Abstract
Background
Cardiovascular disease is the leading cause of death in children with chronic kidney disease (CKD). Serum levels of gut-derived uremic toxins increase with deterioration of kidney function and are associated with cardiac comorbidities in adult CKD patients.
Methods
Indoxyl sulfate (IS) and p-cresyl sulfate (pCS) were measured by high-performance liquid chromatography in serum of children participating in the Cardiovascular Comorbidity in Children with CKD (4C) Study. Results were correlated with measurements of the carotid intima-media thickness (cIMT), central pulse wave velocity (PWV), and left ventricular mass index (LVMI) in children aged 6–17 years with initial eGFR of 10–60 ml/min per 1.73 m2.
Results
The median serum levels of total IS and of pCS, measured in 609 patients, were 5.3 μmol/l (8.7) and 17.0 μmol/l (21.6), respectively. In a multivariable regression model, IS and pCS showed significant positive associations with urea and negative associations with eGFR and uric acid. Furthermore, positive associations of pCS with age, serum albumin, and non-Mediterranean residency and a negative association with glomerular disease were observed. By multivariable regression analysis, only IS was significantly associated with a higher cIMT SDS at baseline and progression of PWV SDS within 12 months, independent of other risk factors.
Conclusions
Serum levels of gut-derived uremic toxins IS and pCS correlated inversely with eGFR in children. Only IS was significantly associated with surrogate markers of cardiovascular disease in this large pediatric CKD cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent studies in adult patients with chronic kidney disease (CKD) suggest that the gut-derived uremic toxins indoxyl sulfate (IS) and p-cresyl sulfate (pCS) correlate inversely with glomerular filtration rate (eGFR) and promote CKD progression and cardiovascular disease (CVD) [1,2,3,4].
In patients with CKD, several factors, including slow intestinal transit time [5], iron therapy, special diets, and frequent use of antibiotics, lead to qualitative and quantitative changes in gut microbiome, or dysbiosis [6]. These changes include an increase of proteolytic bacteria, such as Enterobacteria and Enterococci, and a decrease of saccarolytic bacteria, e.g., Bifidobacterium species, leading to an impaired metabolism of nutrients [6,7,8]. Intestinal catabolism of amino acids leads to formation of indoles and cresols, which are further metabolized by the liver to IS and pCS. Furthermore, high concentrations of urea in body fluids lead to an influx of urea into the gut and to synthesis of ammonium, which alters the epithelial gut cell barrier and promotes systemic toxin accumulation [9]. These protein-bound uremic toxins are eliminated insufficiently due to reduced glomerular and tubular function [6, 7].
CVD is the leading cause of death in children with CKD [10, 11]. In the absence of symptomatic CVD or cardiovascular endpoints, surrogate parameters such as carotid artery intima-media thickness (cIMT), pulse wave velocity (PWV), and left ventricular mass index (LVMI), which have been validated in children [12, 13], are used to detect early CKD-specific changes in the cardiovascular phenotype [14, 15]. In this study, we aimed to assess the determinants of the uremic toxins IS and pCS and their association with cardiovascular surrogate parameters cIMT, PWV, and LVMI in a large cohort of children with CKD.
Materials and methods
Study design and cardiovascular assessment
The Cardiovascular Comorbidity in Children with Chronic Kidney Disease (4C) study is a prospective observational study with 55 participating centers in 12 European countries. Children aged 6–17 years with an initial eGFR of 10–60 ml/min per 1.73 m2 not on renal replacement therapy (RRT) were eligible for the study. Written informed consent was obtained from all patients or parents. The study was approved by the Ethics Commission of the University of Heidelberg (S-032/2009) and subsequently by the local review board of each participating institution (for a complete list of review boards, see [16]). Additional details, including exclusion criteria, were published in detail previously [17].
In this ongoing study, all participating centers are closely monitored by regional coordinators, providing comparable standards in diagnostic procedures and equipment. Every 6 months, medical history, clinical status, anthropometric data, and medication are recorded, and blood and urine samples are collected, stored, and analyzed in a central laboratory. eGFR was calculated using a previously published cystatin C and creatinine-based equation [18]. Physical activity is estimated by hours of physical activity (0/1–2/3–4/> 4 h per week) using a standardized questionnaire.
Starting in 2009, cardiovascular assessment has been performed at baseline and at each annual study visit. Office blood pressure (BP) was documented as an average of three oscillometric measurements using local devices [14]. Carotid IMT was measured according to a standardized protocol using a portable ultrasound device (Acuson P50, Siemens Medical Solutions USA, Inc.) [19]. Carotid-femoral PWV was measured using an oscillometric Vicorder device [12]. Two-dimensional echocardiography was performed, and left ventricular (LV) mass was calculated according to the Devereux equation [14] and indexed to height (LVMI) according to Chinali et al. [20]. Height, body mass index (BMI), BP, cIMT, and PWV were normalized for sex, age, and/or height by calculation of standard deviation scores (SDS) derived from reference values established previously in healthy children [13, 21]. Phosphate SDS was calculated by calculating z-scores with reference values for the two age groups 5 to 12 years and age > 12 years [22].
As surrogate parameter for the influence of nutrition, patients were categorized according to their country of residency as Mediterranean (Turkey, Portugal, France, and Italy) and non-Mediterranean (Germany, Austria, Switzerland, Poland, Lithuania, Serbia, Czech Republic, and UK). Renal diagnoses were grouped into congenital anomalies of the kidney and urinary tract (CAKUT), tubulointerstitial diseases, glomerular diseases, post-AKI CKD, and others.
Laboratory methods
IS and pCS were measured centrally in serum samples obtained at the baseline visit (first visit after study entry) using a reversed-phase separation and fluorescence detection. In brief, serum samples were deproteinized by addition of three parts methanol to one part serum for determination of total IS and pCS. All analyses were performed on a high-performance liquid chromatography (HPLC) system (SL 1000; GOEBEL, Germany), including binary solvent manager, autosampler (AS 5200, GOEBEL, Germany), column compartment, and fluorescence array detector (Shimadzu RF-535), connected with the Geminyx III software (GOEBEL, Germany).
For injection into HPLC, 20 μl of the dissolved samples was used. IS (Potassium salt, Sigma ALDRICH; Nr. I3875) and pCS (Potassium salt, kind gift of Griet Glorieux; University Hospital, Gent, Belgium) were identified using a Shimadzu RF-551 fluorescence detector (λex = 260 nm, λem = 309 nm).
A pre-column LiChroCart-4-4 (Lichrospher 100 RP-18e endcapped, 5 μm, MERCK, Darmstadt, Germany) and a subsequent reversed-phase column LiChroCart-250-4 (Lichrospher 100 RP-18e, 5 μm, MERCK, Darmstadt, Germany) were used for chromatography. The HPLC mobile phases consisted of 10 mM NH4H2PO4 and 20% acetonitrile (pH = 4.0) (buffer A) and 10 mM NH4H2PO4 and 60% acetonitrile (pH = 4.0) (buffer B). Buffer flow was 0.4 mL/min using buffer A for 11 min, buffer 2B for 2 min, and buffer A for 5 min. Under these conditions, IS and pCS eluted at 4.76 and 6.41 min, respectively. Linearity for both derivatives was found between 0 and 130 μM. Coefficient of variation was 3.1% for IS and 1.9% for pCS.
Statistical analyses
Data were stratified by CKD stage and described by mean ± standard deviation (SD) or median with interquartile range (IQR) or frequencies (n, %), as appropriate. ANOVA, Kruskal-Wallis, or chi-squared tests with respective post hoc tests were applied for group comparisons. As the distribution of IS and pCS was skewed, these variables were log-transformed. The associations of log-transformed IS or pCS with baseline cIMT, PWV, or LVMI were analyzed using Pearson correlation and visualized using a scatter plot. The associations of log-transformed IS with the change of cIMT, PWV, and LVMI were analyzed using partial correlations corrected for the respective baseline value. Dependent t test was used to analyze if the change in cIMT, PWV, and LVMI within 1 year of follow-up was statistically significant.
Multivariable linear regression analysis was applied to quantify the association of log-transformed IS or pCS with clinically relevant variables at baseline, including age, sex, diagnosis, residency, physical activity, eGFR, albumin, urea, uric acid, phosphate, iron therapy, and calcium-based phosphate binders. Additionally, multivariable linear regression was used to assess the association between log-transformed IS or pCS levels and cardiovascular parameters including cIMT, PWV, and LVMI corrected for age, sex, body mass index (BMI), BP, eGFR, albumin, log-transformed C-reactive protein (CRP), phosphate, iron therapy, calcium-based phosphate binders, and diagnosis. As cIMT, PWV, and LVMI measurements not always coincide with the regular visit, the time difference (days) was included as a covariate in the model as well. The same models were run to analyze the influence of log-transformed IS or pCS levels with 1-year cIMT, PWV, and LVMI change with start of dialysis (yes/no) as an additional confounder. In these models, the time difference between the first and second measurement was included as a covariate. In addition, we performed mediation analysis to analyze if IS and pCS affected the cardiovascular outcome through a mediator. A subgroup of patients with very high IS levels was identified by determining the upper 95% prediction limit in an univariable regression of eGFR on log-transformed IS. Patient characteristics of this subgroup were compared to the rest of the sample. p Values < 0.05 were considered statistically significant. Data were analyzed using SAS® software version 9.4 (SAS Inc. Cary/NC, USA).
Results
Patient characteristics
A cohort of 704 patients with initial eGFR of 10–60 ml/min per 1.73 m2 was included in the 4C study between October 2009 and August 2011. IS and pCS levels were measured in 609 patients with serum samples available at their baseline visit.
Baseline patient characteristics are given in Table 1. In general, baseline characteristics of our cohort were similar to the complete 4C cohort, which have been described in detail previously [14]. Mean eGFR was 28.1 ± 10.3 ml/min/1.73 m2 and mean age was 12.1 ± 3.3 years. CAKUT was the most prevalent underlying disease category (69.4%), followed by tubulointerstitial diseases (12.8%) and glomerulopathies (8.1%). Male patients comprised 65.6% of the cohort. Approximately 50% of patients were treated in Turkey. There were significant differences in uric acid, phosphate SDS, systolic and diastolic BP SDS, and physical activity between CKD stages. In higher CKD stages, significantly more patients were treated with iron and phosphate binders and the number of children without physical activity increased. Patients with higher CKD stages also had significantly higher LVMI.
Cross-sectional analysis of IS and pCS
Total serum levels of IS and pCS were elevated above the normal range of healthy adults (2.5 μmol/l for total IS and 10.1 μmol/l for total pCS levels) [23]. Mean and median levels of total IS and pCS are shown in Table 1 and illustrated in Online Resource 1. The levels of both toxins increased significantly with higher CKD stages.
Variables associated with IS and pCS levels
In a multivariable regression analysis (Table 2), both IS and pCS levels showed positive associations with urea (p = 0.005 for IS and p = 0.03 for pCS) and negative associations with eGFR (both p < 0.001). Levels of pCS were higher in older patients (p = 0.008), whereas IS levels showed no association with age. Levels of pCS were lower in patients with glomerulopathies compared to patients with CAKUT (p = 0.002) and lower in patients of Mediterranean compared to non-Mediterranean residency (p < 0.001). We also observed negative associations of both toxin levels with uric acid (both p < 0.001) and a positive association of pCS with albumin (p < 0.001). The influence of residency and renal diagnosis on IS and pCS levels is further illustrated in an univariable analysis in Fig. 1.
Indoxyl sulfate and p-cresyl sulfate (logarithmic values; mean ± SD) according to residency in Mediterranean or non-Mediterranean countries (a, b) and according to diagnostic group (c, d) (n = 609). Abbreviations: Log-IS logarithmic value of indoxyl sulfate; Log-pCS logarithmic value of p-cresyl sulfate; Medit. Mediterranean residency; Non-Medit. non-Mediterranean residency; CAKUT congenital anomalies of the kidney and urinary tract, Glom-Path glomerulopathies; Tub-Int tubulointerstitial diseases
Associations of IS and pCS with baseline cardiovascular parameters
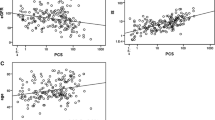
Carotid IMT SDS (n = 571) and LVMI (n = 531), but not PWV SDS (n = 571), at baseline were significantly correlated with the IS (Fig. 2), whereas pCS serum levels were not correlated with any cardiovascular parameters at baseline (data not shown).
Linear correlation of log-transformed indoxyl sulfate levels with cIMT SDS (n = 571), PWV SDS (n = 571), and LVMI (n = 531) and partial correlation of log-transformed indoxyl sulfate levels with the change of cIMT SDS (n = 458), PWV SDS (n = 460), and LVMI (n = 419) corrected for the respective baseline value. Abbreviations: Log-IS logarithmic value of indoxyl sulfate; cIMT carotid intima-media thickness; PWV pulse wave velocity; LVMI left ventricular mass index; SDS standard deviation score
The results of a multivariable linear regression analysis of variables associated with cIMT SDS, PWV SDS, and LVMI are shown in Table 3 and Online Resource 2 and 3, respectively. cIMT SDS was significantly associated with IS (p = 0.05) independently of eGFR, age, sex, BMI, SDS, BP SDS, albumin, CrP, phosphate SDS, therapy with phosphate binders, iron supplementation, and renal diagnosis. PWV SDS and LVMI showed no independent associations with IS at baseline in our multivariable model. There were no significant associations of pCS with either cIMT SDS, PWV SDS, or LVMI.
A subgroup of patients with very high IS levels above the 95% percentile of the distribution in the cohort (n = 43) was identified (Online Resource 4). While the group characteristics of these patients did not differ significantly from the rest of the cohort (Online Resource 5), they had significantly higher baseline cIMT SDS compared to the rest of the cohort (2.16 ± 1.66 vs. 1.60 ± 1.46, p = 0.03).
Follow-up analysis
One year follow-up data for cIMT, PWV, and LVMI was available in 458, 460, and 419 patients, respectively (Online Resource 6). Due to higher drop-out rate and variable influences like deterioration of renal function, renal replacement therapy, or renal transplantation during a longer follow-up, only 1 year follow-up data was evaluated. The change in PWV SDS adjusted for the respective baseline value was significantly correlated with the prevailing IS level (Fig. 2).
The multivariable regression analysis for changes of cIMT SDS, PWV SDS, and LVMI after 12 months is shown in Table 4. The change in PWV SDS was significantly associated with IS levels (p = 0.02). The cIMT SDS was still associated with IS after 12 months in the univariable analysis (r = 0.01, p = 0.04), while there was only a trend for significant association of change in cIMT SDS with IS in the multivariable model (p = 0.07). No associations of change in LVMI with IS levels and no associations of changes in cIMT SDS, PWV SDS, or LVMI with pCS levels were observed (data not shown).
In an additional mediation analysis, the effect of IS on baseline cIMT SDS, but not on progression of PWV SDS, was mediated by phosphate (Online Resource 7).
Discussion
In this comprehensive study of 609 children with different stages of CKD, we found a significant association of IS with cardiovascular surrogate parameters. By multivariable linear regression, IS was associated with cIMT SDS independent of eGFR, age, sex, diagnosis, BMI SDS, systolic BP SDS, serum albumin, phosphate SDS, and CRP. Similar results have been published in a cohort of adult CKD patients [24]. Furthermore, in a multivariable regression analysis, we could demonstrate a significant association of IS levels with 1 year change in PWV SDS, but no association with change in LVMI or cIMT SDS. Further supporting this association, a subgroup analysis of 43 patients with highest IS levels showed a significantly higher cIMT SDS compared to the other patients. Whereas adult CKD patients frequently suffer from diabetic nephropathy, obesity, metabolic syndrome, and other chronic conditions, the patients in this pediatric CKD cohort were largely free of age- and lifestyle-related comorbidities, permitting the study of the effects of “pure” CKD on serum levels of gut-derived uremic toxins and their associations with vascular measurements and demographic and clinical conditions.
There is growing mechanistic knowledge from in vitro studies as to how gut-derived uremic toxins, including IS and pCS, promote cardiovascular toxicity. These manifold mechanisms have been systematically reviewed [25, 26] and include induction of endothelial microparticles [27, 28], inflammation [29], and activation of the aryl hydrocarbon receptor [30, 31].
The mediation analysis suggested some effect of IS on baseline cIMT SDS was mediated by phosphate. This association has not been described previously, but it is known that uremic toxins, especially IS, also affect bone metabolism and are associated with CKD mineral and bone disorder. In this regard, it has been shown that IS induces PTH resistance and leads to repression of osteocyte Wnt/β-catenin signaling and increased expression of Wnt antagonists, a pattern associated with adynamic bone disease [32, 33]. Further, phosphate might be a surrogate parameter for nutrition. However, we can only give first insight into the associations but have not proved a causal relationship since the original study was not designed to prove causal mediation.
Clinical studies, however, have found conflicting evidence regarding the impact of gut-derived uremic toxins on cardiovascular disease and mortality in CKD patients. Several studies suggested that uremic toxins promote CVD and mortality [2, 25, 34]. On the other hand, data from the HEMO study in adult dialysis patients contradicts these findings [35].
Our study shows that subclinical vascular pathology (baseline cIMT SDS, progression of PWV SDS) increased dependent of toxin burden (IS level) and independent of eGFR in children with stage 3–5 CKD. In the absence of other comorbidities, our data might support the role of gut-derived uremic toxins in promoting vascular remodeling. Interestingly, high albumin levels were associated with lower baseline cIMT SDS and LVMI as well as lower progression of LVMI within 12 months. This fact might be partially explained by the lower fraction of free and therefore toxic IS. Nevertheless, high albumin and lower cIMT and LVMI could be due to better nutrition and lower inflammatory state, both of which can also explain higher albumin levels.
We observed a positive association of iron supplementation with cIMT SDS. The role of oral iron supplementation on gut microbiome has been described previously, and hereby, some influence on CVD might be explained [36]. However, the impact of iron therapy on CVD has not been proven. On the one hand, an association with an increase of cIMT has been described [37], while on the other hand, there was no association with cardiovascular events [38].
IS and pCS derive from bacterial colonic fermentation of tryptophan and tyrosine, respectively. In patients with CKD, changes in gut microbiome (dysbiosis), impaired intestinal protein assimilation, and epithelial tight junction breakdown in the gut lead to systemic accumulation of toxins, which are eliminated insufficiently due to glomerular and tubular dysfunction in CKD [3, 6, 22]. Except for two small studies of 57 and 20 patients with CKD, respectively [39, 40], uremic toxins have not been studied previously in children. In our analysis of 609 children, we show representative data for the uremic toxins IS and pCS according to CKD stage in predialysis children. There was a significant inverse correlation of IS and pCS with eGFR, confirming data from previous studies of adult patients [23, 25].
While in adult CKD patients both IS and pCS appear to increase with age [41, 42], the influence of age on gut-derived toxin levels in the pediatric age group has not been studied before. Our study indicates a relatively small but significant increase of pCS levels with age in a multivariable regression analysis (Table 2).
Interestingly, patients with glomerular diseases had lower levels of pCS compared to patients with CAKUT in the multivariable model (Table 2) and also compared to tubulointerstitial diseases in the univariable analysis (Fig. 1d). Since tubular secretion plays a major role in toxin elimination, decreased tubular function might contribute to toxin accumulation in patients with tubulointerstitial disease and CAKUT [3, 43]. However, IS levels did not correlate independently with diagnosis (Table 2). This might be explained by higher concentrations of free solute fractions of IS compared to pCS, which are not bound to albumin and can be excreted by glomerular filtration. Further, different tubular transport systems for both toxins might play a role in toxin elimination [3, 44].
We also found a significant impact of residency on pCS levels. In the multivariable analysis, residence in non-Mediterranean countries and higher albumin levels were independently associated with higher pCS levels (Table 2). These findings might be partially explained by the influence of nutrition. Adult patients with low fiber and high protein intake showed higher gut-derived toxin levels [45, 46], especially higher levels of pCS [47]. This might apply to pediatric patients living in non-Mediterranean countries (i.e., Middle or Eastern Europe), while vice versa, patients in Mediterranean countries have diets with higher fiber and lower protein intake. Further, as mentioned above, free solute concentration and therefore tubular clearance is lower in patients with high albumin. Thus, the impact of albumin on serum levels of uremic toxins on the one hand and its role in promoting cardiovascular disease on the other hand seems opposing and needs to be further investigated. However, genetic variability or other factors might also play a role.
Interestingly, high uric acid was associated with low IS and pCS levels. In our cohort, uric acid increased inversely with eGFR as expected (Table 1). One explanation for the inverse association of uric acid and gut-derived uremic toxins might be a competitive antagonism at tubular excretion mechanisms, leading to saturation of transport channels by the uremic toxin burden and therefore leading to a higher increase of uric acid and vice versa. It has indeed been shown that uric acid and gut-derived toxins are excreted in part by the same organic anion transporters (e.g., OAT3) [48,49,50]. The clinical significance of these findings remains unknown; however, in view of the conflicting evidence regarding the role of uric acid as a risk factor for CVD [51], interactions in the excretion of uric acid with gut-derived uremic toxins could be a confounding factor in the evaluation of cardiovascular risk associated with either uremic toxins or uric acid.
IS and pCS were both independently associated with eGFR and correlated with each other (r = 0.20, p < 0.001). Both solutes are among the best-studied uremic toxins. Several mechanisms leading to cardiovascular damage in vitro have been reported for both toxins and corroborated in clinical studies in adults [25, 52]. We can only speculate why IS, but not pCS, was associated with cardiovascular outcome (baseline IMT SDS, progression of PWV SDS) in our pediatric cohort. Differences in population characteristics such as age, variations in risk factor load, and comorbid conditions such as diabetes might explain the exclusive association of IS and CVD in our cohort. Of note, pCS is independently associated with the presence of diabetes [35, 44].
Several limitations of our study should be mentioned. First, we performed a cross-sectional analysis and therefore cannot prove any causal associations of uremic toxins and cardiovascular phenotype. We cannot rule out the presence of symptomatic or asymptomatic gastrointestinal disorders at the time of blood drawing, which might have influenced intestinal dysbiosis and hereby toxin levels. Furthermore, antibiotic treatment, which also influences gut microbiome, and nutrition were not recorded in our study. Toxin levels were only measured at one single time point. It has been shown that some toxins have a high intra-patient variability, which might affect associations with clinical outcomes [53]. However, this effect might be diminished by the large study population. Finally, we measured only total and not free solute concentrations, which are suspected to play an active role in CVD disease pathophysiology [34].
The results of this study outline potentially fruitful areas of future clinical research. It is presently unclear whether uremic solutes predispose to CVD [54,55,56] or promote [57] CKD progression. To assess this issue, prospective evaluation of free and total levels of uremic toxins and cardiovascular surrogate parameters are necessary. This issue was not targeted in our analysis but should be investigated further in cohort studies. Furthermore, since removal of protein-bound toxins by dialysis is insufficient, the impact of preemptive renal transplantation and different renal replacement strategies should be reevaluated in the future [58, 59]. Finally, the role of pre-, pro-, or antibiotics in influencing the gut microbiome and hereby decreasing or increasing the burden of uremic toxins should be further evaluated in clinical trials [6, 60].
Summarizing, our study shows an increase of uremic gut-derived toxin burden in children with CKD, associated with eGFR, urea, and uric acid. Serum levels of IS, but not pCS, were independently associated with cardiovascular surrogate parameters (baseline cIMT SDS) and their progression within 12 months (PWV SDS), independent of other risk factors.
References
Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA (2009) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4:1551–1558
Lin CJ, Wu V, Wu PC, Wu CJ (2015) Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One 10:e0132589
Poesen R, Viaene L, Verbeke K, Claes K, Bammens B, Sprangers B, Naesens M, Vanrenterghem Y, Kuypers D, Evenepoel P, Meijers B (2013) Renal clearance and intestinal generation of p-cresyl sulfate and indoxyl sulfate in CKD. Clin J Am Soc Nephrol 8:1508–1514
van den Brand JA, Mutsaers HA, van Zuilen AD, Blankestijn PJ, van den Broek PH, Russel FG, Masereeuw R, Wetzels JF (2016) Uremic solutes in chronic kidney disease and their role in progression. PLoS One 11:e0168117
Grant CJ, Harrison LE, Hoad CL, Marciani L, Gowland PA, McIntyre CW (2016) Patients with CKD have abnormal upper gastro-intestinal tract digestive function: a study of uremic enteropathy. J Gastroenterol Hepatol
Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS (2016) Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis 67:483–498
Vaziri ND, Zhao YY, Pahl MV (2016) Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant 31:737–746
Andersen K, Kesper MS, Marschner JA, Konrad L, Ryu M, Kumar Vr S, Kulkarni OP, Mulay SR, Romoli S, Demleitner J, Schiller P, Dietrich A, Muller S, Gross O, Ruscheweyh HJ, Huson DH, Stecher B, Anders HJ (2016) Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD-related systemic inflammation. J Am Soc Nephrol
Vaziri ND, Yuan J, Norris K (2013) Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol 37:1–6
Khouzam N, Wesseling-Perry K (2017) Pathophysiology and treatment of cardiovascular disease in pediatric chronic kidney disease. Pediatr Nephrol
Mitsnefes MM (2012) Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 23:578–585
Kracht D, Shroff R, Baig S, Doyon A, Jacobi C, Zeller R, Querfeld U, Schaefer F, Wuhl E, Schmidt BM, Melk A (2011) Validating a new oscillometric device for aortic pulse wave velocity measurements in children and adolescents. Am J Hypertens 24:1294–1299
Doyon A, Kracht D, Bayazit AK, Deveci M, Duzova A, Krmar RT, Litwin M, Niemirska A, Oguz B, Schmidt BM, Sozeri B, Querfeld U, Melk A, Schaefer F, Wuhl E (2013) Carotid artery intima-media thickness and distensibility in children and adolescents: reference values and role of body dimensions. Hypertension 62:550–556
Schaefer F, Doyon A, Azukaitis K, Bayazit A, Canpolat N, Duzova A, Niemirska A, Sozeri B, Thurn D, Anarat A, Ranchin B, Litwin M, Caliskan S, Candan C, Baskin E, Yilmaz E, Mir S, Kirchner M, Sander A, Haffner D, Melk A, Wuhl E, Shroff R, Querfeld U, Consortium CS (2017) Cardiovascular phenotypes in children with CKD: the 4C study. Clin J Am Soc Nephrol 12:19–28
Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB (2000) Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342:1478–1483
Doyon A, Fischer DC, Bayazit AK, Canpolat N, Duzova A, Sozeri B, Bacchetta J, Balat A, Buscher A, Candan C, Cakar N, Donmez O, Dusek J, Heckel M, Klaus G, Mir S, Ozcelik G, Sever L, Shroff R, Vidal E, Wuhl E, Gondan M, Melk A, Querfeld U, Haffner D, Schaefer F, Consortium CS (2015) Markers of bone metabolism are affected by renal function and growth hormone therapy in children with chronic kidney disease. PLoS One 10:e0113482
Querfeld U, Anarat A, Bayazit AK, Bakkaloglu AS, Bilginer Y, Caliskan S, Civilibal M, Doyon A, Duzova A, Kracht D, Litwin M, Melk A, Mir S, Sozeri B, Shroff R, Zeller R, Wuhl E, Schaefer F (2010) The cardiovascular comorbidity in children with chronic kidney disease (4C) study: objectives, design, and methodology. Clin J Am Soc Nephrol 5:1642–1648
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Desvarieux M, Ebrahim S, Fatar M, Hernandez Hernandez R, Kownator S, Prati P, Rundek T, Taylor A, Bornstein N, Csiba L, Vicaut E, Woo KS, Zannad F (2004) Mannheim intima-media thickness consensus. Cerebrovasc Dis 18:346–349
Chinali M, Emma F, Esposito C, Rinelli G, Franceschini A, Doyon A, Raimondi F, Pongiglione G, Schaefer F, Matteucci MC (2016) Left ventricular mass indexing in infants, children, and adolescents: a simplified approach for the identification of left ventricular hypertrophy in clinical practice. J Pediatr 170:193–198
Thurn D, Doyon A, Sozeri B, Bayazit AK, Canpolat N, Duzova A, Querfeld U, Schmidt BM, Schaefer F, Wuhl E, Melk A (2015) Aortic pulse wave velocity in healthy children and adolescents: reference values for the Vicorder device and modifying factors. Am J Hypertens 28:1480–1488
Group KW (2009) KDOQI clinical practice guideline for nutrition in children with CKD: 2008 update. Executive summary. Am J Kidney Dis 53:S11–S104
Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A (2012) Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23:1258–1270
Rossi M, Campbell K, Johnson D, Stanton T, Pascoe E, Hawley C, Dimeski G, McWhinney B, Ungerer J, Isbel N (2014) Uraemic toxins and cardiovascular disease across the chronic kidney disease spectrum: an observational study. Nutr Metab Cardiovasc Dis 24:1035–1042
Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G (2014) The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol 25:1897–1907
Lau WL, Savoj J, Nakata MB, Vaziri ND (2018) Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Clin Sci (Lond) 132:509–522
Ryu JH, Park H, Kim SJ (2017) The effects of indoxyl sulfate-induced endothelial microparticles on neointimal hyperplasia formation in an ex vivo model. Ann Surg Treat Res 93:11–17
Carmona A, Aguera ML, Luna-Ruiz C, Buendia P, Calleros L, Garcia-Jerez A, Rodriguez-Puyol M, Arias M, Arias-Guillen M, de Arriba G, Ballarin J, Bernis C, Fernandez E, Garcia-Rebollo S, Mancha J, Del Peso G, Perez E, Poch E, Portoles JM, Rodriguez-Puyol D, Sanchez-Villanueva R, Sarro F, Torres A, Martin-Malo A, Aljama P, Ramirez R, Carracedo J (2017) Markers of endothelial damage in patients with chronic kidney disease on hemodialysis. Am J Physiol Renal Physiol 312:F673–f681
Pletinck A, Glorieux G, Schepers E, Cohen G, Gondouin B, Van Landschoot M, Eloot S, Rops A, Van de Voorde J, De Vriese A, van der Vlag J, Brunet P, Van Biesen W, Vanholder R (2013) Protein-bound uremic toxins stimulate crosstalk between leukocytes and vessel wall. J Am Soc Nephrol 24:1981–1994
Wakamatsu T, Yamamoto S, Ito T, Sato Y, Matsuo K, Takahashi Y, Kaneko Y, Goto S, Kazama JJ, Gejyo F, Narita I (2018) Indoxyl sulfate promotes macrophage IL-1beta production by activating aryl hydrocarbon receptor/NF-kappa/MAPK cascades, but the NLRP3 inflammasome was not activated. Toxins (Basel) 10
Dou L, Poitevin S, Sallee M, Addi T, Gondouin B, McKay N, Denison MS, Jourde-Chiche N, Duval-Sabatier A, Cerini C, Brunet P, Dignat-George F, Burtey S (2018) Aryl hydrocarbon receptor is activated in patients and mice with chronic kidney disease. Kidney Int 93:986–999
Drueke TB, Massy ZA (2016) Changing bone patterns with progression of chronic kidney disease. Kidney Int 89:289–302
Yamamoto S, Fukagawa M (2017) Uremic toxicity and bone in CKD. J Nephrol 30:623–627
Shafi T, Meyer TW, Hostetter TH, Melamed ML, Parekh RS, Hwang S, Banerjee T, Coresh J, Powe NR (2015) Free levels of selected organic solutes and cardiovascular morbidity and mortality in hemodialysis patients: results from the retained organic solutes and clinical outcomes (ROSCO) investigators. PLoS One 10:e0126048
Shafi T, Sirich TL, Meyer TW, Hostetter TH, Plummer NS, Hwang S, Melamed ML, Banerjee T, Coresh J, Powe NR (2017) Results of the HEMO study suggest that p-cresol sulfate and indoxyl sulfate are not associated with cardiovascular outcomes. Kidney Int 92:1484–1492
Kortman GAM, Reijnders D, Swinkels DW (2017) Oral iron supplementation: potential implications for the gut microbiome and metabolome in patients with CKD. Hemodial Int 21(Suppl 1):S28–S36
Drueke T, Witko-Sarsat V, Massy Z, Descamps-Latscha B, Guerin AP, Marchais SJ, Gausson V, London GM (2002) Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation 106:2212–2217
Kshirsagar AV, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Brookhart MA (2013) Intravenous iron supplementation practices and short-term risk of cardiovascular events in hemodialysis patients. PLoS One 8:e78930
Snauwaert E, Van Biesen W, Raes A, Glorieux G, Van Bogaert V, Van Hoeck K, Coppens M, Roels S, Vande Walle J, Eloot S (2017) Concentrations of representative uraemic toxins in a healthy versus non-dialysis chronic kidney disease paediatric population. Nephrol Dial Transplant
Hyun HS, Paik KH, Cho HY (2013) p-Cresyl sulfate and indoxyl sulfate in pediatric patients on chronic dialysis. Kor J Pediatr 56:159–164
Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Giebultowicz J, Wroczynski P, Paczek L (2017) Age-dependent increase in serum levels of indoxyl sulphate and p-cresol sulphate is not related to their precursors: tryptophan and tyrosine. Geriatr Gerontol Int 17:1022–1026
Viaene L, Thijs L, Jin Y, Liu Y, Gu Y, Meijers B, Claes K, Staessen J, Evenepoel P (2014) Heritability and clinical determinants of serum indoxyl sulfate and p-cresyl sulfate, candidate biomarkers of the human microbiome enterotype. PLoS One 9:e79682
Deguchi T, Kusuhara H, Takadate A, Endou H, Otagiri M, Sugiyama Y (2004) Characterization of uremic toxin transport by organic anion transporters in the kidney. Kidney Int 65:162–174
Meijers BK, De Loor H, Bammens B, Verbeke K, Vanrenterghem Y, Evenepoel P (2009) p-Cresyl sulfate and indoxyl sulfate in hemodialysis patients. Clin J Am Soc Nephrol 4:1932–1938
Rossi M, Johnson DW, Xu H, Carrero JJ, Pascoe E, French C, Campbell KL (2015) Dietary protein-fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutr Metab Cardiovasc Dis 25:860–865
Poesen R, Mutsaers HA, Windey K, van den Broek PH, Verweij V, Augustijns P, Kuypers D, Jansen J, Evenepoel P, Verbeke K, Meijers B, Masereeuw R (2015) The influence of dietary protein intake on mammalian tryptophan and phenolic metabolites. PLoS One 10:e0140820
Black AP, Anjos JS, Cardozo L, Carmo FL, Dolenga CJ, Nakao LS, de Carvalho FD, Rosado A, Carraro Eduardo JC, Mafra D (2018) Does low-protein diet influence the uremic toxin serum levels from the gut microbiota in nondialysis chronic kidney disease patients? J Ren Nutr
Nigam SK, Bush KT, Martovetsky G, Ahn SY, Liu HC, Richard E, Bhatnagar V, Wu W (2015) The organic anion transporter (OAT) family: a systems biology perspective. Physiol Rev 95:83–123
Bush KT, Wu W, Lun C, Nigam SK (2017) The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut-liver-kidney axis. J Biol Chem 292:15789–15803
Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC, Bhatnagar V (2015) Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol 10:2039–2049
Borghi C, Rosei EA, Bardin T, Dawson J, Dominiczak A, Kielstein JT, Manolis AJ, Perez-Ruiz F, Mancia G (2015) Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens 33:1729–1741 discussion 1741
Gryp T, Vanholder R, Vaneechoutte M, Glorieux G (2017) p-Cresyl sulfate. Toxins (Basel) 9
Eloot S, Van Biesen W, Roels S, Delrue W, Schepers E, Dhondt A, Vanholder R, Glorieux G (2017) Spontaneous variability of pre-dialysis concentrations of uremic toxins over time in stable hemodialysis patients. PLoS One 12:e0186010
Sekula P, Goek ON, Quaye L, Barrios C, Levey AS, Romisch-Margl W, Menni C, Yet I, Gieger C, Inker LA, Adamski J, Gronwald W, Illig T, Dettmer K, Krumsiek J, Oefner PJ, Valdes AM, Meisinger C, Coresh J, Spector TD, Mohney RP, Suhre K, Kastenmuller G, Kottgen A (2016) A metabolome-wide association study of kidney function and disease in the general population. J Am Soc Nephrol 27:1175–1188
Goek ON, Prehn C, Sekula P, Romisch-Margl W, Doring A, Gieger C, Heier M, Koenig W, Wang-Sattler R, Illig T, Suhre K, Adamski J, Kottgen A, Meisinger C (2013) Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol Dial Transplant 28:2131–2138
Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, Yang Q, Cheng S, Pierce K, Deik A, Souza AL, Farrell L, Domos C, Yeh RW, Palacios I, Rosenfield K, Vasan RS, Florez JC, Wang TJ, Fox CS, Gerszten RE (2013) A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol 24:1330–1338
Rhee EP, Clish CB, Wenger J, Roy J, Elmariah S, Pierce KA, Bullock K, Anderson AH, Gerszten RE, Feldman HI (2016) Metabolomics of chronic kidney disease progression: a case-control analysis in the chronic renal insufficiency cohort study. Am J Nephrol 43:366–374
Atherton JG, Hains DS, Bissler JJ, Pendley BD, Lindner E (2018) Generation, clearance, toxicity and monitoring possibilities of unaccounted uremic toxins for improved dialysis prescriptions. Am J Physiol Renal Physiol
Deltombe O, Van Biesen W, Glorieux G, Massy Z, Dhondt A, Eloot S (2015) Exploring protein binding of uremic toxins in patients with different stages of chronic kidney disease and during hemodialysis. Toxins (Basel) 7:3933–3946
Borges NA, Carmo FL, Stockler-Pinto MB, de Brito JS, Dolenga CJ, Ferreira DC, Nakao LS, Rosado A, Fouque D, Mafra D (2017) Probiotic supplementation in chronic kidney disease: a double-blind, randomized, placebo-controlled trial. J Ren Nutr
Funding
This study was made possible by grants from the European Renal Association-European Dialysis and Transplant Association (www.era-edta.org), the Kuratorium für Dialyse und Nierentransplantation (KfH) Foundation for Preventive Medicine, the German Federal Ministry of Education and Research (reference no. 01EO0802), and Pfizer Deutschland GmbH. The entire study was solely initiated and performed by the investigators of the 4C study group. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was partly funded by Pfizer Deutschland GmbH. There are no patents, products in development, or marketed products to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Online Resource 1
(DOCX 161 kb)
Online Resource 2
(DOCX 24 kb)
Online Resource 3
(DOCX 24 kb)
Online Resource 4
(DOCX 121 kb)
Online Resource 5
(DOCX 25 kb)
Online Resource 6
(DOCX 23 kb)
Online Resource 7
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Holle, J., Querfeld, U., Kirchner, M. et al. Indoxyl sulfate associates with cardiovascular phenotype in children with chronic kidney disease. Pediatr Nephrol 34, 2571–2582 (2019). https://doi.org/10.1007/s00467-019-04331-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-019-04331-6