Abstract
Background
The therapeutic potential of adult stem cells in the treatment of chronic diseases is becoming increasingly evident. In the present study, we sought to assess whether treatment with mesenchymal stem cells (MSCs) efficiently retards progression of chronic renal failure (CRF) when administered to experimental models of less severe CRF.
Methods
We used two renal mass reduction models to simulate different stages of CRF (5/6 or 2/3 mass renal reduction). Renal functional parameters measured were serum creatinine (SCr), creatinine clearance (CCr), rate of decline in CCr (RCCr), and 24-h proteinuria (PT24h). We also evaluated renal morphology by histology and immunohistochemistry. MSCs were obtained from bone marrow aspirates and injected into the renal parenchyma of the remnant kidneys of both groups of rats with CRF (MSC5/6 or MSC2/3).
Results
Animals from groups MSC5/6 and CRF2/3 seemed to benefit from MSC therapy because they showed significantly reduction in SCr and PT24h, increase in CCr and slowed the RCCr after 90 days. Treatment reduced glomerulosclerosis but significant improvement did occur in the tubulointerstitial compartment with much less fibrosis and atrophy. MSC therapy reduced inflammation by decreasing macrophage accumulation proliferative activity (PCNA-positive cells) and fibrosis (α-SM-actin). Comparisons of renal functional and morphological parameters responses between the two groups showed that rats MSC2/3 were more responsive to MSC therapy than MSC5/6.
Conclusion
This study showed that MSC therapy is efficient to retard CRF progression and might be more effective when administered during less severe stages of CRF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progressive deterioration observed in chronic kidney disease is the consequence of a series of inflammatory events leading to glomerulosclerosis, interstitial cell infiltration, tubular atrophy, activation of fibrogenic factors, and formation of fibrosis [1]. Since the process of renal repair depends on the severity and extent of kidney injury and the amount of resident stem cells, the recent development of cell therapy aiming to restore or replace chronically injured tissues brought hope for treatment of many chronic diseases [2, 3]. However, in addition to the paucity of reports using mesenchymal stem cells (MSCs) as regenerative therapy for chronic renal failure (CRF), comparisons among results are jeopardized by discrepancies such as the use of different models of renal damage, various amounts of cells administered, and differences in the routes of cell administration [2–7].
The 5/6 nephrectomized rat is the classical model used to simulate human CRF, and is therefore employed in most publications seeking to investigate the effects of cell therapy. However, it causes a significant reduction in nephron number, which in turn results in severe damage and advanced-stage CRF [8, 9]. To our knowledge, there are no reports regarding the efficacy of cell therapy when administered in less severe stages of CRF. We hypothesized that severe reduction of renal mass might compromise the regenerative capacity of the kidney, which would affect cell therapeutic results [10]. To address this issue, we evaluated the ability of MSCs to retard CRF progression when administered to rats with less severe kidney damage (CRF2/3), and endeavored to compare our results with the classical CRF model (CRF5/6).
Materials and methods
Animals
Animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Medical School (FAMERP). Adult female Wistar rats (n = 40 total) weighing 250–300 g underwent 5/6 and 2/3 renal mass reduction. All animals were maintained on standard rat chow and water ad libitum.
Chronic renal failure models
The 5/6 renal mass reduction model was created as previously described to experimentally induce severe CRF. To simulate a less severe CRF model, we reduced total renal mass by two-thirds (the 2/3 CRF model) according to our previous observations [11]. Briefly, female rats were administered ketamine hydrochloride (50 mg/kg) and xilazine (10 mg/kg) anesthesia, and infarction of approximately one-third of the left kidney was performed by microsurgical ligation of one branch of the left renal artery, followed by right-side uninephrectomy [12].
Isolation and characterization of MSCs
MSCs were isolated from the femur and tibiae of male Wistar rats. After bone marrow cells were collected by flushing, nucleated cells were isolated with a density gradient Ficoll-Hypaque (Gibco) and resuspended in DMEM medium (Gibco) supplemented with 1 % penicillin–streptomycin (Invitrogen, Carlsbad, USA) and 10 % fetal bovine serum (FBS; Cultilab, Campinas, Brazil). Cells were incubated (37 °C in 5 % CO2) for 14 days as primary culture. MSCs were recovered by taking advantage of their tendency to adhere tightly to plastic; nonadherent cells were removed by washing. Flow cytometry analyses (FACS Canto; Becton–Dickinson, San Jose, CA) were performed for CD31, CD44, CD90, CD45, CD31, and CD34 (Caltag Laboratories, Carlsbad, CA). We tested the cells’ differentiation potential by assessing their potential for adipogenic and osteogenic differentiation, as previously described [13].
Experimental protocols
Animals were divided according to the amount of renal mass reduced (2/3 and 5/6 models) and further subdivided into 4 groups of 10 animals each according to the amount of renal mass reduced (CRF2/3 or CRF5/6) and treatment given (MSC2/3 or MSC5/6). We assessed renal function by measuring serum creatinine (SCr), creatinine clearance (CCr), and 24-h proteinuria (PT24h) at baseline and at 90 days after surgery. CRF progression was measured by the rate of decline in the CCr (RCCr; mL/min/day). On the day of surgery, MSCs at a concentration of 1 × 106 cells in 0.15 mL of medium were injected into the boundaries of infarcted and healthy parenchyma of the remnant kidney. Culture medium (0.15 mL) was injected into rats in the control group. At the end of 90 days, animals were weighted and killed.
Histological and immunohistochemical analysis
Coronal sections of the kidney were immersion-fixed in paraformaldehyde solution and embedded in paraffin. Light microscopy was performed on 3-mm sections of tissue stained with hematoxylin, eosin (HE), and Masson trichrome to assess glomerulosclerosis (GS) and Tubulointerstitial fibrosis (TI). For grading the induced GS, the numbers of glomeruli forming crescent were counted in a blinded fashion. GS was defined as glomeruli with sclerosis or mesangial expansion and/or focal hyalinosis with tuft adherence. A minimum of 50 glomeruli per rat kidney were evaluated, and the mean value was used as representative for the rats. Glomerular sections were assessed by standard semiquantitative analysis and expressed as the glomerular sclerosis index (GSI) [14]. The extent of GS was graded from 0 to 4 by a semiquantitiative (score: 0, normal glomeruli; 1, mesangial thickening of <25 % of the tuft; 2, moderate GS-mesangial proliferation and thickening up to 50 %; 3, severe GS-obliteration of capillaries and diffuse sclerosis up to 75 %; and 4, complete capillary obliteration and thrombosis with global sclerosis up to 100 %).
TI damage was evaluated using a semiquantitative analysis of 20 cortical fields according to Veniant el al. Lesions were graded from 0 to 4 according to the area with tubulointerstitial changes (tubular atrophy, casts, interstitial inflammation, and fibrosis) [15].
The score index in each rat was expressed as a mean value of all scores obtained. All the histological analyses were performed by an observer unaware of the treatment received by each group.
Immunohistochemistry analysis was performed as previously described [16]. Briefly, the sections were incubated with the following antibodies for immunohistochemical studies: anti-α-SM-actin (Dako, Glostrup, Denmark), and anti-proliferating cell nuclear antigen (PCNA) (Sigma, St. Louis, Mo., USA) or anti-ED1 antibody for 30 min at room temperature. The reaction product was detected with an avidin–biotin–peroxidase complex (Vector Laboratories, Burlingame, CA, USA). The material was counterstained with Hematoxylin, dehydrated and mounted. Nonspecific protein binding was blocked by incubation with 20 % goat serum in phosphate buffered saline (PBS) for 20 min. Negative controls consisted of replacement of primary antibody with normal rabbit IgG or mouse IgG for polyclonal and monoclonal antibodies, respectively, at equivalent concentrations.
For evaluation of immunoperoxidase staining for α-SM-actin, each grid field was graded semi-quantitatively and the mean score per kidney was calculated. Each score reflected mainly changes in the extent, rather than the intensity, of staining and depended on the percentage of grid field showing positive staining: 0 = absent or less than 5 %; I = 5–25 %; II = 25–50 %; III = 50–75 %; IV > 75 %.
The numbers of ED1-positive and PCNA-positive cells in each section were calculated by counting the number of positive cells in 30 sequential (0.245-mm2) grid fields from the renal cortex [17].
Statistical analysis
Data are presented as mean values ± standard deviations. Statistical differences between groups were calculated using analysis of variance (ANOVA) using Bonferroni post-test analysis (GraphPad Prism Software, San Diego, CA). P < 0.05 was considered statistically significant.
Results
Evaluating CRF severity of 2/3 and 5/6 mass reduction models
Rats with CRF2/3 exhibited significantly less functional renal damage than CRF5/6 animals as measured by SCr, CCr, and PT24h (Table 1). After 90 days, renal function in CRF5/6 animals had declined CCr at a rate of 0.0073 ± 0.002 mL/min/day, 38 % faster than in the CRF2/3 group (0.0053 ± 0.002 mL/min/day; P = 0.04) (Table 1).
Effects of MSC treatment in both models
MSC treatment was equally effective in both CRF models. It reduced SCr and PT24h, increased CCr, and slowed RCCr (Table 1). However, while the CCr of CRF5/6 rats increased by 61.5 %, the CCr of CRF2/3 rats increased by 97 %. Similarly, MSC treatment slowed the RCCr of CRF2/3 rats by 60 % (−0.003 ml/min/day) versus 28.5 % (−0.002 ml/min/day) in the CRF5/6 group, while MSC reduced PT24h in the group with severe CRF, it did not significantly decrease in CRF2/3 rats, probably because the less severe disease already presented with reduced baseline values and fewer glomerular lesions.
Histopathological and immunohistochemical findings
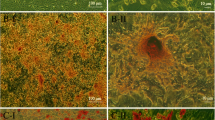
As shown in Figs. 1 and 2, histological semiquantitative analysis showed that tubule interstitial damage and glomerulosclerosis were significantly less severe following MSC therapy in both mass reduction models. However, efficacy of therapy with MSC was much more pronounced in the tubulointerstitial compartment.
Light micrographs of the kidney sections stained with Masson’s trichrome (×200) reagent. a, b Kidney sections from CRF2/3 and CRF5/6 group showed histological features of chronic renal injury, consisting of tubular dilatation and atrophy with thickening of basement membrane, interstitial lymphocyte infiltrates, and tubulointerstitial fibrosis. c, d The renal morphology of CRF2/3 and 5/6 after treatment with MSCs showing marked reduction of chronic renal injury, less interstitial lymphocyte infiltrates, tubular dilatation, and tubulointerstitial fibrosis when compared with the untreated CRF group
Monocytes/macrophages and PCNA
The accumulation of macrophages (ED-1 positive cells) was significantly reduced in rats with less severe chronic kidney disease (CRF2/3 = 9 ± 3 vs. CRF5/6 = 37 ± 10 ED-1–positive cells; p = 0.001) as well as it was the number of PCNA-positive cells (CRF2/3 = 2.4 ± 1 vs. CRF5/6 = 8.3 ± 1 PCNA-positive cells; p = 0.0002) (Fig. 3a, b).
Immunohistochemistry of renal tissue from 2/3 and 5/6 nephrectomized rats. a Immunolocalization of ED-1 positive cells; b immunolocalization of PCNA and c staining for α-SMA. Data are expressed as mean ± SD (*p < 0.01 CRF2/3 vs. CRF5/6, **p < 0.001 CRF5/6 vs. MSC5/6, ***p < 0.001 Sham vs. CRF5/6, • p < 0.01 CRF2/3 vs. MSC2/3, # p < 0.05 Sham vs. CRF5/6)
Treatment with MSC decreased the number of ED-1 positive cells in the CRF2/3 model (Sham = 2.3 ± 0.78 vs. CRF2/3 = 9 ± 3 vs. MSC2/3 = 6 ± 1 p = 0.006), but had no effect on PCNA expression (Figs. 3a, b, 4).
In contrast, a significant decrease in the number of ED-1 positive cells and in the expression of PCNA were observed following MSC treatment in 5/6-nephrectomized rats (p < 0.01) (Figs. 3, 4).
α-Smooth muscle-actin (α-SM-actin)
Staining for α-SM-actin was proportional to the severity of CRF. Much lower expression of α-SM-actin occurred in the CRF2/3 model when compared to the 5/6 model. Treatment with MSC was equally effective at reducing α-SMA expression in both CRF2/3 and CRF5/6 animals (p < 0.05).
Interestingly, efficacy of treatment was possible to be found in CRF2/3 rats in spite of the low expression of this protein in this model (Figs. 3c, 4).
Discussion
Others and we have previously shown that administering MSCs into a chronically damaged kidney improved renal function and retarded progression of chronic kidney disease in 5/6 nephrectomized rats, in part by reducing intra-renal inflammation and suppressing fibrosis [2–7]. Because the severity and extent of kidney injury might influence the number of resident stem cells present in the injured tissue, it is rational to hypothesize that the entire process of renal repair triggered by cell therapy might be influenced by the size of the remnant renal mass [1, 18]. Another important aspect of MSCs’ function is their role in the stabilization of endothelial cells (and hence the microcirculatory vascular bed). CRF is typically associated with microvascular rarefaction and ischemia. Therefore, it is possible that a greater amount of healthy tissue is associated with greater therapeutic effects of the MSCs in the kidney caused by their paracrine effect and new blood vessel formation. Therefore, in the present study we tested whether the amount of remnant renal mass would affect the efficacy of cellular therapy and whether cell therapy might be more effective if administered during a less severe disease stage.
The present study confirms our previous work showing that MSC therapy benefitted rats with severe CRF, and also demonstrates that animals sustained by a remnant renal mass (CRF2/3) twice the size of the traditional 5/6 model can also benefit from MSC therapy. The two CRF models used in the study demonstrated significant differences regarding renal function as a consequence of the renal mass reduction. As expected, CRF2/3 rats presented lower SCr and PT24h levels, better CCr, and slower RCCr than 5/6 nephrectomized rats at baseline and at 90 days after the procedure. Although MSC treatment improved renal functional parameters in the two models, CRF2/3 rats exhibited greater improvement than classical CRF5/6 rats. While an increase of 61.5 % was observed in the CCr of MSC-treated CRF5/6 rats, the same treatment nearly doubled the CCr of CRF2/3 rats. Similarly, cell therapy mildly reduced the RCCr of CRF5/6 rats, but reduced the rate of disease progression by 57 % in CRF2/3 rats. These different results might be caused by the capacity of MSCs to promote repair in response to an inflammatory reaction within the local microenvironment, the number of the host stem cell niche, and ultimately the amount of tissue damage resulting from the renal mass reduction [19, 20].
It has been suggested that MSCs can reduce renal injury and facilitate tissue repair by upregulating the expression of anti-inflammatory cytokines and chemokines by the host infiltrating macrophages, which results in reduced glomerulosclerosis and fibrosis [21, 22]. In fact, comparisons of the morphological findings between the two CRF models revealed greater chronicity (glomerulosclerosis, tubular atrophy, and fibrosis) in CRF5/6 rats than in CRF2/3 rats (Fig. 1). The amelioration of renal function after treatment with MSCs was also associated with significant improvements in the histological features of both groups of CRF animals. In agreement with the histological features immunohistochemistry evaluation showed that rats CRF2/3 have significantly less macrophages and PCNA when compared with animals CRF5/6 group. Treatment with MSC significantly reduced the number of macrophages and the expression of α-SMA in both models. Treatments reduced the PCNA only in animals with CRF5/6, which was probably a consequence of more inflammation and tissue damage in this model.
Conclusion
In conclusion, our results offer strong evidence that cell therapy might be more effective if administered during less severe stages of CRF. However, we understand that many issues remain to be resolved before we can draw any definite conclusions with respect to the therapeutic potential of MSCs.
References
Romagnani P, Kalluri R. Possible mechanisms of kidney repair. Fibrogenesis. Tissue Repair. 2009;26:1–10.
Caldas HC, Fernandes IM, Gerbi F, Souza AC, Baptista MA, Ramalho HJ, Kawasaki-Oyama RS, Goloni-Bertollo EM, Pavarino-Bertelli EC, Braile DM, Abbud-Filho M. Effect of whole bone marrow cell infusion in the progression of experimental chronic renal failure. Transplant Proc. 2008;40:853–5.
Cavaglieri RC, Martini D, Sogayar MC, Noronha IL. Mesenchymal stem cells delivered at the subcapsule of the kidney ameliorate renal disease in the rat remnant kidney model. Transplant Proc. 2009;41:947–51.
Roessger A, Denk L, Minuth WW. Potential of stem/progenitor cell cultures within polyester fleeces to regenerate renal tubules. Biomaterials. 2009;30:3723–855.
Semedo P, Correa-Costa M, Cenedeze MA, Malheiros DM, Reis MA, Shimizu MH, Seguro AC, Pacheco-Silva A, Saraiva Camara NO. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27:3063–73.
Alexandre CS, Volpini RA, Shimizu MH, Sanches TR, Semedo P, Jura DIVL, Câmara NO, Seguro AC, Andrade L. Lineage-negative bone marrow cells protect against chronic renal failure. Stem Cells. 2009;27:682–92.
Choi S, Park M, Kim J, Hwang S, Park S, Lee Y. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev. 2009;18:521–9.
Gabizon D, Goren E, Shaked U, Averbukh Z, Rosenmann E, Modai D. Induction of chronic renal failure in the mouse: a new model. Nephron. 1985;40:349–52.
Szabo AJ, Muller V, Chen GF, Samsell LJ, Erdely A, Baylis C. Nephron number determines susceptibility to renal mass reduction-induced CRF in Lewis and Fisher 344 rats: implications for development of experimentally induced chronic allograft nephropathy. Nephron Dial Transplant. 2008;23:2492–5.
Hishikawa K, Fujita T. Stem cells and kidney disease. Hypertens Res. 2006;29:745–9.
Hostetter TH. Progression of renal disease and renal hypertrophy. Annu Rev Physiol. 1995;57:263–78.
Caldas HC, Fernandes IM, Kawasaki-Oyama RS, Baptista MA, Plepis AM, Martins VA, Coimbra TM, Goloni-Bertollo EM, Braile DM, Abbud-Filho M. Effect of stem cells seeded onto biomaterial on the progression of experimental chronic kidney disease. Exp Biol Med. 2011;236:746–54.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, SimonettI DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;5411:143–7.
Vogelbacher R, Wittmann S, Braun A, Daniel C, Hugo C. The mTOR inhibitor everolimus induces proteinuria and renal deterioration in the remnant kidney model in the rat. Transplantation. 2007;84:1492–9.
Veniant M, Heudes D, Clozel JP, Bruneval P, Menard J. Calcium blockade versus ACE inhibition in clipped and unclipped kidneys of 2K-1C rats. Kidney Int. 1994;46:421–9.
Curtis JJ, Rakowski TA, Argy WP Jr, Schreiner GE. Evaluation of percutaneous kidney biopsy in advanced renal failure. Nephron. 1976;17:259–69.
Coimbra TM, Janssen U, Grone HJ, Ostendorf T, Kunter U, Schmidt H, Brabant G, Floege J. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int. 2000;57:167–82.
Remuzzi A, Gagliardini E, Sangalli F, Bonomelli M, Piccinelli M, Benigni A, Remuzzi G. ACE inhibition reduces glomerulosclerosis and regenerates glomerular tissue in a model of progressive renal disease. Kidney Int. 2006;69:1124–30.
Walker MR, Patel KK, Stappenbeck TS. The stem cell niche. J Pathol. 2009;217:169.
Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–41.
Morigi M, Imberti B, Zoja C, Corna D, TomasonI S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, AlisoN M, Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–804.
Duffield JS, Bonventre JV. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int. 2005;68:1956–61.
Acknowledgments
This work was supported by the Coordination of Improvement of Higher Education Personnel (CAPES) and FAMERP/FUNFARME.
Conflict of interest
All the authors declared no competing interests.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Caldas, H.C., de Paula Couto, T.A.P., Fernandes, I.M.M. et al. Comparative effects of mesenchymal stem cell therapy in distinct stages of chronic renal failure. Clin Exp Nephrol 19, 783–789 (2015). https://doi.org/10.1007/s10157-015-1079-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-015-1079-1