Abstract
Chronic kidney disease (CKD) is a global health problem and is strongly associated with hypertension (HTN) and impaired quality of life. Managing HTN with agents that block the renin angiotensin aldosterone system (RAAS) remains the gold standard, however there is a misleading impression that patients with impaired renal function or those receiving hemodialysis should not be treated with RAAS inhibitors. To date, only a few data in this field are available, given that this population subset is systematically excluded from many major clinical trials. The purpose of this review was to solve the difficult equation regarding the optimal use of RAAS blockade in patients with CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Renin angiotensin aldosterone system (RAAS) inhibitors are undoubtedly considered an effective therapeutic strategy in patients with chronic kidney disease (CKD). |
RAAS inhibitors need to be avoided in very few patients with CKD. |
Close monitoring in all cases is strongly recommended, whereas discontinuing treatment should be considered only after severe renal function impairment. |
1 Introduction
Chronic kidney disease (CKD), defined as kidney damage or an estimated glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 present for ≥ 3 months, is a global public health problem associated with a high prevalence of cardiovascular disease and impaired quality of life. In 2017, the CKD population was estimated at 700 million worldwide, and renal disease resulted in 1.2 million deaths, which is expected to rise to 2.2–4.0 million by 2040 [1]. Approximately 30% of CKD patients have hypertension (HTN). HTN has been recognized as both a cause and a consequence of CKD, with this vicious cycle being an evolving matter of concern given the high prevalence of both conditions [2, 3].
Renin angiotensin aldosterone system (RAAS) inhibitors are generally accepted as first-line antihypertensive medications, regardless of race or diabetes mellitus status, in patients with renal disease. Long-term renal benefits have been identified, such as the slowing of further kidney function decline and proteinuria and the delay in progression to end-stage renal disease (ESRD) independent of blood pressure (BP) reduction. However, the initiation of these agents might result in an acute fall in GFR within the first weeks. Additionally, starting or continuing these agents in patients undergoing hemodialysis, those with advanced CKD (GFR < 30 mL/min/1.73 m2), or those whose kidney function has worsened on RAAS blockade remains debatable [4,5,6,7,8].
The aim of this review was to present recent data for the role of RAAS inhibitors in CKD, unraveling their optimal use in everyday clinical practice based on key studies with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs).
2 Renin Angiotensin Aldosterone System (RAAS) Overactivity in Chronic Kidney Disease
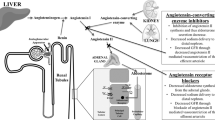
Overactivity of the RAAS has been identified as a risk factor for the onset and progression of CKD. Angiotensin II, the main effector of RAAS, enhances the vascular tone of both afferent and efferent glomerular arterioles, increasing the glomerular hydraulic pressure and ultrafiltration of plasma proteins causing damage to glomerular epithelial, endothelial, and mesangial cells. Today, proteinuria is considered an indicator of glomerular disease severity that increases the degree of glomerulosclerosis, tubular/interstitial inflammation and fibrosis, and leads to progressive renal function loss [9, 10].
The non-hemodynamic effects of angiotensin II, including activation of the pathways associated with inflammation, fibrosis, extracellular matrix accumulation, reactive oxygen species, and endothelial dysfunction, might also promote renal disease progression [9, 11].
In addition, angiotensin II augments the adrenal production of aldosterone, the principal mineralocorticoid produced in the zona glomerulosa layer of the adrenal cortex. Aldosterone is also produced in endothelial and vascular smooth muscle cells in the heart, blood vessels, and brain. Acting through epithelial mineralocorticoid receptors, aldosterone plays a central role in salt and water homeostasis and potassium excretion, and mediates renal and vascular remodeling. Aldosterone strengthens some of the effects of angiotensin II and therefore might also directly contribute to endothelial dysfunction [9].
Aside from traditional circulating RAAS, a locally expressed RAAS in kidneys is involved in the pathogenesis of CKD. Intrarenal formation of angiotensin II not only controls glomerular hemodynamics and tubule sodium transport but also activates a number of inflammatory and fibrotic pathways [10, 11].
3 RAAS Inhibitors and Long-Term Renoprotective Benefits
Pharmacological inhibition of RAAS attenuates the decline in renal function associated with CKD. Table 1 summarizes several studies that investigated the long-term renoprotective effects of these agents. In the majority of these studies, patients had hypertension, one of the most common causes of renal disease.
Almost 3 decades ago, a randomized trial was the first to clearly demonstrate the protective effect of captopril against the deterioration of renal function among patients with insulin-dependent diabetic nephropathy compared with placebo. In the captopril group, a decreased risk of death, dialysis, and transplantation was recorded, while a higher baseline creatinine value was associated with a lower risk of creatinine doubling. The renoprotective effect of ACEIs was independent of the drug’s antihypertensive action [12].
The AIPRI study investigated the effect of benazepril in 583 patients with renal insufficiency and showed a 53% reduction in the risk of doubling of serum creatinine or requiring dialysis in those receiving an ACEI. The risk reduction was higher among males and those with baseline proteinuria >1 g/24 h, glomerular disease, diabetic nephropathy, nephrosclerosis, or interstitial nephritis, but not among those with polycystic kidney disease. After only 2 months of treatment, the serum creatinine concentration and urinary protein excretion decreased to a greater extent in the benazepril group compared with the placebo group. The protective effect of benazepril was partially due to the better control and substantial decrease in BP [13].
The REIN study examined the efficacy of the ACEI ramipril among non-diabetic CKD patients. Patients were stratified before randomization by level of 24 h proteinuria (stratum 1: ≥ 1 and < 3 g/day; stratum 2: ≥ 3 g/day). Treatment with ramipril or placebo plus conventional antihypertensive therapy was targeted at the same BP control. In both stratums, ramipril halved the risk of progression to ESRD, indicating that the changes in GFR were irrespective of BP control in both groups, but dependent on the changes in proteinuria. As expected, the rate of decline in GFR and the frequency of ESRD were much lower in stratum 1 than in stratum 2 [14, 15].
In the RENAAL study, treatment with losartan offered renal benefits in patients with type 2 diabetes and nephropathy (proteinuria >0.5 g/day or serum creatinine 1.3–3 mg/dL). In the losartan group, a 16% reduction in the risk of the primary composite endpoint (defined as the composite of a doubling of the baseline serum creatinine value, ESRD, or death) was noted compared with placebo. The risk of ESRD and twofold increase in the creatinine concentration was 28% and 25% lower, respectively, in the losartan group than in the placebo group. The ARB also caused an average reduction in proteinuria by 35%, decreased the rate of decline in renal function by 18%, and slowed the decline in GFR by 15%. There was no difference in cardiovascular morbidity and mortality between the two groups, although the rate of first hospitalization for heart failure was significantly decreased with losartan (risk reduction 32%) [16].
The IRMA-2 study tested the effectiveness of the ARB irbesartan in delaying or preventing the development of diabetic nephropathy among 590 hypertensive patients with persistent microalbuminuria and serum creatinine level < 1.5 mg/dL in males and < 1.1 mg/dL in females. Approximately 5% of patients in the irbesartan 300 mg group, 9.7% of patients in the irbesartan 150 mg group, and 14.9% of patients in the placebo group developed nephropathy. Irbesartan reduced albuminuria by 24% and 38% among those receiving 150 mg and 300 mg, respectively, and restored normoalbuminuria in 24% and 34% of patients, respectively. These benefits seemed to be independent of systemic BP since the average BP value during the study was only slightly lower in the irbesartan groups compared with the placebo group [17].
The renoprotective effects of irbesartan were also demonstrated in the IDNT trial. This study assessed whether the addition of irbesartan, amlodipine, or placebo to standard antihypertensive regimens protected against the progression to nephropathy among patients with type 2 diabetes and HTN. The achieved BP in the irbesartan, amlodipine, and placebo groups was 140/77, 141/77, and 144/80 mmHg, respectively. Creatinine increased 24% more slowly with irbesartan compared with placebo, and 21% more slowly compared with amlodipine. A lower risk of doubling of baseline creatinine or developing ESRD was noted in the irbesartan group than in the other groups. There was no significant difference among the three treatment groups regarding the risk of death from any cause, whereas irbesartan was associated with a rate of congestive heart failure necessitating hospitalization approximately 23% lower than placebo. The differences in BP lowering could explain the better renal outcomes in the irbesartan group [18].
The AASK study, which included African American patients with hypertensive renal disease, found that lowering BP with the ACEI ramipril was more effective in slowing the deterioration of renal function compared with amlodipine. Patients with a urinary protein to creatinine ratio > 0.22 had a 48% risk reduction in clinical endpoints (composite of decline in GFR, ESRD, and death), and a 36% slower decline in GFR [19, 20].
The antiproteinuric effect of valsartan was demonstrated in the MARVAL study among 332 patients with type 2 diabetes and persistent microalbuminuria. This multicenter, randomized, double-blind trial showed a 44% reduction in albuminuria in ARB users, compared with only an 8% reduction in amlodipine users. A similar pattern was found in subgroup analyses for hypertensive and normotensive patients at baseline. Valsartan also induced regression to normoalbuminuria in a greater proportion of patients than amlodipine by week 24 (30% vs. 15%). Mean reductions in BP were close for both treatments (systolic BP: valsartan − 11.2 mmHg, amlodipine − 11.6 mmHg; diastolic BP: valsartan − 6.6 mmHg, amlodipine − 6.5 mmHg). The changes in urine albumin excretion were independent of BP-lowering differences [21].
The BENEDICT trial indicated that diabetic nephropathy can be prevented by the early administration of an ACEI. Among hypertensive patients with type 2 diabetes and normal renal function, the progression to microalbuminuria was significantly lower in those treated with the ACEI trandolapril or the combination of trandolapril and verapamil (6% and 5.7%, respectively) compared with those treated with either verapamil or placebo (11.9% and 10%, respectively). Thereafter, the study aimed to assess the efficacy of trandolapril alone compared with the combination of trandolapril and verapamil in preventing the development of macroalbuminuria in hypertensive patients with type 2 diabetes and persistent microalbuminuria. Fifteen patients (10.5%) in the trandolapril group and 18 (13.0%) in the trandolapril and verapamil group progressed to macroalbuminuria, while 71 (49.7%) and 62 (44.9%) participants regressed to normoalbuminuria, respectively. Patients who regressed to normoalbuminuria were mainly heavier and with greater BP values, shorter duration of diabetes, and lower albuminuria at inclusion compared with those with persistent microalbuminuria or progressing to macroalbuminuria [22, 23].
The INNOVATION study revealed that the ARB telmisartan conferred superior renoprotection compared with placebo among Japanese patients with type 2 diabetes and microalbuminuria. The transition rates to nephropathy were 16.7% in the telmisartan 80 mg group, 22.6% in the telmisartan 40 mg group, and much higher, almost 50%, in the placebo group. Remission to microalbuminuria occurred in 21.2% among those patients treated with telmisartan 80 mg, 12.8% among those patients treated with telmisartan 40 mg, and 1.2% among those patients treated with placebo. Regarding the decrease in BP, systolic and diastolic BP fell from 138/78 to 128/72 mmHg with telmisartan 80 mg, from 137/78 to 128/72 mmHg with telmisartan 40 mg, and from 137/77 to 132/74 mmHg with placebo [24].
A prespecified secondary analysis of the ALTITUDE trial investigated the efficacy and safety of the direct renin inhibitor aliskiren. When added to an ACEI or an ARB in patients with type 2 diabetes and CKD (GFR 30–60 mL/min/1.73 m2), this drug postponed the progression to macroalbuminuria and favored the regression of albuminuria. An initial acute decline in GFR was caused by aliskiren, but, after 6 months, a similar gradient of reduction was noted between aliskiren and placebo. However, aliskiren did not show any benefit on the primary composite renal endpoint (ESRD, renal death, serum creatinine doubling) [25].
Ultimately, these data support the effectiveness of ACEI or ARB therapy in delaying CKD progression. Conversely, the ABCD study and the ALLHAT and DREAM trials showed that RAAS inhibitors conferred no protection on renal function [26,27,28].
The ABCD trial demonstrated that there was no significant difference in renal outcomes between hypertensive patients with type 2 diabetes receiving enalapril or nisoldipine after the first 36 months of treatment. The percentage of patients advancing from normoalbuminuria to microalbuminuria, and from microalbuminuria to macroalbuminuria, was similar for both groups [26].
The ALLHAT trial showed that the ACEI lisinopril was not superior to chlorthalidone or amlodipine in preventing the deterioration of renal function and the development of ESRD among hypertensive patients with at least one additional risk factor for coronary heart disease. Nevertheless, the ALLHAT trial was designed to evaluate cardiovascular outcomes rather than renal outcomes and these results were derived from a post hoc analysis [27].
Finally, in the DREAM study, the ACEI ramipril had no significant impact on renal outcomes among patients with impaired glucose tolerance and/or impaired fasting glucose without known cardiovascular disease or renal insufficiency. It is likely this finding may be due to the short follow-up of 3 years [28].
4 Are All RAAS Inhibitors the Same?
ACEIs and ARBs act in a similar way and it is hypothesized that they share similar properties; however, different medications have distinct effects that can either add further advantage or reduce efficacy. Agents with a longer half-life have a sustained result lasting until the next administration. Some medications have additional benefits beyond RAAS blockade. For example, several ACEIs lower the reabsorption of uric acid in the proximal tubule, increasing uricosuria. Telmisartan has peroxisome proliferator-activated receptor-γ-modulating activity [29].
In the ONTARGET trial, the effect of the ARB telmisartan on primary renal outcome (composite of dialysis, doubling of serum creatinine, and death) did not differ to that of the ACEI ramipril among approximately 17,000 patients at high vascular risk. GFR declined most with telmisartan (− 4.1 mL/min/1.73 m2) compared with ramipril (− 2.8 mL/min/1.73 m2) [30]. A network meta-analysis of 119 randomized controlled trials revealed that both ACEIs and ARBs decreased the risk for renal failure and cardiovascular events among CKD patients [31].
On the other hand, in the REACH study ARBs were superior to ACEIs, reducing the cardiovascular events irrespective of baseline GFR among patients with more than three risk factors for atherosclerosis or established cardiovascular disease [32]. Furthermore, a trial of predialytic stage 5 CKD patients revealed that ARB users were associated with a lower mortality rate compared with ACEI users [33].
Regarding different ARBs, a large study of 860 patients with type 2 diabetes and overt nephropathy found that telmisartan (a highly lipophilic agent with a long half-life) was superior to losartan (with low lipophilicity and a short half-life) in reducing proteinuria despite a similar reduction pattern in BP [34]. Another study of 855 hypertensive patients with type 2 diabetes and overt nephropathy that compared telmisartan with valsartan concluded that the renoprotection afforded by both ARBs was similar [35].
ACEIs have also been associated with a 90% greater risk of angioedema compared with ARBs. A meta-analysis of 40 trials including approximately 200,000 patients with a mean follow-up of 123 weeks found that the incidence of angioedema was 0.3% among ACEI users and 0.1% among ARB users. From a pathophysiological point of view, ACEI-induced angioedema is due to the accumulation to bradykinin. Unlike ACEIs, ARBs do not inhibit the degradation of bradykinin and the involved mechanisms might include the upregulation of angiotensin type 2 receptors by the increased level of angiotensin II, the involvement of prostaglandins, or the deficiency of complement cascade mediators [36,37,38]. Even if single agents have separate properties, it is likely this does not translate into meaningful clinical differences. Hence, due to conflicting evidence, it is not clear that one RAAS agent is more beneficial than another.
5 RAAS Inhibitors and Early Glomerular Filtration Rate Decline
An acute fall in baseline GFR, or a rise in baseline serum creatinine < 30%, is often accepted as a physiological effect of RAAS inhibitors. It is generally considered well tolerated and is due to the greater vasodilatation of the efferent arterioles in the glomeruli and the reduction of intraglomerular pressure. This phenomenon, if it occurs, typically happens during the first 2 weeks after treatment initiation, and renal parameters are usually stabilized within 2–4 weeks [6].
Nonetheless, an early GFR decline or a creatinine increase of > 30% is not negligible. This might occur as a result of volume depletion, renal artery stenosis, or coadministration with medications such as nonsteroidal anti-inflammatory drugs (NSAIDs). In a study of approximately 2000 patients with a creatinine rise >30%, the majority were females and elderly, had renal/cardiac comorbidities (moderate to severe CKD, previous myocardial infarction, heart failure, arrhythmias, peripheral arterial disease), or were mainly receiving NSAIDs, loop diuretics, or potassium-sparing diuretics. In addition, there was a graduated greater risk of ESRD, myocardial infarction, heart failure, and death for each 10% increase in serum creatinine [6, 39].
Elderly individuals receiving RAAS inhibitors are at a greater risk for further deterioration of renal function and developing acute kidney injury due to some unique characteristics, such as reduction in renal blood flow, impaired renal regulation, diminished repaired response, and hormonal and vascular changes. Moreover, the age-associated decrease in aldosterone and plasma renin activity make these patients more susceptible to hyperkalemia, while those with stage 3 or 4 CKD are more prone to hypotensive episodes [40].
To date, there is no consensus on whether to stop these agents in CKD patients. Most clinicians are reluctant to use them in subjects with GFR < 30 mL/min/1.73 m2 or in hemodialysis because of concern that serum creatinine or potassium levels will rise. It is also usual practice to reduce or interrupt RAAS blockade in those with excessive decline in kidney function.
RAAS inhibitor cessation in patients with advanced CKD restores the capacity for kidney autoregulation and leads to a rise in GFR, delaying the onset of renal replacement therapy (RRT). This theory was confirmed in a study of 43 subjects with a mean GFR of 19.3 ± 8.1 mL/min/1.73 m2. Patients who were improved were mainly those whose BP rose the most after stopping treatment, indicating that the beneficial effect on renal function might be the consequence of increased BP and perfusion pressure [41]. Likewise, an observational study of 52 patients with advanced CKD (GFR 16.4 ± 1 mL/min), in preparation for RRT, demonstrated that the withdrawal of RAAS inhibitors led to an overall mean increase in GFR by 10 mL/min/1.73 m2 over 12 months. Almost 62% of these patients had a more than 25% increase in GFR, while 37% of patients had an increase exceeding 50%. A modest rise in mean BP by 4 mmHg was also recorded without change in cardiovascular events and proteinuria [42]. Thus, it is mandatory to balance the benefits of RAAS inhibitor therapy with the potential to accelerate the need for RRT.
Nowadays, neither the National Kidney Foundation/Kidney Disease Outcomes Quality Initiative Clinical Practice Guidelines on HTN nor the Kidney Disease: Improving Global Outcomes 2020 Clinical Practice Guideline on the Management of BP in CKD recommend the cessation of treatment in patients with advanced CKD. Instead, they suggest more careful monitoring of renal function and serum potassium. Only in subjects with advanced CKD who experienced uremic symptoms or excessive high serum potassium levels is it reasonable to stop RAAS blockade therapy temporarily to allow time for RRT preparation. The 2018 European guidelines for the management of HTN propose the withdrawal of antihypertensive therapy if the decline in GFR continues and suggest examining patients for renovascular disease [5, 6, 8, 43].
A retrospective cohort of subjects treated with RAAS inhibitors showed that therapy discontinuation owing to a fall in GFR below 30 mL/min/1.73 m2 was associated with a higher risk of mortality (35.1% vs. 29.4%) and major adverse cardiovascular events, without a significant difference in the risk of ESRD. Similar patterns also held for those with a ≥ 40% decrease in GFR [44].
RAAS inhibitors should be considered first-line antihypertensive medications in patients undergoing hemodialysis due to their favorable effects on left ventricular hypertrophy, arterial stiffness, endothelial function, and oxidative stress [45]. In a study of 12 countries from North America, Europe and Japan, a small but significant survival benefit was recorded among hemodialysis subjects receiving RAAS inhibitors. It was found a lower all-cause mortality by 11% in incident (< 120 days) and 6% in prevalent (> 120 days) dialysis subjects. This advantage appeared greater with ARBs versus ACEIs [46]. Conversely, their impact on residual renal function remains inconclusive [47, 48].
The STOP-ACEi trial is an ongoing project including subjects with advanced CKD that will aim to answer whether stopping these medications results in stabilization or improvement of renal function. It will also show whether this intervention can improve the progression to ESRD without causing any increase in cardiovascular events [49].
For all patients planned for the institution of RAAS blockade treatment, creatinine, GFR, and potassium should be estimated at baseline. The frequency of follow-up measurement of GFR to detect an early decrease is dependent on its baseline value. If baseline GFR is > 60 mL/min/1.73 m2, the proposed time interval is 4–12 weeks; if it is 30–59 mL/min/1.73 m2, the interval is 2–4 weeks; and if it is < 30 mL/min/1.73 m2, the interval is ≤ 2 weeks [6, 43].
Differentiations in the management or dose of RAAS inhibitors are determined by the magnitude of early decrease in GFR. In case of mild decreases (< 30%), the antihypertensive therapy should be continued and GFR should be evaluated after 10–14 days. If GFR falls 30–50% or > 50% over baseline, the ACEI or ARB dose should be reduced or discontinued, respectively. GFR should be reassessed every 5–7 days until kidney function returns to baseline. If GFR does not return to baseline level, an alternative antihypertensive agent is selected. A proposed approach for patients initiating RAAS inhibitors is illustrated in Fig. 1 [6, 43]. The combination of an ACEI with an ARB is not recommended due to serious adverse effects, such as hyperkalemia and acute kidney injury [30, 50].
6 Conclusions
Given the central role of RAAS inhibitors in the onset and progression of CKD, these medications are undoubtedly considered an effective therapeutic strategy. Their beneficial effect on renal outcomes in the long-term appears to be independent of or supplementary to their BP-lowering properties. Evidence indicates that RAAS inhibitors do not need to be avoided in CKD; however, close monitoring in all of these cases is strongly recommended, whereas discontinuing treatment should be considered only after severe renal function impairment.
References
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–33. https://doi.org/10.1016/S0140-6736(20)30045-3.
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–37. https://doi.org/10.1038/s41581-019-0244-2.
NCD Risk Factor Collaboration. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet. 2017;389(10064):37–55. https://doi.org/10.1016/S0140-6736(16)31919-5.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–324. https://doi.org/10.1161/HYP.0000000000000066.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104. https://doi.org/10.1093/eurheartj/ehy339.
KDIGO Executive Committee. Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guideline on the management of blood pressure in chronic kidney disease. KDIGO. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-BP-Management-in-CKD-GL-public-review-draft-Jan-31-2020.pdf. Accessed Jan 2020.
Pugh D, Gallacher PJ, Dhaun N. Management of hypertension in chronic kidney disease. Drugs. 2019;79(4):365–79. https://doi.org/10.1007/s40265-019-1064-1.
Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28(9):2812–23. https://doi.org/10.1681/ASN.2017020148.
Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;99:S57-65. https://doi.org/10.1111/j.1523-1755.2005.09911.x.
Zhang F, Liu H, Liu D, Liu Y, Li H, Tan X, et al. Effects of RAAS inhibitors in patients with kidney disease. Curr Hypertens Rep. 2017;19(9):72. https://doi.org/10.1007/s11906-017-0771-9.
Siragy HM, Carey RM. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am J Nephrol. 2010;31(6):541–50. https://doi.org/10.1159/000313363.
Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–62. https://doi.org/10.1056/NEJM199311113292004.
Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334(15):939–45. https://doi.org/10.1056/NEJM199604113341502.
Ruggenenti P, Perna A, Mosconi L, Matalone M, Tognoni G, Remuzzi G, et al. Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet. 1997;349(9069):1857–63.
Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354(9176):359–64. https://doi.org/10.1016/S0140-6736(98)10363-X.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9. https://doi.org/10.1056/NEJMoa011161.
Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–8. https://doi.org/10.1056/NEJMoa011489.
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60. https://doi.org/10.1056/NEJMoa011303.
Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285(21):2719–28. https://doi.org/10.1001/jama.285.21.2719.
Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–31. https://doi.org/10.1001/jama.288.19.2421.
Viberti G, Wheeldon NM. Microalbuminuria reduction with VSI. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation. 2002;106(6):672–8. https://doi.org/10.1161/01.cir.0000024416.33113.0a.
Ruggenenti P, Fassi A, Ilieva A, Iliev IP, Chiurchiu C, Rubis N, et al. Effects of verapamil added-on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT-B randomized trial. J Hypertens. 2011;29(2):207–16.
Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351(19):1941–51.
Makino H, Haneda M, Babazono T, Moriya T, Ito S, Iwamoto Y, et al. Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care. 2007;30(6):1577–8. https://doi.org/10.2337/dc06-1998.
Heerspink HJ, Persson F, Brenner BM, Chaturvedi N, Brunel P, McMurray JJ, et al. Renal outcomes with aliskiren in patients with type 2 diabetes: a prespecified secondary analysis of the ALTITUDE randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(4):309–17. https://doi.org/10.1016/S2213-8587(15)00469-6.
Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. 2000;23(Suppl 2):B54-64.
Rahman M, Ford CE, Cutler JA, Davis BR, Piller LB, Whelton PK, et al. Long-term renal and cardiovascular outcomes in Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) participants by baseline estimated GFR. Clin J Am Soc Nephrol. 2012;7(6):989–1002. https://doi.org/10.2215/CJN.07800811.
DREAM Trial Investigators, Dagenais GR, Gerstein HC, Holman R, Budaj A, Escalante A, et al. Effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care. 2008;31(5):1007–14. https://doi.org/10.2337/dc07-1868.
Locatelli F, Del Vecchio L, Cavalli A. Inhibition of the renin-angiotensin system in chronic kidney disease: a critical look to single and dual blockade. Nephron Clin Pract. 2009;113(4):c286–93. https://doi.org/10.1159/000235946.
Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372(9638):547–53. https://doi.org/10.1016/S0140-6736(08)61236-2.
Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou W, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67(5):728–41. https://doi.org/10.1053/j.ajkd.2015.10.011.
Potier L, Roussel R, Elbez Y, Marre M, Zeymer U, Reid CM, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in high vascular risk. Heart. 2017;103(17):1339–46. https://doi.org/10.1136/heartjnl-2016-310705.
Lin CC, Wu YT, Yang WC, Tsai MJ, Liu JS, Yang CY, et al. Angiotensin receptor blockers are associated with lower mortality than ACE inhibitors in predialytic stage 5 chronic kidney disease: a nationwide study of therapy with renin-angiotensin system blockade. PLoS ONE. 2017;12(12):e0189126. https://doi.org/10.1371/journal.pone.0189126.
Bakris G, Burgess E, Weir M, Davidai G, Koval S, AMADEO Study Investigators. Telmisartan is more effective than losartan in reducing proteinuria in patients with diabetic nephropathy. Kidney Int. 2008;74(3):364–9. https://doi.org/10.1038/ki.2008.204.
Galle J, Schwedhelm E, Pinnetti S, Boger RH, Wanner C, Investigators V. Antiproteinuric effects of angiotensin receptor blockers: telmisartan versus valsartan in hypertensive patients with type 2 diabetes mellitus and overt nephropathy. Nephrol Dial Transplant. 2008;23(10):3174–83. https://doi.org/10.1093/ndt/gfn230.
Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377–82. https://doi.org/10.1111/jch.13097.
Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6(5):604–11. https://doi.org/10.1161/CIRCOUTCOMES.113.000359.
Makani H, Messerli FH, Romero J, Wever-Pinzon O, Korniyenko A, Berrios RS, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012;110(3):383–91. https://doi.org/10.1016/j.amjcard.2012.03.034.
Schmidt M, Mansfield KE, Bhaskaran K, Nitsch D, Sorensen HT, Smeeth L, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ. 2017;356:j791. https://doi.org/10.1136/bmj.j791.
Turgut F, Balogun RA, Abdel-Rahman EM. Renin-angiotensin-aldosterone system blockade effects on the kidney in the elderly: benefits and limitations. Clin J Am Soc Nephrol. 2010;5(7):1330–9. https://doi.org/10.2215/CJN.08611209.
Goncalves AR, Khwaja A, Ahmed AK, El Kossi M, El Nahas M. Stopping renin-angiotensin system inhibitors in chronic kidney disease: predictors of response. Nephron Clin Pract. 2011;119(4):c348–54. https://doi.org/10.1159/000330289.
Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant. 2010;25(12):3977–82. https://doi.org/10.1093/ndt/gfp511.
Kidney Disease Outcomes Quality Initiative. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 Suppl 1):S1-290.
Qiao Y, Shin JI, Chen TK, Inker LA, Coresh J, Alexander GC, et al. Association between renin-angiotensin system blockade discontinuation and all-cause mortality among persons with low estimated glomerular filtration rate. JAMA Intern Med. 2020;180(5):718–26. https://doi.org/10.1001/jamainternmed.2020.0193.
Inrig JK. Antihypertensive agents in hemodialysis patients: a current perspective. Semin Dial. 2010;23(3):290–7. https://doi.org/10.1111/j.1525-139X.2009.00697.x.
Karaboyas A, Xu H, Morgenstern H, Locatelli F, Jadoul M, Nitta K, et al. DOPPS data suggest a possible survival benefit of renin angiotensin-aldosterone system inhibitors and other antihypertensive medications for hemodialysis patients. Kidney Int. 2018;94(3):589–98. https://doi.org/10.1016/j.kint.2018.03.013.
Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11(3):556–64.
Yoo KD, Kim CT, Kwon S, Lee J, Oh YK, Kang SW, et al. Renin angiotensin aldosterone system blockades does not protect residual renal function in patients with hemodialysis at 1 year after dialysis initiation: a prospective observational cohort study. Sci Rep. 2019;9(1):18103. https://doi.org/10.1038/s41598-019-54572-6.
Bhandari S, Ives N, Brettell EA, Valente M, Cockwell P, Topham PS, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant. 2016;31(2):255–61. https://doi.org/10.1093/ndt/gfv346.
Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369(20):1892–903. https://doi.org/10.1056/NEJMoa1303154.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was used to assist in the preparation of this review.
Conflicts of interest
Elias Sanidas, Dimitrios Papadopoulos, Michalis Chatzis, Maria Velliou, and John Barbetseas have no conflicts of interest to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The authors declare that all relevant data are available within the article.
Code availability
Not applicable.
Author contributions
All authors contributed equally to this review.
Rights and permissions
About this article
Cite this article
Sanidas, E., Papadopoulos, D., Chatzis, M. et al. Renin Angiotensin Aldosterone System Inhibitors in Chronic Kidney Disease: A Difficult Equation. Am J Cardiovasc Drugs 21, 619–627 (2021). https://doi.org/10.1007/s40256-021-00467-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-021-00467-9