like CYP. Vester et al. showed polymorphic expression of GST-M1 null or GST-P1 would increase or decrease efficacy of CYP respectively [19]. Bhimma et al. highlighted the influence of race on CYP sensitivity, with Indian children with SRNS responding better to treatment than black children (69.0 vs. 20.2%) [20].
Children with high steroid-dependent dose are especially prone to an unfavourable response to CYP. Patients who had steroid-dependent dose ≤ 1.4 mg/kg showed a significant sustained remission of 43% at 2 years vs. 22.5% in those with prednisone > 1.4 mg/kg [21].
Oral CYP is an effective steroid-sparing agent. Alkylating agents (CYP and chlorambucil) reduce the number of children who relapse at 6–12 months (RR 0.44, 95% CI 0.32–0.60) and at 12–24 months (RR 0.20, 95% CI 0.09–0.46) [22, 23].
Vester et al., in their study, showed prolonged sustained remission of > 10 years (24%) with oral CYP treatment. Factors significantly correlating with prolonged sustained remission were again older age at CYP therapy (> 5.5 years), FR status, leukopenia during treatment and CYP cumulative dose ≥ 5040 mg/m2 [17]. On the other hand, a study by Kemper et al. did not show such prolonged sustained remission. Only 30% sustained remission (> 2 years) on oral CYP in children with SDNS and many patients (86%) again developed steroid dependency, requiring further alternative treatment [24]. Thus, different studies show varied results. Our results were similar to those of Kemper et al., with only 11% children showing sustained remission (> 2 years). Data on previous studies on oral CYP in children with SSNS is shown in Table 4 [1, 13, 17, 18, 21, 24,25,26,27].
Second-line therapies including CYP do not often cure SDNS. They may cause a temporary phase shift in relapse pattern, which may be revealed if patients are followed for a prolonged period [28]. This temporary phase shift was also seen in our study, where the remission rate is not sustained after 6 months after CYP. A similar short-term positive effect was observed by adding cyclosporin A to initial treatment of NS for 8 weeks, but the effect disappeared after 6–9 months [29].
CYP use has been associated with safety issues. A meta-analysis of cytotoxic drugs showed a fatality rate of approximately 1%. Side effects seen were leukopenia (32.4%), alopecia (17%), haemorrhagic cystitis (2.2%) and severe bacterial infections in 1.5% patients during CYP use. Malignancies were observed in 0.2% children after high doses. Females rarely developed permanent gonadal damage; however, in males, there was a marked increase in risk of oligospermia or azoospermia with higher cumulative doses [30]. In our study, 24% of patients developed reversible leukopenia. It may be unsafe to repeat CYP courses because of the risk of serious side effects [10].
A positive impact on median height and weight was also seen [31], with median height increasing significantly from −0.5 to −0.1 SDS over 11 months and BMI decreasing significantly and remaining stable [1]. This positive impact on height was also seen in our patients although not significantly.
Recent studies support the use of MMF as a steroid-sparing agent with a lack of kidney, hemodynamic and metabolic toxicity, making it an attractive alternative. Treatment with CYP could be preferred in patients where 12 weeks of therapy would be possible rather than long-term compliance [12].
Although alternative medications are available for relapsing NS, CYP still seems to play a role even when comparing it to rituximab. Kari et al. showed 1-year relapse-free survival was reached in 58.6% patients treated with CYP compared to 84.2% with rituximab (adjusted HR 0.36; 95% CI 0.09–1.45; p = 0.151) [26]. Webb et al. showed longer remission time and fewer side effects than CYP, with median time to first relapse of 7 months after CYP and 14 months after rituximab [27]. While a number of alternative steroid-sparing treatments are available, including levamisole [32], calcineurin inhibitors, mycophenolate salts and rituximab, CYP is still an important option, especially in older children where better response is expected.
There are some limitations to our study, this being a retrospective single-centre study, albeit with a good sample size. A comparatively greater proportion of children with SDNS in the study may have affected the response in our children. The comparatively lower cumulative dose/BSA used in the study group compared to the cut-off dose (5040 mg/BSA) described by Vester et al. [17] could also be one reason for lower response rate in this study.
The study highlights that treatment of young children with CYP should be discouraged because it does not improve their course, and exposes them to an unnecessary risk. The authors suggest preference to MMF as the first-choice therapy, especially for children less than 5 years old, and if need be CYP can be given later in the course of disease before considering cyclosporin A/tacrolimus or rituximab as alternative treatment. The present observations may also direct future research into the pathogenetic mechanisms of NS in relation to age. Today the indications for CYP in glomerular diseases are more restricted than in the past [10], although it continues to be used to treat NS in developing countries because of cost advantage, while carefully addressing safety issues of the drug.
This is one of the few studies of oral CYP, analysing predictors of response in Indian children with SSNS.
References
Cammas B, Harambat J, Bertholet-Thomas A, Bouissou F, Morin D, Guigonis V, Bendeddouche S, Afroukh-Hacini N, Cochat P, Llanas B, Decramer S, Ranchin B (2011) Long-term effects of cyclophosphamide therapy in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. Nephrol Dial Transplant 26:178–184. https://doi.org/10.1093/ndt/gfq405
Nishi S, Ubara Y, Utsunomiya Y, Okada K, Obata Y, Kai H, Kiyomoto H, Goto S, Konta T, Sasatomi Y, Sato Y, Nishino T, Tsuruya K, Furuichi K, Hoshino J, Watanabe Y, Kimura K, Matsuo S (2016) Evidence-based clinical practice guidelines for nephrotic syndrome 2014. Clin Exp Nephrol 20:342–370. https://doi.org/10.1007/s10157-015-1216-x
Martinelli R, Pereira L, Silva O, Okumura A, Rocha H (2004) Cyclophosphamide in the treatment of focal segmental glomerulosclerosis. Braz J Med Biol Res 37:1365–1372. https://doi.org/10.1590/s0100-879x2004000900011
Shohet I, Meyerovitch J, Aladiem M, Boichis H (1988) Cyclophosphamide in treatment of minimal change nephrotic syndrome. Eur J Pediatr 147:239–241. https://doi.org/10.1007/BF00442686
Ponticelli C, Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, Lama G, Zacchello G, Confalonieri R, Altieri P, Bettinelli A, Maschio G, Cinotti GA, Fuiano G, Schena FP, Castellani A, Delia Casa-Alberighi O (1993) Cyclosporin versus cyclophosphamide for patients with steroid-dependent and frequently relapsing idiopathic nephrotic syndrome: a multicentre randomized controlled trial. Nephrol Dial Transplant 8:1326–1332
Sümegi V, Haszon I, Bereczki C, Papp F, Túri S (2008) Long-term follow-up after cyclophosphamide and cyclosporine-A therapy in steroid-dependent and resistant nephrotic syndrome. Pediatr Nephrol 23:1085–1092. https://doi.org/10.1007/s00467-008-0771-8
Siegel N, Gaudio K, Krassner L, McDonald B, Anderson F, Kashgarian M (1981) Steroid-dependent nephrotic syndrome in children: histopathology and relapses after cyclophosphamide treatment. Kidney Int 19:454–459
Moncrieff MW, White RH, Oggs CS, Cameron JS (1969) Cyclophosphamide therapy in the nephrotic syndrome in childhood. Br Med J 1:666–671. https://doi.org/10.1136/bmj.1.5645.666
Tejani A, Phadke K, Nicastri A, Adamson O, Chen CK, Trachtman H, Tejani C (1985) Efficacy of cyclophosphamide in steroid-sensitive childhood nephrotic syndrome with different morphological lesions. Nephron 41:170–173. https://doi.org/10.1159/000183575
Ponticelli C, Escoli R, Moroni G (2018) Does cyclophosphamide still play a role in glomerular diseases? Autoimmun Rev 17:1022–1027. https://doi.org/10.1016/j.autrev.2018.04.007
Al Salloum AA, Muthanna A, Bassrawi R, Al Shehab AA, Al Ibrahim A, Islam MZ, Al Hasan K (2012) Long-term outcome of the difficult nephrotic syndrome in children. Saudi J Kidney Dis Transpl 23:965–972. https://doi.org/10.4103/1319-2442.100877
Indian Pediatric Nephrology Group, Indian Academy of Pediatrics, Bagga A, Ali U, Banerjee S, Kanitkar M, Phadke KD, Senguttuvan P, Sethi S, Shah M (2008) Management of steroid sensitive nephrotic syndrome: revised guidelines. Indian Pediatr 45:203–214
Azib S, Macher MA, Kwon T, Dechartres A, Alberti C, Loirat C, Deschênes G, Baudouin V (2011) Cyclophosphamide in steroid-dependent nephrotic syndrome. Pediatr Nephrol 26:927–932. https://doi.org/10.1007/s00467-011-1830-0
Watson AR, Rance CP, Bain J (1985) Long term effects of cyclophosphamide on testicular function. Br Med J (Clin Res Ed) 291:1457–1460. https://doi.org/10.1136/bmj.291.6507.1457
Yule SM, Boddy AV, Cole M, Price L, Wyllie R, Tasso MJ, Pearson AD, Idle JR (1996) Cyclophosphamide pharmacokinetics in children. Br J Clin Pharmacol 41:13–19. https://doi.org/10.1111/j.1365-2125.1996.tb00153.x
Van Husen M, Kemper MJ (2011) New therapies in steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome. Pediatr Nephrol 26:881–892. https://doi.org/10.1007/s00467-010-1717-5
Vester U, Kranz B, Zimmermann S, Hoyer P (2003) Cyclophosphamide in steroid-sensitive nephrotic syndrome: outcome and outlook. Pediatr Nephrol 18:661–664. https://doi.org/10.1007/s00467-003-1170-9
Ueda N, Kuno K, Ito S (1990) Eight and 12 week courses of cyclophosphamide in nephrotic syndrome. Arch Dis Child 65:1147–1150. https://doi.org/10.1136/adc.65.10.1147
Vester U, Kranz B, Zimmermann S, Büscher R, Hoyer PF (2005) The response to cyclophosphamide in steroid-sensitive nephrotic syndrome is influenced by polymorphic expression of glutathion-S-transferases-M1 and -P1. Pediatr Nephrol 20:478–481. https://doi.org/10.1007/s00467-004-1759-7
Bhimma R, Adhikari M, Asharam K (2006) Steroid-resistant nephrotic syndrome: the influence of race on cyclophosphamide sensitivity. Pediatr Nephrol 21:1847–1853. https://doi.org/10.1007/s00467-006-0276-2
Zagury A, de Oliveira AL, de Moraes CA, de Araujo Montalvão JA, Novaes RH, de Sá VM, Monteiro de Carvalho Dde B, Matuck T (2011) Long-term follow-up after cyclophosphamide therapy in steroid-dependent nephrotic syndrome. Pediatr Nephrol 26:915–920. https://doi.org/10.1007/s00467-011-1825-x
Durkan A, Hodson E, Willis N, Craig J (2001) Immunosuppressive agents in childhood nephrotic syndrome: a meta-analysis of randomized controlled trials. Kidney Int 59:1919–1927. https://doi.org/10.1046/j.1523-1755.2001.0590051919.x
Larkins NG, Liu ID, Willis NS, Craig JC, Hodson EM (2020) Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst Rev 4:CD002290. https://doi.org/10.1002/14651858.CD002290.pub5
Kemper MJ, Altrogge H, Ludwig K, Timmermann K, Müller-Wiefel DE (2000) Unfavorable response to cyclophosphamide in steroid-dependent nephrotic syndrome. Pediatr Nephrol 14:772–775. https://doi.org/10.1007/pl00013435
Report of Arbeitsgemeinschaft für Pädiatrische Nephrologie (1987) Cyclophosphamide treatment of steroid dependent nephrotic syndrome: comparison of eight week with 12 week course. Arch Dis Child 62:1102–1106. https://doi.org/10.1136/adc.62.11.1102
Kari JA, Alhasan KA, Albanna AS, Safdar OY, Shalaby MA, Böckenhauer D, El-Desoky SM (2020) Rituximab versus cyclophosphamide as first steroid-sparing agent in childhood frequently relapsing and steroid-dependent nephrotic syndrome. Pediatr Nephrol 35:1445–1453. https://doi.org/10.1007/s00467-020-04570-y
Webb H, Jaureguiberry G, Dufek S, Tullus K, Bockenhauer D (2016) Cyclophosphamide and rituximab in frequently relapsing/steroid-dependent nephrotic syndrome. Pediatr Nephrol 31:589–594. https://doi.org/10.1007/s00467-015-3245-9
Hoyer PF (2015) New lessons from randomized trials in steroid-sensitive nephrotic syndrome: clear evidence against long steroid therapy. Kidney Int 87:17–19. https://doi.org/10.1038/ki.2014.354
Hoyer PF, Brodeh J (2006) Initial treatment of idiopathic nephrotic syndrome in children: prednisone versus prednisone plus cyclosporine A: a prospective, randomized trial. J Am Soc Nephrol 17:1151–1157. https://doi.org/10.1681/ASN.2005090922
Latta K, von Schnakenburg C, Ehrich J (2001) A meta-analysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr Nephrol 16:271–282. https://doi.org/10.1007/s004670000523
Berns JS, Gaudio KM, Krassner LS, Anderson FP, Durante D, McDonald BM, Siegel NJ (1987) Steroid-responsive nephrotic syndrome of childhood: a long-term study of clinical course, histopathology, efficacy of cyclophosphamide therapy, and effects on growth. Am J Kidney Dis 9:108–114. https://doi.org/10.1016/s0272-6386(87)80087-2
Moorani KN, Zubair AM, Veerwani NR, Hotchandani HJ (2020) Efficacy of levamisole in children with frequent relapsing and steroid dependent nephrotic syndrome at tertiary care center-Karachi. Pak J Med Sci 36:1193–1198. https://doi.org/10.12669/pjms.36.6.2337
Acknowledgements
The authors acknowledge all of the parents round the world who are constantly involved in the care of children with nephrotic syndrome.
Author information
Authors and Affiliations
Contributions
JS, DB: recruited the subjects, collected the data, carried out the literature review and prepared the initial draft of the manuscript; PAP, SB, SK: contributed to manuscript writing; GSD: conceived and designed the study, analysed the data and finalized the manuscript. All authors have approved the manuscript submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical clearance
Institutional ethics committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sandhu, J., Bhat, D., Dhooria, G.S. et al. Oral cyclophosphamide therapy in 100 children with steroid-sensitive nephrotic syndrome: experience from a developing country. Pediatr Nephrol 36, 2759–2767 (2021). https://doi.org/10.1007/s00467-021-05052-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05052-5