Abstract
Renin-angiotensin-aldosterone inhibitors (RAASi) are the mainstay therapy in both adult and paediatric chronic kidney disease (CKD). RAASi slow down the progression of kidney failure by optimization of blood pressure and reduction of proteinuria. Despite recommendations from published guidelines in adults, the evidence related to the use of RAASi is surprisingly scarce in children. Moreover, their role in advanced CKD remains controversial. Without much guidance from the literature, paediatric nephrologists may discontinue RAASi in patients with advanced CKD due to apparent worsening of kidney function, hyperkalaemia and hypotension. Current data suggest that this strategy may in fact lead to a more rapid decline in kidney function. The optimal approach in this clinical scenario is still not well defined and there are varying practices worldwide. We will in this review describe the existing evidence on the use of RAASi in CKD with particular focus on paediatric data. We will also address the use of RAASi in advanced CKD and discuss the potential benefits and harms. At the end, we will suggest a practical approach for the use of RAASi in children with CKD based on current state of knowledge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
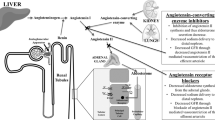
Chronic kidney disease (CKD) in children is often characterized by gradual deterioration of kidney function, resulting in kidney failure and the need of kidney replacement therapy (KRT). Over the years, a number of strategies have been shown to slow CKD progression in children, including strict blood pressure control, reducing proteinuria, optimizing anaemia and achieving normal 25-hydroxyvitamin D levels [1,2,3,4]. Renin-angiotensin-aldosterone-system inhibitors (RAASi), consisting of angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB), lower blood pressure and also urinary protein excretion by reducing intra-glomerular pressure and enhancing the barrier-size selection function in the slit pore membrane [5]. To date, RAASi is the mainstay therapy to attenuate kidney progression in both adult and paediatric CKD by attaining desirable blood pressure and proteinuria control [6,7,8,9,10,11,12,13,14,15]. The anti-fibrotic and anti-inflammatory properties of RAASi provide additional benefits to preserve kidney function [6]. Despite an undisputed efficacy in delaying disease progression in multiple adult studies, evidence supporting the renoprotective effect of RAASi is less substantial in paediatric CKD. Their role in advanced CKD, defined as glomerular filtration rates (GFR) less than 30 ml/1.73 m2/min, also remains controversial [16]. With scant evidence from the literature, paediatric nephrologists may incline to discontinue RAASi due to worsening kidney function, hyperkalaemia and hypotension [17, 18]. While the intention of drug cessation is to restore kidney function and hopefully delay dialysis, discontinuation of RAASi in advanced CKD may accelerate the decline in GFR [19,20,21]. These controversies were highlighted in a recently published KDOQI survey in which 703 physicians’ decisions on whether to consider RAASi discontinuation when patients’ GFR fell below 20 ml/1.73 m2/min were examined [16]. Findings revealed that 46.2% would stop the drug while 53.8% would instead opt for continuation.
Relevant data in the paediatric population are scarce, and this warrants careful extrapolation from the adult data. In this review, we describe the evidence related to the use of RAASi in reducing CKD progression in paediatric and adult populations. We carefully examine the available data on the efficacy and safety of RAASi therapy in advanced CKD. By the end of this review, we attempt to formulate an optimal approach to the use of RAASi in paediatric CKD and identify future research directions.
RAASi in adult CKD: does one size fit all?
In adults, the control of blood pressure and proteinuria are the two main therapeutic targets to delay CKD progression. For decades, the use of RAASI has become an integral part of the standard management in diabetic nephropathy [22,23,24,25,26], which is a rare condition in children. Importantly, such beneficial effects in slowing down the progression of CKD appear to be independent of blood pressure control and extend to patients with microalbuminuria [27]. The renoprotective effect, however, is not confirmed in those who are non-albuminuric.
Clinical trials and meta-analysis have similarly demonstrated efficacy in the preservation of kidney function for non-diabetic CKD [9,10,11]. This effect was best illustrated by the benazepril trial, where 583 predominantly non-diabetic CKD patients were randomly assigned to benazepril or placebo, in addition to conventional antihypertensive therapies [10]. Fewer patients in the benazepril group experienced doubling of serum creatinine levels or progressed to dialysis, with an overall risk reduction of 53%. In a meta-analysis that reviewed 11 randomized controlled trials, including the aforementioned benazepril trial, ACEi therapy was found to be associated with a 30% relative risk reduction for reaching a doubled serum creatinine or kidney failure [9]. Importantly, multiple studies reported that a higher degree of proteinuria at baseline and reduction in urinary protein excretion following RAASi initiation were associated with better kidney outcomes [8, 9, 28,29,30,31]. The best renoprotective effects are indeed observed in those with proteinuria above 1000 mg per day [29, 30]. The treatment strategy with RAASi, however, is not universally efficacious in all types of CKD. In particular, the use of RAASi does not appear to confer clinical benefit in patients with polycystic kidney diseases and patients who have less severe proteinuria (less than 500–1000 mg protein per day) [9,10,11].
The Ramipril Efficacy in Nephropathy (REIN) trial examined the rates of GFR decline in adult patients (18–70 years) with chronic non-diabetic nephropathies and proteinuria who were randomly assigned to ramipril or placebo plus conventional anti-hypertensives to achieve a diastolic blood pressure less than 90 mmHg [8, 32, 33]. While both groups attained comparable blood pressure, ramipril effectively reduced the rates of GFR decline and patients reaching kidney failure, suggesting an effect beyond hypertension control. With time, the tendency of GFR to decline could be effectively halted, and some patients even showed an improvement in GFR.
Results of the African American Study of Kidney Disease and Hypertension (AASK) showed that ACEi was better than beta blockers and calcium channel blockers in slowing the rate of CKD progression in African American (AA) patients with CKD secondary to hypertension [12, 34]. In this study, 1094 African Americans aged 18 to 70 years with hypertensive CKD (GFR 20–65 ml/1.73 m2/min) were recruited. The clinical composite endpoint comprised 50% drop in GFR, development of kidney failure or death. Compared with metoprolol and amlodipine, the ramipril group showed a risk reduction in reaching the clinical composite endpoint by 22% and 38% respectively. Of note, there was no difference on the composite outcome between the two blood pressure targets (102–107 mmHg versus 92 mmHg or less). This study supports the use of RAASi in an AA adult population and it also suggests a potential application in AA children who seem to have a faster decline in GFR and progression to kidney failure [35].
The ONTARGET trial evaluated the efficacy of monotherapy with ramipril or telmisartan, or both. There were 25,620 patients with atherosclerosis or diabetes recruited and the primary kidney outcomes were similar for ACEi or ARB monotherapy, but the outcome was worse in the group on combination therapy despite a higher degree of reduction in proteinuria [36, 37]. Concurring with these findings, in the VA NEPHRON-D study, combination therapy also led to a higher likelihood of adverse events, such as hospitalizations due to acute kidney injury or severe hyperkalaemia [36, 38].
The 2012 KDIGO guideline on CKD recommends either ACEi or ARB to be used in both diabetic and non-diabetic adults with CKD and urine albumin excretion over 300 mg per day [14]. Either agent can be used as monotherapy, while combination therapy is not advisable. Although there is substantial evidence to support RAASi in delaying progression of CKD, it is not clear how early these agents should be started. Nonetheless, collective findings from a few studies do suggest that maximal renoprotection is attained with earlier treatment and a higher basal GFR [10, 23].
RAASi and paediatric CKD: what is the evidence?
CKD may arise from a number of kidney diseases which are associated with different degrees of nephron loss. Adult patients present with mainly glomerular disorders, while congenital anomalies of kidney and urinary tract (CAKUT) is the leading cause of chronic kidney diseases in children. CAKUT is associated with a substantially lower degree of proteinuria [39]. Progression of kidney disease is often multifactorial and contributed to by multiple secondary factors [40]. After the initial loss of nephrons, compensatory glomerular hyperfiltration occurs in the remaining functional units of the kidney and this results in glomerular hypertension and hypertrophy in order to maintain GFR. With time, secondary glomerular injury ensues, which leads to the development of glomerulosclerosis, proteinuria and eventually progression of CKD. Therefore, children with CAKUT, even though it is a non-glomerular condition, may benefit from RAAS blockade through reduction of intra-glomerular pressure as well as optimization of blood pressure and proteinuria.
In contrast to the adult population, evidence of RAASi use in paediatric CKD is limited and is predominantly derived from the Chronic Kidney Disease Children (CKiD) study and Effect of Strict Blood Pressure Control and ACE inhibition on the Progression of CRF in Pediatric Patients (ESCAPE) trial. The efficacy of RAAS blockade has not been directly examined in most paediatric studies. Therefore, the beneficial effect of RAASi in children is mostly based on assumption and extrapolation to a certain extent.
The CKiD study prospectively enrolled patients aged 1 to 16 years, with an estimated GFR ranging from 30 to 90 ml/1.73 m2/min, from 54 participating centres in North America. Factors associated with CKD progression, defined as KRT initiation or 50% decline in GFR, were examined among 496 children [1]. Notably, nephrotic-range proteinuria (urinary protein-to-creatinine ratio > 2 mg/mg), hypoalbuminaemia and elevated blood pressure (systolic or diastolic blood pressure > 90th centile for age, sex and height) were the common risk factors for progression in both non-glomerular and glomerular CKD groups. The risk and rate of progression were more prominent in CKD of glomerular origin. In children with CKD of non-glomerular origin, a twofold higher basal urine protein-to-creatinine ratio was associated with an accelerated GFR decline of 0.3 ml/1.73 m2/min per year, in addition to the 1.3 ml/1.73 m2/min per year average decline in the entire cohort [2]. This detrimental effect was observed even among normotensive children. Regarding blood pressure, the elevation of every unit of z score of the baseline systolic blood pressure was associated with an additional GFR decline of 0.4 ml/1.73 m2/min per year [2].
Based on these findings, hypertension and proteinuria are similarly considered as potential treatment targets in paediatric CKD, independent of the underlying disease. However, despite publication of relevant guidelines, hypertension remains prevalent in paediatric CKD, even at early stages, and is frequently under- or untreated [41,42,43]. RAASi is the preferred antihypertensive therapy as its use is associated with lower incidence of uncontrolled hypertension and a higher likelihood of normal ambulatory blood pressure results [41, 44]. RAASi also have an added benefit of reducing proteinuria, which may further improve blood pressure control [44, 45]. In another report by Abraham et al. from the CKiD study, 851 children (median GFR 52 ml/1.73 m2/min) were evaluated to test the effect of RAASi use on time to KRT [46]. Over a median of 4.1 years, 217 patients required initiation of KRT. In comparison to non-users, 472 (55%) RAASi users had a risk reduction of 21–37% for KRT with different statistical models. The authors concluded there was a substantial benefit of RAASi in paediatric CKD in delaying dialysis.
Another paediatric landmark study was the ESCAPE trial, which demonstrated that intensified blood pressure control successfully slowed the progression of CKD [4]. In this trial, 385 children with CKD (GFR 15–80 ml/1.73 m2/min) were recruited across the European Union. All children received ramipril at a dose of 6 mg per meter square body surface area per day, with additional antihypertensives as indicated to reach the target blood pressure. Children who underwent intensified blood pressure control with a target 24-h mean arterial blood pressure of less than the 50th percentile were less likely to develop 50% decline in GFR or kidney failure (30% vs. 41%, hazard ratio 0.65), compared to the group receiving conventional blood pressure control (50–95th percentile). An initial 50% reduction in protein excretion with ramipril also predicted a protective effect on CKD progression (hazard ratio 0.46). Notably, children with underlying glomerulopathy, a urine protein-to-creatinine ratio > 1.5 mg/mg, baseline GFR < 45 ml/1.73 m2/min and a decline of GFR > 3 ml/min per year benefited most from strict blood pressure control. The anti-proteinuric effect of ramipril was noted to wean over time and the protein excretion was similar to baseline values after three years. While the results of this trial support that intensified blood pressure control slows down the progression of CKD in children, it is important to be aware that both treatment and control groups received ACEi and therefore the effect of RAASi on kidney outcomes was not directly assessed. In a post hoc analysis of the ESCAPE trial, ramipril reduced proteinuria by 43.5% and a higher initial proteinuria reduction was associated with a lower risk of CKD progression [47].
In 2012, KDIGO recommended ACEi or ARB to be used in children with CKD in whom anti-hypertensive therapy is indicated, irrespective of proteinuria level [14]. The 2017 AAP Clinical Practice Guidelines for screening and management of high blood pressure in children and adolescents recommends ACEi or ARB as the preferred treatments for children with CKD who are hypertensive and proteinuric [15]. However, these recommendations are based on very limited evidence and there are no specifications from either guidelines under what circumstances RAASi would be indicated or not.
There are specific concerns regarding RAASi use in the paediatric population. While Snauwaert et al. reported favourable side effect profile in a systematic review, there were insufficient safety data in neonates and infants [48]. These young patients are susceptible to volume depletion and develop complications such as acute kidney injury, hyperkalaemia and hypotension [49]. Profound hypotension is particularly prevalent in premature neonates receiving captopril, presumably due to a significant role of RAAS on blood pressure regulation and reduced drug clearance in this patient population [48, 50]. RAAS also regulates nephron development and there is concern that RAAS blockade during early stages of life may impair kidney maturation [51, 52]. The administration of RAASi to infants should be cautious until they have reached a corrected age of 44 weeks [53]. Besides, RAASi are potentially teratogenic and are contraindicated in young pregnant women [54]. In adolescents of child-bearing age, the use of these agents should be balanced with the risk of pregnancy. Appropriate patient education is essential. Finally, renoprotective is not a licensed indication and there is a limited choice of RAASi that are approved for use in children, especially for those younger than 6 years (Table 1). There is also a lack of long-term safety data of the RAASi on growth and maturation in children [48].
RAASi in advanced CKD: what are the concerns?
The primary concerns regarding RAASi in advanced CKD are hyperkalaemia and an inadvertent decline in GFR, which would lead to earlier initiation of KRT. The Cardiovascular Co-morbidity in Children with CKD (4C) study is an ongoing, multicentre, prospective observational study that enrols children aged from 6 to 17 years with an estimated GFR (eGFR) of 10–60 ml/1.73 m2/min [60]. 298 (42%) and 82 (12%) of the 704 participants received RAASi at baseline and during the follow-up phase of the study, respectively [17]. Seventy-three (19%) of these 380 children discontinued RAASi without reaching the composite kidney endpoint. The leading reasons to discontinue RAASi were worsening of kidney function (33%), hyperkalaemia (23%) and hypotension (17%). In the adult population, prospective trials showed that only a minority of patients required drug cessation due to a significant fall in kidney function and hyperkalaemia (Table 2) [19, 20].
Traditionally, an increase of less than 30% of the serum creatinine level following drug initiation has been generally expected [61]. However, this figure typically refers to earlier stages of CKD and it is uncertain if this limit is applicable to patients with advanced CKD. For instance, in a patient with baseline serum creatinine level of 300 μmol/L, a 30% increment will correspond to a significant and worrying increase of 100 μmol/L. The clinical significance of an acute change in serum creatinine associated with RAASi is also largely controversial [62, 63]. Schmidt et al. argued that the increase in creatinine following the initiation of RAASi would lead to adverse kidney outcomes in a graduated fashion, even when the increase was far less than 30% [64]. On the other hand, the GFR decline is caused by a fall in the intra-glomerular pressure, which may also be a modifiable factor for CKD progression. In a post hoc analysis of ONTARGET and TRANSCEND trials, a GFR decline of 15% or more at 2 and 8 weeks following drug initiation was observed in 16% and 7% of patients, respectively [62]. While the acute fluctuation in GFR was common, it did not predict subsequent kidney and cardiovascular outcomes. Furthermore, in a systemic review of 12 randomized clinical trials, a strong association was noted between preservation of kidney function and acute increase in serum creatinine up to 30% after ACEi therapy, although these patients had a milder degree of kidney impairment (serum creatinine > 124 μmol/L or 1.4 mg/dL) [63]. In fact, an initial acute fall in GFR has been shown to predict a slower kidney function decline in the long term [65].
In our experience, the decline in GFR is usually modest and is rarely severe (> 30%). This decline usually occurs a few days after the start or modification of RAASi therapy and therefore close monitoring is warranted. Patients with bilateral renal artery stenosis and CKD patients with atherosclerosis are particularly at risk due to the pre-existing impaired intra-renal perfusion. Although these conditions are uncommon in the paediatric population, one should be extremely careful about using RAASi in young children who are susceptible to intravascular volume depletion. These patients would bear an increased risk of RAASi-related drug toxicity and mortality has also been reported [66].
Under normal circumstances, aldosterone is released in response to the elevation of serum potassium levels which, in turn, promotes urinary excretion of potassium. RAAS blockade reduces aldosterone secretion and action in the face of impaired potassium excretion related to CKD, leading to hyperkalaemia. Although the risk of hyperkalaemia is definite, most patients only present with mild hyperkalaemia which is amendable to nutritional and medical interventions [19, 20, 67]. Clinicians are advised to proactively prevent hyperkalaemia and balance the risk of hyperkalaemia against the potential benefit of RAASi. Besides, inhibition of RAAS reduces kidney net acid excretion in the cortical collecting tubule and can potentially result in metabolic acidosis [68].
In one observational study, up to 50% patients did not tolerate dual therapy with ACEi and ARB [69]. Combination therapy with ACEi and ARB should be avoided since this approach failed to show any added benefit in adult patients and was associated with an increased likelihood of adverse events, including hypotension, syncope and kidney dysfunction [70]. Finally, patients with advanced CKD are susceptible to acute kidney injury. The risk is particularly high in the presence of concomitant medication, such as diuretics.
RAASi in adult advanced CKD: should it be started?
Although the available evidence supports the use of RAASi in early stages of CKD, there are very few studies addressing their efficacy in patients with a GFR less than 30 ml/1.73 m2/min [19, 20, 34]. Given the concerns of hyperkalaemia and worsening of kidney function, the clinical questions are whether the benefits would extend to advanced CKD. A summary of currently available data on kidney outcomes and adverse events is presented in Table 2.
In a post hoc analysis of the REIN trial, the benefit of ACEi in patients with advanced CKD was evaluated by comparing the change in GFR and the incidence of stage 5 CKD (CKD 5) in three different ranges of baseline kidney function [19]. In the group of lowest basal GFR (11 to 33 ml/min/1.73 m2), ramipril reduced GFR decline by 22% and the CKD 5 incidence by 33%. Of the 52 patients in the lowest GFR group who received ramipril, only one had worsening of kidney function and two developed hyperkalaemia that required drug cessation. The adverse events for both treatment arms were also comparable across the three groups.
In one Chinese study, Hou et al. evaluated 422 non-diabetic CKD patients aged 18–70 years by assigning them into two groups according to serum creatinine levels (Group 1, 1.5 to 3 mg/dL (133 to 265 μmol/L)); Group 2, 3.1 to 5 mg/dL (74 to 442 μmol/L)) [20]. All patients initially received benazepril at 10 mg/day and increased to 20 mg/day if kidney function tolerated. Ninety-four patients who developed marked alteration in kidney function and severe hyperkalaemia were excluded from the study. After a 3-week wash-out period, the remaining 224 patients in Group 2 (GFR 26 ± 5 ml/1.73 m2/min) were randomly assigned to benazepril (10 mg twice daily) or placebo, alongside conventional antihypertensives. The primary outcome measure was the time to first composite kidney end point of a doubled creatinine level, CKD 5 or death. Over a mean follow-up of 3.4 years, fewer patients receiving benazepril reached the primary endpoint (41% versus 60%) with a 43% risk reduction, which was not attributable to blood pressure control. It was also associated with a 23% reduction in the rate of GFR decline (6.8 versus 8.8 ml/1.73 m2/min per year). The incidence of adverse events among the two arms was similar. While six patients developed hyperkalaemia and one developed acute decline of GFR out of the 112 patients in the benazepril group, only two patients were eventually withdrawn from the study due to persistent hyperkalaemia. It is, however, worth noting that this study was at risk of selection bias because a large proportion of patients did not tolerate ACEi and were excluded before study commencement [20].
In a controversies report presented by NKF-KDOQI, a careful review of available clinical data concluded that the decline in GFR and rise in serum potassium levels related to RAAS blockade in advanced CKD did not lead to adverse kidney outcomes [16]. ACEi or ARB monotherapy initiation appeared to be effective in slowing down the progression of CKD.
RAASi in adult advanced CKD: should it be stopped?
The aforementioned trials focused on the initiation of RAASi in advanced CKD. However, there are even fewer data in patients who are already receiving RAASi and have now reached CKD stage 4 or 5. To date, there are only a few studies addressing whether or not RAASi should be discontinued (or continued) in this setting [18, 57, 58].
In a small open randomized control trial, Pita-Fernandez et al. evaluated patients with GFR < 60 ml/1.73 m2/min and heart failure treated with RAASi during hospitalization [57]. Patients were assigned to a 50% reduction of RAASi dose in the intervention group or standard basal dose in the control group. Following 1 to 3 months after discharge, the intervention group had an improved kidney function, with GFR increasing from 33 to 43 ml/1.73 m2/min. The findings from this study are limited by very short-term patient outcomes. In an observational study, Ahmed et al. followed 52 elderly patients (mean age 73.3 ± 1.8) with a baseline GFR of 16.38 ± 1 ml/1.73 m2/min in whom ACEi or ARB were stopped [18]. After drug discontinuation for 12 months, 62% patients demonstrated GFR improvement of more than 25% and the overall eGFR was shown to be increased to 26.6 ± 2.2 ml/1.73 m2/min from baseline. Hansen et al. reported a similar improvement of kidney function shortly after ACEi discontinuation in a group of patients with diabetic nephropathy (n = 42, mean age 40 ± 7 years) [71]. The Mayo clinic group reported worsening of kidney function with RAASi in older CKD patients with renal artery stenosis [58]. These patients were generally older with different comorbidities, such as diabetes and renovascular disease, and are therefore at higher risk of vascular disease and impaired kidney perfusion. Withdrawal of ACEi or ARB may revert this phenomenon and explain the improvement observed in kidney function. It may however not be appropriate to extrapolate these findings to the paediatric population who have different pathophysiology of their primary kidney disease.
However, in a number of observational studies, where there was no specification regarding drug initiation or continuation, the use of RAASi appeared to have beneficial effect on kidney outcomes (Table 2) [21, 55]. Hsu et al. conducted a large-scale population-based longitudinal cohort study to investigate the role of RAASi in 28497 patients (20–100 years old) with pre-dialysis CKD 5 (serum creatinine > 6 mg/dL or 530 μmol/L), hypertension and renal anaemia [21]. Although most patients progressed to long-term dialysis (71%) or died (20%) over a short period of 7 months, the use of ACEi/ARB amongst patients, compared to non-users, was associated with a small but significantly reduced risk for long-term dialysis and death by 6% (hazard ratio 0.94, 95% CI 0.92–0.97). This suggests the benefit of RAASi extends even to very advanced CKD and at least does not hasten the start of KRT.
To summarize, there is currently insufficient evidence to support continuation or cessation of RAASi in this clinical context. It is uncertain whether the outcomes will be different in subjects who are younger and at lower risk of vascular disease, as in those RAASi-naïve patients presented above (predominantly less than 70 years) who attained promising results with drug initiation compared to those who are older and with more vascular disease. A multicentre, open-label, randomized controlled trial, the STOP-ACEi trial (trial registration number: ISRCTN62869767), is underway in the UK to examine the effect on progression of CKD following drug continuation versus discontinuation in 410 patients with stage 4 or 5 progressive CKD receiving ACEi or ARB [59]. The follow-up period is 3 years and the primary outcome is the difference in kidney function between the two groups. A similar randomized controlled trial is being conducted in the USA and is estimated to be completed by September 2020 (trial identifier: NCT03957161). Results of these trials will hopefully shed light on this controversial area in CKD management.
RAASi in children with advanced CKD: what are the data?
To our knowledge, the only study that has specifically assessed the effect of RAASi (ACEi or ARB) continuation or withdrawal in advanced paediatric CKD is the recent 4C study [17]. The composite kidney endpoint in this study was defined as a sustained 50% eGFR decline or eGFR < 10 ml/1.73 m2/min or KRT initiation. Sixty-nine children aged 13.7 ± 3.2 years who discontinued RAASi (eGFR 27 ± 12 ml/1.73 m2/min prior to drug termination) were included. Congenital anomalies of kidney and urinary tract were the predominant primary kidney disease. The reasons for drug cessation were due to hyperkalaemia, symptomatic hypotension and raised creatinine. Upon drug withdrawal, blood pressure and albuminuria were increased by 2.8 mmHg and 115%, respectively, while potassium was only minimally decreased (0.17 meq/L).
More than a twofold decline in eGFR was reported following the discontinuation of RAASi (− 1.5 to − 3.9 ml/1.73 m2/min per year, p = 0.005), over a median of 1.9 years and 1.2 years’ follow-up before and after drug withdrawal, respectively. In order to confirm this change in eGFR to be independent of natural disease progression, a group of control patients who continued RAASi was selected for comparison by means of propensity score matching. The accelerated rate of decline in eGFR seen in the study cohort was not observed in the well-matched control cohort (− 1.8 to − 1.2 ml/1.73 m2/min per year, p = 0.30). Of note, rebound albuminuria was significant (115%) and a more marked increase in urinary protein excretion was associated with worse subsequent kidney outcomes. Although the study is limited by its observational nature and selection bias, these findings demonstrated a strong anti-proteinuric effect of RAASi even in advanced paediatric CKD and that their withdrawal could accelerate the decline of kidney function.
While the continuation of RAASi appears to be promising in paediatric advanced CKD, randomized trials and separate studies on drug initiation in this group of patients are very much required to provide further guidance.
Practical approach
The evidence related to the use of RAASi in children is much less substantial than in the adult population and this is particularly true in advanced CKD (GFR ≤ 30 ml/1.73 m2/min). There are many outstanding questions regarding the optimal approach and careful consideration is required when trying to extrapolate adult data to children. Moreover, different patients respond differently to RAASi and the potential benefits of reducing CKD progression have to be balanced against potential complications. Therefore, the decision on the use of RAASi in patients with advanced CKD should be individualized. Different factors including patient characteristics, treatment response such as changes in proteinuria and side effect profiles should be considered. These factors are presented in Fig. 1. Based on our clinical experience and limited existing data, we suggest an approach to RAASi therapy in paediatric CKD. A summary of these suggestions is presented in Table 3.
Factors to consider for RAASi use in advanced chronic kidney disease. The decision on RAASi use in advanced CKD should be individualized and based on factors including baseline patient characteristics, treatment response and development of complications. AKI, acute kidney injury; CKD, chronic kidney disease; GFR, glomerular filtration rate; K, potassium; RAASi, renin-angiotensin-aldosterone-system inhibitors
Early CKD (stage I to III)
In children, there is more robust evidence to support strict blood pressure control (24-h mean arterial pressure less than 50 to 75th percentiles) than reduction of proteinuria in slowing kidney progression. We suggest early use of RAASi for renoprotection in all children with CKD I to III with high blood pressure (≥ 90th centile) and/or significant proteinuria/albuminuria (urinary protein-to-creatinine ratio ≥ 0.5 mg/mg or 50 mg/mmol; albumin-to-creatinine ratio ≥ 0.3 mg/mg or 30 mg/mmol). The administration of RAASi to young infants should be cautious, and contraindications include neonates, renal artery stenosis, pregnancy, hyperkalaemia despite treatment, history of angioedema and allergic reaction related to RAASi [53, 61]. Monotherapy with ACEi is the preferred option to ARB since more paediatric data are available on efficacy and safety of ACEi. It is important to monitor serum creatinine and potassium levels 3 to 5 days after drug initiation or adjustment. RAASi should be stopped in case of GFR decline > 30%, persistent hyperkalaemia or hypotension. We also suggest RAASi to be temporarily withheld in acute situations with intravascular volume depletion related to dehydration, gastroenteritis, diuretic use or nephrotic state.
Advanced CKD (stage IV and V, without KRT)
For children progressing to advanced stages of CKD, the limited paediatric data support continuation of RAASi to attenuate CKD progression. Therapy should be individualized, with careful monitoring to limit potential complications. Of note, worsening of kidney function can be difficult to interpret as it may simply reflect the natural course of the disease. We do, however, recommend cessation of RAASi if the patient develops hypotension, episodes of acute kidney injury or rapidly declining kidney function progressing to CKD 5. Hyperkalaemia should be actively prevented and managed before withdrawal of RAASi. Potential strategies include dietary restriction, acid-base correction, modification of concomitant medications associated with hyperkalaemia, judicious use of diuretics and potassium-binders. In the future, the availability of newer generation potassium-binding medications, such as patiromer, may potentially allow extended RAAS blockade in patients with advanced CKD.
There is currently no paediatric data to guide our decisions in RAASi-naïve children with advanced CKD. Judging from the adult data, it is reasonable to attempt RAAS blockade in children without aforementioned contraindications. In those without significant proteinuria, we would suggest considering alternative antihypertensives for blood pressure control. Close monitoring of kidney function is mandatory following drug initiation or adjustment, and we suggest adopting a lower threshold to discontinue these agents in this vulnerable population. In line with the NKF-KDOQI controversies report, a GFR decline of > 20%, rather than 30%, can be adopted in this context [16]. If patients develop hypotension, uncontrolled hyperkalaemia, significant uraemia or GFR persistently falls more than 20% with dose adjustment, RAASi therapy should be terminated.
Uncertainties and future research directions
The optimal use of RAASi in paediatric CKD remains controversial. Prospective investigations in children are very much needed to answer the outstanding questions related to the use of RAASi:
-
1.
To address the efficacy of RAAS blockade in delaying kidney progression in children with CKD, especially for those who are non-proteinuric
-
2.
To identify the optimal timing and dosing of RAASi in children to attain the maximal effect in delaying disease progression
-
3.
High-quality double-blinded randomized controlled trials testing RAASi initiation and its continuation versus cessation in advanced paediatric CKD
-
4.
To identify the predictive factors of good response to RAASi in children with CKD
-
5.
To develop and validate new potassium-binding agents in children to facilitate use of RAASi in those with hyperkalaemia
-
6.
To investigate the relationship between kidney function changes from the use of RAASi and the long-term kidney outcome in children with CKD.
Conclusion
RAAS blockade is an effective and important therapy in attenuating CKD progression by controlling blood pressure and reducing proteinuria. The evidence supporting its use in children is much less substantial than adults and its role in advanced CKD is also controversial. Despite a conventional concern that RAASi may worsen kidney function and potentially hasten the start of dialysis, recent findings suggest that the initiation or continuation ACEi or ARB monotherapy in paediatric and adult advanced CKD may improve kidney outcomes. Therefore, RAASi should be considered with close monitoring of blood pressure, serum creatinine and potassium levels. The degree of initial proteinuria reduction and rebound albuminuria upon drug cessation are both important predictors of worsening treatment outcomes. The use of RAASi should be individualized and based on factors including baseline patient characteristics, treatment response and side effect profiles. High-quality prospective randomized trials are very much needed to answer outstanding research questions and identify the optimal strategy for the use of RAASi in children.
Key summary points
-
1.
Although the evidence is much less substantial than adults, RAAS blockade is recommended in children with CKD with hypertension and/or proteinuria to delay CKD progression.
-
2.
Monotherapy with ACEi or ARB is preferred to combination therapy, which is associated with higher risk of adverse events.
-
3.
The degree of initial proteinuria reduction and rebound proteinuria after drug cessation are important predictors for treatment outcomes.
-
4.
While the role of RAASi in advanced CKD is controversial, current data support its use with close monitoring of kidney function, blood pressure and potassium level.
-
5.
Individualized approach is recommended, and one should consider factors including baseline patient characteristics, treatment response and side effect profiles.
Multiple-choice questions (answers are provided following the reference list)
-
1.
Which of the following is not predictive for kidney disease progression in both glomerular and non-glomerular CKD?
-
A.
High blood pressure
-
B.
Significant proteinuria
-
C.
Age
-
D.
Hypoalbuminaemia
-
A.
-
2.
Which of the following is not preferred in the management of paediatric CKD?
-
A.
ACEi monotherapy
-
B.
ACEi and ARB combination therapy
-
C.
Normal protein intake
-
D.
Attaining a normal vitamin D level
-
A.
-
3.
Which of the following predicts good kidney outcomes after RAAS blockade?
-
A.
Significant initial reduction in proteinuria
-
B.
Acute changes of GFR
-
C.
Hyperkalaemia
-
D.
Hypotension
-
A.
-
4.
What factors should be considered when one decides on the use of RAASi in advanced CKD?
-
A.
Baseline patient characteristics e.g. underlying renal diagnosis
-
B.
Treatment response, e.g. proteinuria reduction
-
C.
Side effect profiles, e.g. hyperkalaemia
-
D.
All of the above
-
A.
References
Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, Mitsnefes M, Kaskel F, Greenbaum LA, Mak RH (2015) Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the chronic kidney disease in children (CKiD) cohort. Am J Kidney Dis 65:878–888
Fathallah-Shaykh SA, Flynn JT, Pierce CB, Abraham AG, Blydt-Hansen TD, Massengill SF, Moxey-Mims MM, Warady BA, Furth SL, Wong CS (2015) Progression of pediatric CKD of nonglomerular origin in the CKiD cohort. Clin J Am Soc Nephrol 10:571–577
Shroff R, Aitkenhead H, Costa N, Trivelli A, Litwin M, Picca S, Anarat A, Sallay P, Ozaltin F, Zurowska A (2016) Normal 25-hydroxyvitamin D levels are associated with less proteinuria and attenuate renal failure progression in children with CKD. J Am Soc Nephrol 27:314–322
ESCAPE Trial Group (2009) Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361:1639–1650
Remuzzi A, Perticucci E, Ruggenenti P, Mosconi L, Limonta M, Remuzzi G (1991) Angiotensin converting enzyme inhibition improves glomerular size-selectivity in IgA nephropathy. Kidney Int 39:1267–1273
Wühl E, Schaefer F (2008) Therapeutic strategies to slow chronic kidney disease progression. Pediatr Nephrol 23:705–716
Kunz R, Friedrich C, Wolbers M, Mann JF (2008) Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin–angiotensin system on proteinuria in renal disease. Ann Intern Med 148:30–48
The GISEN Group (1997) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349:1857–1863
Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, Maschio G, Brenner BM, Kamper A, Zucchelli P (2001) Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease: a meta-analysis of patient-level data. Ann Intern Med 135:73–87
Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P (1996) Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med 334:939–945
Kent DM, Jafar TH, Hayward RA, Tighiouart H, Landa M, De Jong P, De Zeeuw D, Remuzzi G, Kamper A-L, Levey AS (2007) Progression risk, urinary protein excretion, and treatment effects of angiotensin-converting enzyme inhibitors in nondiabetic kidney disease. J Am Soc Nephrol 18:1959–1965
Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R (2002) Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 288:2421–2431
Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, Lesti M, Perticucci E, Chakarski IN, Leonardis D (2005) Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 365:939–946
Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco AL, De Jong PE, Griffith KE, Hemmelgarn BR, Iseki K, Lamb EJ (2013) Kidney disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int (Suppl 3):1-150
Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK (2017) Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140:e20171904
Weir MR, Lakkis JI, Jaar B, Rocco MV, Choi MJ, Kramer HJ, Ku E (2018) Use of renin-angiotensin system blockade in advanced ckd: An NKF-KDOQI controversies report. Am J Kidney Dis 72:873–884
van den Belt SM, Heerspink HJ, Kirchner M, Gracchi V, Thurn-Valsassina D, Bayazit AK, Niemirska A, Canpolat N, Bulut IK, Azukaitis K (2020) Discontinuation of RAAS inhibition in children with advanced CKD. Clin J Am Soc Nephrol 15:625–632
Ahmed AK, Kamath NS, El Kossi M, El Nahas AM (2010) The impact of stopping inhibitors of the renin–angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant 25:3977–3982
Ruggenenti P, Perna A, Remuzzi G (2001) ACE inhibitors to prevent end-stage renal disease: when to start and why possibly never to stop: a post hoc analysis of the REIN trial results. J Am Soc Nephrol 12:2832–2837
Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, Jiang JP, Liang M, Wang GB, Liu ZR (2006) Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 354:131–140
Hsu T-W, Liu J-S, Hung S-C, Kuo K-L, Chang Y-K, Chen Y-C, Hsu C-C, Tarng D-C (2014) Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med 174:347–354
Lewis EJ, Hunsicker LG, Bain RP, Rohde RD (1993) The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329:1456–1462
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I (2001) Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345:851–860
Brenner BM, Cooper ME, De Zeeuw D, Keane WF, Mitch WE, Parving H-H, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S (2001) Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345:861–869
Patel A, ADVANCE Collaborative Group, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B (2007) Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 370:829–840
Fink HA, Ishani A, Taylor BC, Greer NL, MacDonald R, Rossini D, Sadiq S, Lankireddy S, Kane RL, Wilt TJ (2012) Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 156:570–581
Parving H-H, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P (2001) The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345:870–878
Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL (1995) Blood pressure control, proteinuria, and the progression of renal disease: the Modification of Diet in Renal Disease Study. Ann Intern Med 123:754–762
Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, De Jong PE, De Zeeuw D, Shahinfar S, Toto R, Levey AS (2003) Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 139:244–252
Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G (1999) Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 354:359–364
Lea J, Greene T, Hebert L, Lipkowitz M, Massry S, Middleton J, Rostand SG, Miller E, Smith W, Bakris GL (2005) The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med 165:947–953
Ruggenenti P, Perna A, Gherardi G, Gaspari F, Benini R, Remuzzi G (1998) Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet 352:1252–1256
Ruggenenti P, Perna A, Benini R, Bertani T, Zoccali C, Maggiore Q, Salvadori M, Remuzzi G (1999) In chronic nephropathies prolonged ACE inhibition can induce remission: dynamics of time-dependent changes in GFR. J Am Soc Nephrol 10:997–1006
Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG (2001) Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 285:2719–2728
Ng DK, Moxey-Mims M, Warady BA, Furth SL, Muñoz A (2016) Racial differences in renal replacement therapy initiation among children with a nonglomerular cause of chronic kidney disease. Ann Epidimiol 26:780–787.e781
Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S (2008) Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372:547–553
Tobe SW, Clase CM, Gao P, McQueen M, Grosshennig A, Wang X, Teo KK, Yusuf S, Mann JF (2011) Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 123:1098–11073
Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O'Connor T, Palevsky PM (2013) Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369:1892–1903
Wong CS, Pierce CB, Cole SR, Warady BA, Mak RH, Benador NM, Kaskel F, Furth SL, Schwartz GJ (2009) Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the chronic kidney disease in children study. Clin J Am Soc Nephrol 4:812–819
Metcalfe W (2007) How does early chronic kidney disease progress? A background paper prepared for the UK Consensus Conference on early chronic kidney disease. Nephrol Dial Transplant 22(suppl_9):ix26–ix30
Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA (2008) Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension 52:631–637
Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, Kimball T, Furth S, Warady B, CKiD Study Group (2010) Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol 21:137–144
Barletta G-M, Pierce C, Mitsnefes M, Samuels J, Warady BA, Furth S, Flynn J (2018) Is blood pressure improving in children with chronic kidney disease? A period analysis. Hypertension 71:444–450
Samuels J, Ng D, Flynn JT, Mitsnefes M, Poffenbarger T, Warady BA, Furth S (2012) Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension 60:43–50
Kogon AJ, Pierce CB, Cox C, Brady TM, Mitsnefes MM, Warady BA, Furth SL, Flynn JT (2014) Nephrotic-range proteinuria is strongly associated with poor blood pressure control in pediatric chronic kidney disease. Kidney Int 85:938–944
Abraham AG, Betoko A, Fadrowski JJ, Pierce C, Furth SL, Warady BA, Muñoz A (2017) Renin–angiotensin II–aldosterone system blockers and time to renal replacement therapy in children with CKD. Pediatr Nephrol 32:643–649
van den Belt SM, Heerspink HJ, Gracchi V, de Zeeuw D, Wühl E, Schaefer F, ESCAPE Trial Group (2018) Early proteinuria lowering by angiotensin-converting enzyme inhibition predicts renal survival in children with CKD. J Am Soc Nephrol 29:2225–2233
Snauwaert E, Walle JV, De Bruyne P (2017) Therapeutic efficacy and safety of ACE inhibitors in the hypertensive paediatric population: a review. Arch Dis Child 102:63–71
Ku LC, Zimmerman K, Benjamin DK, Clark RH, Hornik CP, Smith PB (2017) Safety of Enalapril in infants admitted to the neonatal intensive care unit. Pediatr Cardiol 38:155–161
Tack ED, Perlman JM (1988) Renal failure in sick hypertensive premature infants receiving captopril therapy. J Pediatr 112:805–810
Tufro-McReddie A, Romano L, Harris J, Ferder L, Gomez R (1995) Angiotensin II regulates nephrogenesis and renal vascular development. Am J Phys 269:F110–F115
Frölich S, Slattery P, Thomas D, Goren I, Ferreiros N, Jensen BL, Nüsing RM (2017) Angiotensin II-AT1–receptor signaling is necessary for cyclooxygenase-2–dependent postnatal nephron generation. Kidney Int 91:818–829
Starr MC, Flynn JT (2019) Neonatal hypertension: cases, causes, and clinical approach. Pediatr Nephrol 34:787–799
Becker GJ, Wheeler DC, De Zeeuw D, Fujita T, Furth SL, Holdaas H, Mendis S, Oparil S, Perkovic V, Rodrigues CIS (2012) Kidney disease: improving global outcomes (KDIGO) blood pressure work group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2:337–414
Jovanovich AJ, Chonchol MB, Sobhi A, Kendrick JB, Cheung AK, Kaufman JS, Smits G, Jablonski KL, HOST Investigators (2015) Mineral metabolites, angiotensin II inhibition and outcomes in advanced chronic kidney disease. Am J Kidney Dis 42:361–368
Oh YJ, Kim SM, Shin BC, Kim HL, Chung JH, Kim AJ, Ro H, Chang JH, Lee HH, Chung W (2017) The impact of renin-angiotensin system blockade on renal outcomes and mortality in pre-dialysis patients with advanced chronic kidney disease. PLoS One 12:e0170874
Pita-Fernández S, Chouciño-Fernández T, Juega-Puig J, Seoane-Pillado T, López-Calviño B, Pértega-Díaz S, Pedreira-Andrade JD, Gil-Guillén V (2014) A randomized clinical trial to determine the effect of angiotensin inhibitors reduction on creatinine clearance and haemoglobin in heart failure patients with chronic kidney disease and anaemia. Int J Clin Pract 68:1231–1238
Onuigbo M, Onuigbo N (2008) Worsening renal failure in older chronic kidney disease patients with renal artery stenosis concurrently on renin angiotensin aldosterone system blockade: a prospective 50-month Mayo-Health-System clinic analysis. QJM 101:519–527
Bhandari S, Ives N, Brettell EA, Valente M, Cockwell P, Topham PS, Cleland JG, Khwaja A, El Nahas M (2016) Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 31:255–261
Querfeld U, Anarat A, Bayazit AK, Bakkaloglu AS, Bilginer Y, Caliskan S, Civilibal M, Doyon A, Duzova A, Kracht D (2010) The Cardiovascular Comorbidity in Children with Chronic Kidney Disease (4C) study: objectives, design, and methodology. Clin J Am Soc Nephrol 5:1642–1648
Kidney Disease Outcomes Quality Initiative (2004) K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 43(5 Suppl 1):S1-290
Clase CM, Barzilay J, Gao P, Smyth A, Schmieder RE, Tobe S, Teo KK, Yusuf S, Mann JF (2017) Acute change in glomerular filtration rate with inhibition of the renin-angiotensin system does not predict subsequent renal and cardiovascular outcomes. Kidney Int 91:683–690
Bakris GL, Weir MR (2000) Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med 160:685–693
Schmidt M, Mansfield KE, Bhaskaran K, Nitsch D, Sørensen HT, Smeeth L, Tomlinson LA (2017) Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 356:j791
Holtkamp FA, De Zeeuw D, Thomas MC, Cooper ME, De Graeff PA, Hillege HJ, Parving H-H, Brenner BM, Shahinfar S, Heerspink HJL (2011) An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80:282–287
Tullus K (2011) Safety concerns of angiotensin II receptor blockers in preschool children. Arch Dis Child 96:881–882
Hou FF, Xie D, Zhang X, Chen PY, Zhang WR, Liang M, Guo ZJ, Jiang JP (2007) Renoprotection of Optimal Antiproteinuric Doses (ROAD) Study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol 18:1889–1898
Pham AQT, Xu LHR, Moe OW (2015) Drug-induced metabolic acidosis. F1000Res 4:F1000
Frimodt-Møller M, Høj Nielsen A, Strandgaard S, Kamper A-L (2010) Feasibility of combined treatment with enalapril and candesartan in advanced chronic kidney disease. Nephrol Dial Transplant 25:842–847
ONTARGET Investigators (2008) Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358:1547–1559
Hansen HP, Rossing P, Tarnow L, Nielsen FS, Jensen BR, Parving H-H (1995) Increased glomerular filtration rate after withdrawal of long-term antihypertensive treatment in diabetic nephropathy. Kidney Int 47:1726–1731
(FDA) FDA New Pediatric Labeling Information Database. https://www.accessdata.fda.gov/scripts/sda/sdNavigation.cfm?sd=labelingdatabase
Acknowledgements
The authors would like to thank Ms. Fiona Lai and Ms. Serena Wong for preparing the summary table on licensed use of RAASi in the paediatric population.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Answers
1. C; 2. B; 3. A; 4.D
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chan, E.Yh., Ma, A.Lt. & Tullus, K. When should we start and stop ACEi/ARB in paediatric chronic kidney disease?. Pediatr Nephrol 36, 1751–1764 (2021). https://doi.org/10.1007/s00467-020-04788-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04788-w