Abstract
Acute kidney injury (AKI) is a significant source of morbidity and mortality in pediatric patients, affecting more than one quarter of critically ill children. Despite significant need, there are no targeted therapies to reliably prevent or treat AKI. Recent advances in our understanding of renal injury and repair signaling pathways have enabled the development of several targeted pharmaceuticals. Here we review emerging pharmacotherapies for AKI that are currently in clinical trials. Categorized by their general mechanism of action, the therapies discussed include anti-inflammatory agents (recAP, AB103, ABT-719), antioxidants (iron chelators, heme arginate), vasodilators (levosimendan), apoptosis inhibitors (QPI-1002), and repair agents (THR-184, BB-3, mesenchymal stem cells).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is a significant cause of morbidity and mortality in the pediatric population, the extent of which has been better characterized in recent years with the standardization of serum creatinine and urine output-based definitions [1]. Using Kidney Disease: Improving Global Outcomes (KDIGO) creatinine-based criteria, the incidence of AKI in nonacute pediatric hospital patients was 5% [2]. The incidence in critically ill patients is much higher: in a recent multinational prospective study evaluating critically ill children, 27% of patients were diagnosed with AKI, 11% of whom were severe, as indicated by KDIGO stage 2 or greater [3]. Patients with AKI have longer hospital stays and higher health-care expenditures, and AKI severity confers a step-wise increase in mortality [3, 4]. Furthermore, there is emerging evidence that pediatric AKI confers an increased risk of developing chronic kidney disease (CKD) [5,6,7].
The most common etiologies of AKI in pediatric patients are renal ischemia, nephrotoxic medications, and sepsis [8]. The frequency of AKI in hospitalized children can be successfully decreased by systematically reducing nephrotoxic exposures [9]. However, beyond thoughtful medication selection and supportive hemodynamic optimization, there are no targeted therapies to prevent or treat kidney injury. Several agents that have been used in the past, including dopamine, furosemide, and mannitol, are no longer recommended as routine preventive or therapeutic strategies [10]. Thus, the 13th Acute Dialysis Quality Initiative (ADQI) Consensus Conference in 2014 focused on identifying knowledge gaps and potential new therapeutic targets of human AKI [11,12,13,14].
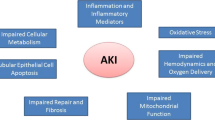
As described by Basile et al., the initiation phase of kidney injury is marked by energetic failure and structural alterations of tubular epithelial cells, including loss of both the brush border and cellular polarity [15] (Fig. 1). Inflammatory cytokines and chemokines are released, and apoptotic and necrotic cells, as well as viable epithelial cells, slough off and cause tubular obstruction. During the extension phase, inflammatory infiltration, oxidative stress, and coagulation activation lead to microvascular congestion, vascular endothelial cell damage, sustained hypoxia, and further amplification of inflammatory cascades. Return of adequate blood flow ushers in the maintenance phase. Cells undergo repair and reorganization, including dedifferentiation, migration, and proliferation. Recovery occurs when cells differentiate and re-establish their polarity, and function returns to normal. Perpetuated injury, dysregulated injury signaling, or maladaptive repair mechanisms can lead to the development of fibrosis and CKD. Advances in our understanding of the renal injury and repair signaling pathways have enabled the development of several targeted pharmaceuticals. This review briefly summarizes the emerging pharmacotherapies for AKI that are currently in, or have recently completed, human clinical trials. They are categorized by their general mechanism of action: anti-inflammatory agents, antioxidants, vasodilators, apoptosis inhibitors, and repair agents (Fig. 1).
A simplified model of the pathophysiology of acute kidney injury (AKI) and targets of emerging pharmacotherapies: Energetic failure and cellular structural disruption trigger local and systemic inflammatory cascades, oxidative stress, activation of cell-death pathways, and renal microvascular circulation dysfunction. Anti-inflammatory and cellular-repair signaling pathways lead to either cellular recovery or maladaptive repair and progressive fibrosis. Emerging pharmacotherapies currently in clinical trials include anti-inflammatory agents, antioxidants, vasodilators, apoptosis inhibitors, and repair agents
Anti-inflammatory agents
Inflammatory cell infiltration is a prominent feature of early AKI, noted within 2 h of ischemic injury [15]. Extension of injury is thought to be due in part to leukocyte adhesion to activated endothelial cells, which may impair blood flow via physical congestion of the lumen, exacerbation of vasoconstriction, and increased intraluminal pressures from interstitial edema [15]. Interrupting inflammatory cascades may preserve the glomerular filtration rate (GFR) and limit the ultimate scope of injury.
Recombinant alkaline phosphatase
Alkaline phosphatase, which is naturally expressed along the brush border of the proximal tubule, reduces renal inflammation via dephosphorylation of extracellular adenosine triphosphate (ATP) and adenosine diphosphate (ADP) to adenosine, which has anti-inflammatory effects [16]. Additionally, just as intestinal alkaline phosphatase detoxifies bacterial endotoxins via dephosphorylation, alkaline phosphatase in the kidney inhibits bacterial activation of proinflammatory toll-like receptor 4 (TLR4) via dephosphorylation of their lipopolysaccharide (LPS) cell membranes [17].
AM-Pharma developed a bovine intestinal alkaline phosphatase, which completed a phase 2b placebo-controlled study in 36 critically ill adults with severe sepsis or septic shock with evidence of AKI [18]. An intravenous bolus followed by a 48-h continuous infusion of either alkaline phosphatase or placebo was initiated within 48 h of onset of AKI. The treatment group recovered normal creatinine clearance in the first 7 days, while the creatinine clearance in the placebo group remained impaired throughout the 28-day follow-up period. Clinical outcomes were supported by laboratory findings of reduced systemic markers of inflammation [C-reactive protein, interleukin-6 (IL-6), LPS-binding protein] and urinary biomarkers of renal damage [kidney injury molecule 1 (KIM-1) and IL-18] in patients treated with alkaline phosphatase vs. controls. There were no safety concerns.
AM-Pharma subsequently developed a recombinant human chimeric alkaline phosphatase, recAP, which contains domains of placental and intestinal alkaline phosphatase. It has completed a phase 1 safety and tolerability study, and enrollment in an adaptive phase IIa/b trial in critically ill adults with highly suspected bacterial sepsis and AKI is currently in progress (NCT02182440). The study is estimated to be complete during the fall of 2017.
CD28 receptor antagonist
The T-cell costimulatory receptor CD28 is critical to the cytokine storm released in response to bacterial toxins. Preclinical work has demonstrated that CD28 receptor blockade attenuates the systemic inflammatory response [19]. Atox Bio Ltd. has completed a phase II safety, pharmacokinetics, and efficacy study with AB103 (previously p2TA), a CD28 receptor antagonist, in adult patients with necrotizing soft tissue infections (NCT01417780). With one dose of medication administered within 6 h of clinical diagnosis, patients were found to have significantly less global organ dysfunction at 14 days than those that received placebo, as represented by Sequential Organ Failure Assessment (SOFA) scores. There were no safety concerns [20].
A phase III randomized control trial is currently enrolling patients down to 12 years of age with necrotizing soft tissue infections (NCT02469857). Although the primary outcome of the study is not AKI prevention, end-organ function will again be evaluated via the SOFA score. Additionally, a secondary outcome measure will be recovery from any AKI by day 28. It is estimated to be completed in 2019.
Alpha-melanocortin-stimulating hormone agonist
Another anti-inflammatory drug target is the alpha melanocortin (α-MSH) 1 receptor. When bound by α-MSH, it exerts anti-inflammatory and organ-protective effects [21]. An α-MSH agonist, ABT-719 (formerly AP214 acetate), licensed by Abbott/AbbVie, had promising preclinical data, and a phase II trial of 77 patients undergoing cardiopulmonary bypass (CPB) surgery showed a reduced incidence of AKI in patients who received ABT-719 (NCT01256372). However, a phase IIb, multicenter study of ABT-719 in 231 patients with CKD who underwent CPB surgery failed to show a significant reduction in the incidence of AKI in patients who received the drug, as assessed by both serum creatinine measurements and novel biomarkers of tubular injury (NCT01777165) [22]. Potential explanations for the discordance with earlier, promising studies include differences in the pathophysiology or severity of AKI in the animal models versus human, and potentially false positive results in the earlier phase II study due to small sample size (treatment n = 26).
Antioxidants
Poor oxygenation during the initiation of AKI leads to the production of reactive oxygen species, which are thought to play a significant role in AKI initiation and extension via augmenting vasoconstriction and alterations in renal blood [15]. Antioxidants are likely to play a significant protective role in AKI.
Iron chelators
Iron is an essential element, critical to oxygen transport, cellular energy metabolism, and a wide variety of enzymatic reactions. While most iron is intracellular and stored in iron-containing proteins, there is a small extracellular portion that is bound loosely to albumin and low-molecular-weight chelates, such as citrate, acetate, and phosphate [23]. Known as catalytic or labile iron, this small pool circulates, participating in redox reactions by cycling between its ferrous and ferric states. When oxygen is incompletely reduced to water, this iron pool is responsible for converting the resulting hydrogen peroxide to free-radical ions (Fenton’s reaction), which cause oxidative damage [24].
Iron is filtered by the glomerulus and reabsorbed in the renal tubules. In animal models, the amount of catalytic iron in the kidney rapidly increases with a variety of injuries, including ischemia–reperfusion, aminoglycosides, cisplatin, rhabdomyolysis, and contrast exposure [23]. In humans, blood levels of catalytic iron have been used as a surrogate marker for renal levels of catalytic iron and were likewise found to reflect risk for AKI in cardiopulmonary bypass, critical illness, and contrast exposure [23]. Iron chelators have been effective at reducing AKI in animal models. However, trials in adults have been limited in number, size, and positive outcomes. Deferoxamine, a parenteral iron chelator administered alone prior to CPB surgery and with N-acetylcysteine in critically ill patients with sustained hypotension (NCT00870883), did not reduce AKI rates [23, 25]. Results from a phase 2 randomized controlled trial evaluating safety and efficacy of deferiprone, an oral iron chelator for preventing contrast nephropathy during angiography, remain unpublished since study completion in 2011 (NCT01146925).
Even if efficacy could be shown, iron chelation may be limited by the side-effect profiles of this class of medications. These include renal tubular dysfunction and acquired Fanconi syndrome with deferasirox, visual and auditory neurotoxicity with deferoxamine, and arthropathy, neutropenia, agranulocytosis, and hepatic fibrosis with deferiprone [26,27,28,29,30,31]. Attempts at neutralizing the damaging effects of catalytic iron via sequestration by plasma-derived haptoglobin produced mixed results [23]. Other proposed strategies, including increasing sequestration of free iron by ferritin, increasing scavenging of heme from circulation, and regulating heme breakdown via heme oxygenase 1 gene expression are nowhere near clinical application [23].
Heme arginate
Heme oxygenase 1 (HO-1) is an inducible enzyme that exhibits antioxidant effects via rapid breakdown of free heme and generation of protective byproducts, such as carbon monoxide, bilirubin, and ferritin [32]. During AKI, HO-1 exhibits anti-inflammatory effects by regulating immune cell gene expression, maturation, and migration. Additionally, it is a potent regulator of autophagy and has antiapoptotic effects via reduction of cell-cycle-arrest molecule p21 activity. Preclinical studies have shown that HO-1 induction is protective in cisplatin and ischemia–reperfusion renal injury models [33, 34].
Heme arginate (HA), used to treat acute porphyria, is an inducer of HO-1. In a proof-of-concept phase III placebo-controlled trial, 40 adult recipients of deceased donor kidney transplants were randomized to receive either HA or placebo preoperatively and again on postoperative day 2 (NCT01430156) [35]. HA patients demonstrated significant upregulation of HO-1 protein and messenger RNA (mRNA) expression. Although the study was not powered to assess clinical outcomes, there was a trend toward lower levels of urinary AKI biomarkers neutrophil gelatinase-associated lipocalin (NGAL) and KIM-1. However, histopathology on postoperative day 5 did not appear different.
Vasodilators
Early loss of GFR and injury extension are attributed to decreased renal blood flow and hypoxia. Although the extent to which alterations in local and global perfusion may play a role in enhancing later tissue repair, early preservation of filtration and perfusion could limit the extension of injury [11].
Levosimendan
Levosimendan, produced by the Orion Corporation, is a calcium sensitizer with inotropic and vasodilatory effects. It is clinically indicated as an inotrope for acutely decompensated heart failure in patients with low cardiac output, as it increases cardiac contractility while decreasing both pre- and afterload on the heart [36]. It works on vascular smooth muscle by opening ATP-sensitive potassium channels.
In animal models of endotoxemia and ischemic reperfusion, levosimendan appeared to have renoprotective effects [37]. Several small clinical studies have since looked at its ability to prevent AKI after CPB surgery. A meta-analysis of results of 13 trials with a total of 1345 individuals found levosimendan may significantly reduce the incidence of postoperative AKI, need for renal replacement (RRT) therapy, days of mechanical ventilation, days of intensive care, and 30-day mortality [38]. Limitations of the meta-analysis included small sample sizes in the primary studies, different definitions of AKI, different dosing regimens, lack of access to potentially confounding clinical data, potential for biased reporting, and lack of long-term outcome data.
An expert panel recently released a position paper expressing optimism about the pleiotropic effects of levosimendan, encouraging further mechanistic, proof-of-concept, and clinical studies to evaluate possible expanded application of the drug [36]. There are three ongoing randomized trials assessing the impact of levosimendan on renal function. The first, a phase III trial set to complete in December 2017, is comparing the effect of a 75-min infusion of levosimendan versus dobutamine on renal hemodynamics and GFR in adult patients with cardiorenal syndrome (NCT02133105). The second is a phase IV study evaluating the effect of a 3.5-h infusion of levosimendan versus placebo on renal hemodynamics and GFR of adults after CPB surgery (NCT02531724). The third is a phase II study notably enrolling children between 1 and 12 months of age with congenital cardiac disease. It will use serum creatinine levels collected for 4 days post-CPB surgery, as well as 30-day mortality rates, to evaluate whether a 24-h infusion of levosimendan postbypass confers kidney and/or survival benefits over milrinone (NCT02232399). Results are anticipated by the end of 2017.
Apoptosis inhibitors
Cell death via apoptosis, necrosis, and autophagy are prominent arbiters of tubular injury in AKI. The severity and duration of AKI may be related to the proportion of tubular cells that can maintain viability and contribute to the repair process [15].
p53 siRNA
Apoptosis is an important pathogenic mechanism for kidney injury in both ischemia–reperfusion and DNA damage from cisplatin [39, 40]. Induction of the proapoptotic transcription factor p53 is common to both etiologies. Quark Pharmaceuticals, which specializes in RNA interference technology to inhibit gene expression or translation, developed QPI −1002, a small interfering RNA (siRNA) targeted at p53. Preclinical trials showed rapid and specific localization of intravenously administered QPI −1002 to renal proximal tubular cells, significant reduction in p53 gene expression when administered 4 h after bilateral renal clamp, and a 24- to 48-h duration of siRNA effect [41]. Repeated doses of siRNA were also shown to attenuate cisplatin-induced kidney injury.
Quark Pharmaceuticals completed a phase I, dose escalation, safety and pharmacokinetic study of QPI-1002 in adults undergoing major cardiovascular surgery, making it the first siRNA to be systematically administered to humans (NCT00554359). However, their follow-up phase I study targeting cardiovascular surgery patients at high risk of AKI was terminated, citing lack of an available patient population (NCT00683553). There is a phase II, randomized control trial currently ongoing to evaluate the efficacy and safety of a single dose of QPI-1002 in cardiovascular surgery patients at high risk of AKI (NCT02610283). Estimated outcome measures from the trial, which is to be completed in 2018, will include the proportion of subjects who develop AKI in the first 5 postoperative days, and the proportion of individuals who require RRT, have more than 25% decrease in GFR, or die during the 90-day postoperative period.
Quark Pharmaceuticals completed a phase I/II multicenter dose-escalation study for prophylaxis of delayed graft function (DGF) in adults undergoing renal transplant with organ donations after cardiac death, expanded criteria donors, or standard criteria donors with >24 h of cold ischemia time (NCT00802347). Patients treated with QPI-1002, particularly those with older donors, had significantly reduced risk for DGF, increased time to first dialysis, reduced mean duration of dialysis, and reduced number of dialysis sessions required in the first 30 days posttransplant; there were no safety concerns. Recruitment into a phase III randomized controlled trial to evaluate the safety and efficacy of QPI-1002 for reducing the incidence and severity of DGF with kidney allografts from deceased donors (brain death criteria) >45 years old (NCT02610296) is in progress and estimated to end in 2019.
Repair agents
Early activation and augmentation of adaptive repair mechanisms may lessen the severity of AKI, while maladaptive or downregulated repair mechanisms can lead to the development of fibrosis and CKD [15, 42].
BMP receptor agonist
Bone morphogenetic protein-7 (BMP7) is critical for determining the final nephron number and size of developing kidneys [43]. In the mature organ, its roll shifts to that of protection and regeneration. Administration of recombinant BMP7 to animal models of ischemia–reperfusion and obstructive injuries showed blunted AKI and enhanced recovery via decreased inflammation, apoptosis, and fibrosis [44, 45]. However, development of a BMP7 protein for systemic administration was met with production and pharmacokinetic obstacles, and the strategy shifted to that of small-molecule mimetics [46].
THR-123 and THR-184, produced by Thrasos Therapeutics, are agonists of the activin-like kinase-3 receptor (Alk3), the main BMP7 receptor expressed in tubular epithelial cells. Preclinical work with the oral formulation, THR-123, showed it suppressed inflammation, apoptosis, and epithelial-to-mesenchymal transition and reversed fibrosis in five mouse models of acute and chronic renal injury, including ischemia–reperfusion injury, unilateral ureteral obstruction, and nephrotoxic serum-induced nephritis [47]. A multicenter phase II study with THR-184, the intravenous formulation, in which the drug was administered around the time of CPB in patients at high risk for AKI, was completed in 2015 (NCT01830920). There was a reduction in the incidence of AKI in patients treated with the highest of four dosing regimens, and the effect was most prominent in patients with underlying CKD. There were no safety concerns.
Hepatocyte growth factor
Hepatocyte growth factor (HGF) is a pleiotropic growth factor and cytokine that promotes cell proliferation, survival, motility, and differentiation. HGF actions are exerted by binding exclusively to the proto-oncogenic cell-surface receptor c-met, which is expressed in the epithelial cells of many organs during both embryogenesis and adulthood [48]. In the kidney, HGF is secreted by renal mesenchymal cells, with c-met receptors located on both local fibroblasts and tubular epithelial cells. Upregulation of HGF has been observed in the setting of ischemia–reperfusion injury [49]. In preclinical studies, HGF was renoprotective in a variety of animal models of AKI and CKD, exerting anti-inflammatory and regenerative effects and preventing tubular cell apoptosis, epithelial-to-mesenchymal transition, and fibrosis [50,51,52].
BB-3, produced by Angion Biomedica Corporation, is a small molecule with HGF-like activity. In preclinical trials, BB-3 given 24 h after ischemic injury mitigated AKI, as evidenced by lesser changes in urine output, creatinine, and blood urea nitrogen (BUN), reduced urinary KIM-1 and NGAL excretion, and less tubular apoptosis and necrosis on histopathology [53]. In the interim analysis of a multicenter phase II study in renal transplant patients with low or no urine output posttransplant, patients who received three doses of BB-3 had increased urine output, lower serum creatinine, and reduced need for dialysis (NCT01286727). Therefore, the company transitioned to a phase III trial in which all patients receiving a suboptimal kidney received BB-3 infusions postoperatively (NCT02474667). It is estimated to be completed in 2018.
Additionally, a multicenter phase II study to evaluate the safety and efficacy of administering four doses of BB-3 to individuals at high risk of AKI after CBP surgery is currently enrolling (NCT02771509) and is estimated to be completed in 2018.
Mesenchymal stem cells
As described by Peired et al., mesenchymal stem cells (MSC) are fibroblast-like cells capable of self-renewal [54]. Originally discovered in bone marrow, they can be harvested from multiple tissues, including tooth pulp, adipose tissue, umbilical cord scrapings, amniotic fluid, and the kidney. MSC can migrate to areas of injury and modulate local injury response by releasing soluble factors such as interleukins, insulin-like growth factor, and HGF, as well as via microvesicles, which are released by membrane budding and intracytoplasmic multivesicular bodies. These microvesicles contain mRNA, micro-RNA (miRNA), surface receptors, and proteins that can reprogram the activities of other cells.
Preclinical studies in AKI have used MSC harvested from different tissues, as well as microvesicles obtained from cultures of MSC. There have been >600 clinical studies using different forms of MSC in different clinical contexts [54]. In nephrology, these include CKD, focal segmental glomerular sclerosis (FSGS), diabetic kidney disease, lupus nephritis, and kidney transplantation; to date, there have been three studies in AKI [54].
The Mario Negri Institute for Pharmacological Research is aiming to complete a phase I study in adult oncology patients receiving cisplatin who develop AKI as evidenced by an increase in urine NGAL concentration at day 2 post cisplatin infusion (NCT01275612). Patients are being treated with donor ex vivo expanded MSC, with a dose escalation schema to assess safety and efficacy. Estimated date of completion is 2018.
AlloCure Inc. completed a phase I dose-escalation study of postoperatively infused allogenic bone-marrow-derived MSC in patients at high risk of AKI during CPB surgery (NCT00733876). They reported no safety concerns, early and late protection of kidney function, and reduced length of hospital stay and need for readmission [55]. However, the phase II study using their allogenic bone-marrow-derived MSC, AC607, was terminated early reportedly due to no difference in outcomes between therapy and placebo groups, although the full trial report has yet to be published (NCT01602328).
Conclusions
Current treatment of AKI remains largely supportive. However, recent advances in our understanding of renal injury and repair signaling pathways in preclinical models have enabled the development of several promising pharmaceuticals that are currently in human clinical trials. These include anti-inflammatory agents (recAP, AB103, ABT-719), antioxidants (iron chelators, heme arginate), vasodilators (levosimendan), apoptosis inhibitors (QPI-1002), and restorative agents (THR-184, BB-3, MSCs). However, given the complex multifactorial pathophysiology of human AKI, it is unlikely that any single agent will elicit a profound and consistent salutary response. It is anticipated that any future pharmacotherapy for AKI will need to be individualized based on etiology and primary mechanism. Different combinations of agents will likely need to be deployed for different clinical scenarios.
The timing of renal insult, along with the ability to predict and diagnose AKI early, are also critical for personalized therapy. Preclinical studies revealed much greater success when the agents described herein were administered early in the course of AKI, well before serum creatinine rises. While this is easy to achieve in patients with anticipated kidney injuries, such as CPB surgery or nephrotoxic chemotherapy, it is more difficult to achieved in most clinical scenarios. Indeed, several previous pharmaceutical approaches have failed in human trials due to a delay in institution of therapy [56]. The availability of novel noninvasive biomarkers that predict AKI before a rise in serum creatinine, and also differentiate between various forms of AKI, holds promise for the rational choice of patient populations in whom emerging therapies are most likely to be successful.
It is noteworthy that the majority of the AKI clinical trials underway are being performed in adults only. While there are obvious safety and economic considerations driving industry to initiate testing therapeutic agents in adults, there are several advantages of including children in such investigations. First, children frequently represent a more pristine population, which is not burdened by the confounding chronic conditions that plague adult clinical trials, such as diabetes, hypertension, polypharmacy, and CKD. Second, children tend to develop serious complications such as AKI early in the course of other critical illnesses, which facilitates early and reliable entry into clinical trials. Third, children generally display a more rapid and real-time trend toward recovery from illnesses, which can translate to shorter durations for pediatric clinical trials. It will be important for the pediatric nephrology community to advocate and lobby for inclusion of children in current and future AKI clinical trials.
Summary points
-
1.
AKI is a significant cause of morbidity and mortality in the pediatric population, for which there are currently no targeted therapies available.
-
2.
Advances in understanding renal injury and repair signaling pathways has enabled the development of several targeted pharmaceuticals that are currently in human clinical trials.
-
3.
General mechanisms of action for these medications include anti-inflammatory agents, antioxidants, vasodilators, apoptosis inhibitors, and repair agents.
-
4.
Successful pharmacotherapy for AKI will likely require individualized deployment of different combinations of agents based on the primary mechanisms and timing of injury in different clinical scenarios.
Multiple-choice questions (answers are provided following the reference list)
-
1.
In critically ill hospitalized children, the incidence of AKI is estimated to be:
-
a.
5%
-
b.
10%
-
c.
15%
-
d.
25%
-
e.
50%
-
a.
-
2.
The mechanism of action of alkaline phosphatase in treating AKI is:
-
a.
Conversion of ATP and ADP to adenosine, which has anti-inflammatory effects
-
b.
Phosphorylation of bacterial endotoxins
-
c.
Alkalinization of the urine
-
d.
Antioxidant effect
-
e.
Unknown
-
a.
-
3.
The use of iron chelators in treating AKI is most likely to be hampered by:
-
a.
Lack of pharmaceutical agents that can chelate labile iron in humans
-
b.
Lack of efficacy of iron chelation in humans
-
c.
Lack of preclinical data on iron chelation
-
d.
High cost of iron chelators
-
e.
Multiple systemic side effects
-
a.
-
4.
Of the following, a promising pharmaceutical approach in AKI will likely include:
-
a.
Dopamine
-
b.
Mannitol
-
c.
Calcium channel blockade
-
d.
Furosemide
-
e.
A different combination of agents for different clinical scenarios
-
a.
-
5.
Of the following, a promising strategy in AKI will likely include:
-
a.
Continuing current supportive care strategies
-
b.
Initiating pharmacotherapies after serum creatinine has doubled
-
c.
Initiating pharmacotherapies after acute dialysis has begun
-
d.
Using novel biomarkers of AKI to trigger early interventions
-
e.
Treating only the underlying cause
-
a.
References
Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, Dutta S, Garcia P, Goldstein SL (2015) AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol 10:554–561
McGregor TL, Jones DP, Wang L, Danciu I, Bridges BC, Fleming GM, Shirey-Rice J, Chen L, Byrne DW, Van Driest SL (2016) Acute kidney injury incidence in Noncritically ill hospitalized children, adolescents, and young adults: a retrospective observational study. Am J Kidney Dis 67:384–390
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376:11–20
Moffett BS, Goldstein SL (2011) Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol 6:856–863
Goldstein SL (2012) Acute kidney injury in children and its potential consequences in adulthood. Blood Purif 33:131–137
Chaturvedi S (2017) The path to chronic kidney disease following acute kidney injury: a neonatal perspective. Pediatr Nephrol 32:227–241
Greenberg JH, Coca S, Parikh CR (2014) Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: a systematic review. BMC Nephrol 15:184–184
Hui-Stickle S, Brewer ED, Goldstein SL (2005) Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis 45:96–101
Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, Kirkendall ES (2016) A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int 90:212–221
(2012) Section 3: Prevention and Treatment of AKI. Kidney Int Suppl 2:37–68
Matejovic M, Ince C, Chawla LS, Blantz R, Molitoris BA, Rosner MH, Okusa MD, Kellum JA, Ronco C (2016) Renal hemodynamics in AKI: in search of new treatment targets. J Am Soc Nephrol 27:49–58
Okusa MD, Rosner MH, Kellum JA, Ronco C (2016) Therapeutic targets of human AKI: harmonizing human and animal AKI. J Am Soc Nephrol 27:44–48
Humphreys BD, Cantaluppi V, Portilla D, Singbartl K, Yang L, Rosner MH, Kellum JA, Ronco C, for the Acute Dialysis Quality Initiative (ADQI) XIII Work Group (2016) Targeting endogenous repair pathways after AKI. J Am Soc Nephrol 27:990–998
Rabb H, Griffin MD, DB MK, Swaminathan S, Pickkers P, Rosner MH, Kellum JA, Ronco C (2016) Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol 27:371–379
Basile DP, Anderson MD, Sutton TA (2012) Pathophysiology of acute kidney injury. Compr Physiol 2:1303–1353
Peters E, Heemskerk S, Masereeuw R, Pickkers P (2014) Alkaline phosphatase: a possible treatment for sepsis-associated acute kidney injury in critically ill patients. Am J Kidney Dis 63:1038–1048
Peters E, Geraci S, Heemskerk S, Wilmer MJ, Bilos A, Kraenzlin B, Gretz N, Pickkers P, Masereeuw R (2015) Alkaline phosphatase protects against renal inflammation through dephosphorylation of lipopolysaccharide and adenosine triphosphate. Br J Pharmacol 172:4932–4945
Pickkers P, Heemskerk S, Schouten J, Laterre P-F, Vincent J-L, Beishuizen A, Jorens PG, Spapen H, Bulitta M, Peters WH, van der Hoeven JG (2012) Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care 16:R14
Kaempfer R, Arad G, Levy R, Hillman D, Nasie I, Rotfogel Z (2013) CD28: direct and critical receptor for superantigen toxins. Toxins (Basel) 5:1531–1542
Bulger EM, Maier RV, Sperry J, Joshi M, Henry S, Moore FA, Moldawer LL, Demetriades D, Talving P, Schreiber M, Ham B, Cohen M, Opal S, Segalovich I, Maislin G, Kaempfer R, Shirvan A (2014) A novel drug for treatment of necrotizing soft-tissue infections: a randomized clinical trial. JAMA Surg 149:528–536
Star RA, Rajora N, Huang J, Stock RC, Catania A, Lipton JM (1995) Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc Natl Acad Sci U S A 92:8016–8020
PA MC, Bennett-Guerrero E, Chawla LS, Beaver T, Mehta RL, Molitoris BA, Eldred A, Ball G, Lee HJ, Houser MT, Khan S (2016) ABT-719 for the prevention of acute kidney injury in patients undergoing high-risk cardiac surgery: a randomized phase 2b clinical trial. J Am Heart Assoc. doi:10.1161/JAHA.116.003549
Leaf DE, Swinkels DW (2016) Catalytic iron and acute kidney injury. Am J Physiol Renal Physiol 311:F871–F876
Walker VJ, Agarwal A (2016) Targeting iron homeostasis in acute kidney injury. Semin Nephrol 36:62–70
Fraga CM, Tomasi CD, Damasio DC, Vuolo F, Ritter C, Dal-Pizzol F (2016) N-acetylcysteine plus deferoxamine for patients with prolonged hypotension does not decrease acute kidney injury incidence: a double blind, randomized, placebo-controlled trial. Crit Care 20:331
Papneja K, Bhatt MD, Kirby-Allen M, Arora S, Wiernikowski JT, Athale UH (2016) Fanconi syndrome secondary to Deferasirox in Diamond-Blackfan anemia: case series and recommendations for early diagnosis. Pediatr Blood Cancer 63:1480–1483
Chuang GT, Tsai IJ, Tsau YK, Lu MY (2015) Transfusion-dependent thalassaemic patients with renal Fanconi syndrome due to deferasirox use. Nephrology (Carlton) 20:931–935
Dee CM, Cheuk DK, Ha SY, Chiang AK, Chan GC (2014) Incidence of deferasirox-associated renal tubular dysfunction in children and young adults with beta-thalassaemia. Br J Haematol 167:434–436
Freedman MH, Boyden M, Taylor M, Skarf B (1988) Neurotoxicity associated with deferoxamine therapy. Toxicology 49:283–290
Cases A, Kelly J, Sabater F, Torras A, Grino MC, Lopez-Pedret J, Revert L (1990) Ocular and auditory toxicity in hemodialyzed patients receiving desferrioxamine. Nephron 56:19–23
Cohen AR, Galanello R, Piga A, DiPalma A, Vullo C, Tricta F (2000) Safety profile of the oral iron chelator deferiprone: a multicentre study. Br J Haematol 108:305–312
Bolisetty S, Zarjou A, Agarwal A (2017) Heme Oxygenase 1 as a therapeutic target in acute kidney injury. Am J Kidney Dis 69:531–545
Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, Nick HS, Agarwal A (2000) Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol 278:F726–F736
Tracz MJ, Juncos JP, Croatt AJ, Ackerman AW, Grande JP, Knutson KL, Kane GC, Terzic A, Griffin MD, Nath KA (2007) Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int 72:1073–1080
Thomas RA, Czopek A, Bellamy CO, SJ MN, Kluth DC, Marson LP (2016) Hemin preconditioning upregulates Heme Oxygenase-1 in deceased donor renal transplant recipients: a randomized, controlled, phase IIB trial. Transplantation 100:176–183
Farmakis D, Alvarez J, Gal TB, Brito D, Fedele F, Fonseca C, Gordon AC, Gotsman I, Grossini E, Guarracino F, Harjola V-P, Hellman Y, Heunks L, Ivancan V, Karavidas A, Kivikko M, Lomivorotov V, Longrois D, Masip J, Metra M, Morelli A, Nikolaou M, Papp Z, Parkhomenko A, Poelzl G, Pollesello P, Ravn HB, Rex S, Riha H, Ricksten S-E, RHG S, Vrtovec B, Yilmaz MB, Zielinska M, Parissis J (2016) Levosimendan beyond inotropy and acute heart failure: evidence of pleiotropic effects on the heart and other organs: an expert panel position paper. Int J Cardiol 222:303–312
Zager RA, Johnson AC, Lund S, Hanson SY, Abrass CK (2006) Levosimendan protects against experimental endotoxemic acute renal failure. Am J Physiol Renal Physiol 290:F1453–F1462
Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B (2016) Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 67:408–416
Devarajan P (2006) Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17:1503–1520
Yan M, Tang C, Ma Z, Huang S, Dong Z (2016) DNA damage response in nephrotoxic and ischemic kidney injury. Toxicol Appl Pharmacol 313:104–108
Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, Kalinski H, Skaliter R, Erlich S, Feinstein E (2009) siRNA targeted to p53 attenuates ischemic and Cisplatin-induced acute kidney injury. J Am Soc Nephrol 20:1754–1764
Chawla LS, Eggers PW, Star RA, Kimmel PL (2014) Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371:58–66
Tsujimura T, Idei M, Yoshikawa M, Takase O, Hishikawa K (2016) Roles and regulation of bone morphogenetic protein-7 in kidney development and diseases. World J Stem Cells 8:288–296
Vukicevic S, Basic V, Rogic D, Basic N, Shih MS, Shepard A, Jin D, Dattatreyamurty B, Jones W, Dorai H, Ryan S, Griffiths D, Maliakal J, Jelic M, Pastorcic M, Stavljenic A, Sampath TK (1998) Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest 102:202–214
Morrissey J, Hruska K, Guo G, Wang S, Chen Q, Klahr S (2002) Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol 13(Suppl 1):S14–S21
Tampe D, Zeisberg M (2014) Potential approaches to reverse or repair renal fibrosis. Nat Rev Nephrol 10:226–237
Sugimoto H, LeBleu VS, Bosukonda D, Keck P, Taduri G, Bechtel W, Okada H, Carlson W Jr, Bey P, Rusckowski M, Tampe B, Tampe D, Kanasaki K, Zeisberg M, Kalluri R (2012) Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat Med 18:396–404
Organ SL, Tsao M-S (2011) An overview of the c-MET signaling pathway. Ther Adv Med Oncol 3(1 Suppl):S7–S19
Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S (1997) Enhanced expression of hepatocyte growth factor/c-met by myocardial ischemia and reperfusion in a rat model. Circulation 95:2552–2558
Zhou D, Tan RJ, Lin L, Zhou L, Liu Y (2013) Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int 84:509–520
Tan RJ, Zhou D, Liu Y (2016) Signaling crosstalk between tubular epithelial cells and interstitial fibroblasts after kidney injury. Kidney Dis (Basel) 2:136–144
Gong R, Rifai A, Tolbert EM, Biswas P, Centracchio JN, Dworkin LD (2004) Hepatocyte growth factor ameliorates renal interstitial inflammation in rat remnant kidney by modulating tubular expression of macrophage chemoattractant protein-1 and RANTES. J Am Soc Nephrol 15:2868–2881
Narayan P, Duan B, Jiang K, Li J, Paka L, Yamin MA, Friedman SL, Weir MR, Goldberg ID (2016) Late intervention with the small molecule BB3 mitigates postischemic kidney injury. Am J Physiol Renal Physiol 311:F352–F361
Peired AJ, Sisti A, Romagnani P (2016) Mesenchymal stem cell-based therapy for kidney disease: a review of clinical evidence. Stem Cells Int 2016:4798639
Tögel FE, Westenfelder C (2012) Kidney protection and regeneration following acute injury: progress through stem cell therapy. Am J Kidney Dis 60:1012–1022
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Disclosure
No further disclosures.
Additional information
Responses to Multiple-choice Questions
1. d; 2. a; 3. e; 4. e; 5. d
Rights and permissions
About this article
Cite this article
Benoit, S.W., Devarajan, P. Acute kidney injury: emerging pharmacotherapies in current clinical trials. Pediatr Nephrol 33, 779–787 (2018). https://doi.org/10.1007/s00467-017-3695-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3695-3