Abstract
Acute kidney injury (AKI) is a common complication with far-reaching consequences. There is currently no approved therapy to prevent or treat this condition but there are numerous potential agents on the horizon. At a cellular level, important targets include mitochondria, the cell membrane, and the endoplasmic reticulum. In particular, mitochondria have been shown to play a pivotal role in the pathogenesis of AKI. Several novel therapies have been developed to target these organelles. A variety of cell surface receptors have been implicated in the processes of injury and repair in AKI. Several new drugs are targeting receptors to treat or prevent AKI. A number of novel agents with antisepsis properties are also in development, and some have the potential to treat, as well as prevent, AKI. Many of these agents are at an advanced stage of development and may become therapeutic options in the near future. However, there have been many proposed therapies that have failed to yield positive results in clinical trials despite promising preclinical studies. Flaws in trial design may be partly to blame. In addition, the complex and multisystemic nature of AKI makes research in this area very challenging. The ideal therapy is safe and efficacious and has the ability to target a common feature in the pathogenesis of AKI, regardless of the triggering injury.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
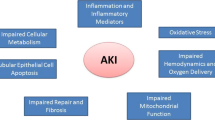
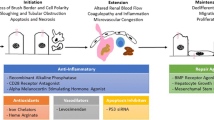
Acute kidney injury (AKI) is a multisystem disease with many potential causes. The mortality and morbidity associated with AKI remain unacceptably high despite advances in critical care and dialysis technologies . There is an expanding body of knowledge of the cellular and molecular mechanisms of AKI , which has led to the discovery of many potential therapeutic targets (see Table 21.1). Despite varied triggers, there are certain common features in the renal response to injury. Here we present the data on emerging therapies, potential drug targets and recent negative trials. We have categorized potential therapies according to their site and mode of action (see Figs. 21.1 and 21.2).
2 Mitochondria
The renal tubular cells, particularly those that are highly involved in active solute transport, have a rich concentration of mitochondria. Mitochondrial injury has been found to be a common feature of AKI, regardless of the inciting injury. It is an integral component in the pathogenesis of AKI, but may also play a role in promoting kidney injury. Given this, mitochondria are important potential therapeutic targets (see Fig. 21.2). To date, mitochondria-specific pharmacotherapies have largely been prophylactic rather than therapeutic.
2.1 Agents Targeting MPTP Opening
2.1.1 Cyclosporine
Mitochondrial permeability transition pore (MPTP) is a cyclosporine-sensitive channel located in the inner mitochondrial membrane. In the setting of ischaemia-reperfusion (IR) injury, MPTP opening mediates cell death. Due to its ability to inhibit MPTP opening, cyclosporine A (CsA) has anti-apoptotic properties and may prevent IR-mediated cell injury. A single dose of CsA has also been shown to reduce inflammatory cell infiltration and tubular cell injury [1]. In animal models of IR, the administration of CsA at the time of resuscitation limited the extent of kidney dysfunction [2]. Lemoine et al. recently proposed that the nephroprotective effect of CsA depends on both the dose and the timing of administration, relative to IR injury [3]. Unfortunately, there are distinct disadvantages to the use of cyclosporine as a prophylactic agent for AKI. The potent immunosuppressive and renal vasoconstrictor properties of this drug are likely to limit its clinical utility in the prevention of AKI. A phase II trial exploring the ability of cyclosporine to reduce the risk and degree of AKI in the context of cardiac surgery is underway.
2.1.2 Other Cyclophilin Inhibitors
N-Methyl-4-isoleucine cyclosporine (NIM-811) is a non-immunosuppressive cyclophilin inhibitor. NIM-811 has been found to improve kidney dysfunction significantly following IR injury in rabbits [2]. It had comparable efficacy to cyclosporine but without the systemic side effects. Preclinical studies suggest that it may have a role in preventing irreversible cellular injury. Early clinical trials report a favourable safety profile.
2.2 Agents Targeting Mitochondrial Oxidative Damage
2.2.1 Mitochondrial-Targeted Antioxidants
Oxidative injury to mitochondria is a prominent feature of IR injury. The inability of damaged mitochondria to recover ATP leads to tubular cell injury and promotes AKI. Most antioxidant agents are clinically ineffective as they are not taken up by mitochondria. Novel mitochondria-specific antioxidants are showing promising results in early clinical trials.
MitoQ is a mitochondria-targeted antioxidant agent that accumulates in mitochondria, localizing within the inner mitochondrial membrane (IMM). Once there, it is continually reduced by the respiratory chain and prevents mitochondrial oxidative damage. It was the first mitochondria-specific antioxidant agent to undergo clinical trials in humans. It has been found to be effective in reducing tubular damage and cell death during cold storage of porcine kidneys [4]. Pretreatment with MitoQ protected mice kidneys from IR-mediated damage and dysfunction [5]. MitoQ has undergone early clinical trials in Parkinson’s disease and hepatitis without any serious adverse events [6, 7]. MitoCP, another mitochondria-targeted antioxidant, may have similar protective properties. It has been shown to prevent cisplatin-induced renal dysfunction in mice in a dose-dependent manner [8]. These potent mitochondrial antioxidants hold promise as an effective preventative therapy for AKI.
2.2.2 Bendavia
Cardiolipin is a phospholipid, located in the IMM and involved in many essential mitochondrial functions. Ischaemic injury causes peroxidation of cardiolipin through the generation of reactive oxygen species (ROS). Structural and functional defects in the mitochondria occur as a result. The peroxidation of cardiolipin also promotes the dissociation of cytochrome c from the IMM into the cytosol, activating programmed cell death pathways.
Bendavia (SS-31 or MTP-131) is a tetrapeptide that inhibits the peroxidation of cardiolipin [9]. It has been shown to accelerate the recovery of ATP after ischaemic insult and ameliorate kidney injury [10]. Pretreatment of rats with bendavia resulted in improved repair of mitochondrial morphology and reduced tubular apoptosis and necrosis after IR injury. Early studies look promising, and phase 2 trials are underway.
2.3 Sildenafil
Sildenafil is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), which increases endogenous nitric oxide (NO) activity. The preservation of NO levels has been found to protect the kidney against a range of insults. There is evidence that PDE inhibitors can induce mitochondrial biogenesis, a key step in the recovery of renal function in AKI [11]. Another postulated mechanism for the renoprotective effect of PDE inhibitors is through local vasodilation. In an experimental model of ischaemic kidneys, a more favourable haemodynamic pattern was evident in animals pretreated with sildenafil [12].
Sildenafil may have the potential to accelerate the recovery from AKI in addition to having a prophylactic effect. In animal models, pretreatment with sildenafil has been shown to lessen histological injury, attenuate serum creatinine levels and reduce reactive oxygen species generation [13,14,15]. Recently, a phase 1 study reported that sildenafil was well-tolerated in cardiac surgery patients [16]. Unfortunately, a randomized placebo controlled trial by Krane et al. did not observe a significant renoprotective effect with a single preoperative dose of sildenafil [17]. The lack of an observed beneficial effect may relate to the dosing regimen used in this study. Sildenafil has a short half-life, and it may be necessary to administer repeated doses for a protective effect. This potential disadvantage of sildenafil could limit its clinical utility.
2.4 Mitochondrial Division Inhibitor-1
Mitochondrial dynamics are governed by two key processes: fission and fusion. There is evidence to indicate that proteins involved in mitochondrial fission actively participate in apoptosis. Dynamin-related protein 1 (Drp-1) is an integral mitochondrial fission protein. Inhibition of Drp-1 inhibits mitochondrial fission and delays programmed cell death.
Mitochondrial division inhibitor-1 (mdivi-1) is a selective inhibitor of Drp-1 that partially inhibits apoptosis [18]. Experimental studies have demonstrated that it can prevent mitochondrial fragmentation and tubular cell apoptosis during kidney injury [19]. However, Sumida et al. did not identify a significant renoprotective effect when they administered mdivi-1 to mice with IR injury [20]. In addition to these inconclusive preclinical results, there is concern that permanent inhibition of mitochondrial fission may have detrimental effects on mitochondrial and cellular function.
3 Endoplasmic Reticulum
The endoplasmic reticulum (ER) is a network of tubules within the cytoplasm. ER stress contributes to AKI. Tunicamycin is an antibiotic that induces extensive ER stress and has been shown to induce substantial proximal tubular damage. Many researchers have utilized it to induce ER stress and AKI in animal studies. There is evidence to suggest that males may be more vulnerable to ER stress than females [21], which may partly explain gender differences in the response to renal injury.
3.1 4-Phenylbutyrate
C/EBP homologous protein (CHOP) is a protein that mediates ER stress-induced apoptosis. Prolonged ER stress in renal cells results in upregulation of CHOP. The chemical chaperone, 4-phenylbutyrate (4-PBA) , reduces the expression of CHOP. In mice with tunicamycin-induced AKI , 4-PBA has been shown to protect the kidney and reduce the extent of tubular injury [15, 22]. It appears to protect the kidney through the inhibition of ER stress. Taurodeoxycholic acid is another chemical chaperone, which appears to protect kidney cells through a similar mechanism [23].
4-PBA is approved for use in urea cycle disorders and has undergone clinical trials in non-renal conditions such as neurodegenerative diseases, liver cirrhosis and certain cancers. It has been reported to have an acceptable safety profile in these conditions. At present, its use in renal diseases has not progressed past preclinical testing. It may prove to be an effective prophylactic agent. Those most likely to benefit are patients at high risk of AKI in which ER stress is a prominent pathogenic feature, e.g. cisplatin- and contrast-induced AKI.
3.2 Epoxyeicosatrienoic Acid Analogs
Epoxyeicosatrienoic acids (EETs) are metabolites of arachidonic acid, with anti-inflammatory and antioxidant effects. Also, they have potent vasodilatory and antihypertensive properties. Based on the results of experimental models, EET analogues can protect against organ injury in conditions such as diabetes and cardiovascular disease. They have also been found to protect the kidney from cisplatin-induced apoptosis through various mechanisms. Amongst these mechanisms is its ability to reduce ER stress and attenuate renal inflammation secondary to cisplatin [24]. It accomplishes this without attenuating the chemotherapeutic effects of cisplatin. Unfortunately, EETs may promote tumour growth and metastasis [25, 26]. This concerning finding may limit their clinical utility.
4 Cell Membrane
The cell membrane is a primary site of damage in AKI. Both necrosis and apoptosis feature alterations in the cell membrane and both forms of cell death can exist in AKI. The plasma membrane is an exciting potential target for therapies to prevent or attenuate AKI.
Fingolimod
Sphingolipids are integral components of the cell membrane. The metabolites of sphingolipids, which include sphingosine-1-phosphate (S1P), act as important signalling molecules. S1P is a ligand for a family of five G-protein-coupled receptors. Through its actions on these receptors, it is involved in many cell processes, including cell growth and the suppression of apoptosis. S1P plays a pivotal role in determining cell fate. Activation of the S1P receptors has been shown to protect the proximal tubular cells from IR injury.
Fingolimod (also known as FTY720) is an orally active immunomodulatory agent that activates the S1P receptor. When administered before IR injury, fingolimod was shown to attenuate kidney injury [27,28,29]. It has been approved for the treatment of multiple sclerosis in many countries. The results of several phase 3 trials of fingolimod in renal transplant patients are awaited.
5 Receptors
Many cell surface receptors have been implicated in epithelial injury, and subsequent repair, in AKI. Signalling through certain receptors (e.g. epidermal growth factor receptor and the hepatocyte growth factor receptor) has a protective or regenerative effect. Others, such as TGF-β, can increase apoptosis. There is scope to attenuate or prevent kidney injury by chemically targeting receptors. Here we outline drugs that have successfully manipulated receptor pathways to protect against and treat AKI in experimental models.
5.1 Nicotinic Agonists
The cholinergic pathway has been linked with an anti-inflammatory effect, in particular through activation of the α7 nicotinic receptor (α7nAChR) . Nicotine is a directly acting cholinergic agonist that mediates its actions through stimulation of the nicotinic acetylcholine receptors . Although chronic nicotine exposure has been linked with adverse renal effects, nicotine has also been found to have a powerful anti-inflammatory effect. Through α7nAChR-dependent regulation of the immune response, nicotine may limit tubular damage and protect renal function after IR injury [30]. Interestingly, although a single pretreatment dose of nicotine prior to IR injury had a protective effect, repeated administration over several days before injury had the opposite effect in a mouse model of IR injury [31].
Nicotinic agonists may prove to be beneficial in the prevention of AKI. However, it has yet to be determined if the protective effects of nicotine are species-specific. GTS-21 is an agent that selectively stimulates the α7nAChR. It has been shown to reduce the infiltration of leucocytes into the kidney [32]. In animal models, GTS-21 has been found to attenuate renal injury in both IR and sepsis-induced AKI. Due to its selective α7nAChR agonist effects, it may have greater potential as a therapeutic agent for the prevention of AKI. Phase 1 studies have commenced.
Exposure to a modified ultrasound regime has been found to attenuate kidney injury in mice that were subject to IR injury. It is postulated to mediate this effect through its actions on the splenic cholinergic anti-inflammatory pathways , particularly via the α7nAChR [33]. At present, data is limited and is insufficient to propose ultrasound as a prophylactic strategy against AKI.
5.2 Endothelin Receptor Antagonists
The kidney is extremely sensitive to endothelin and has abundant endothelin receptors. Endothelin plays an integral role in regulating kidney function through its ability to control global and local renal blood flow. During AKI, there is an imbalance between endothelin and nitric oxide, a potent vasodilator. The effect of endothelin dominates and plays an important role in mediating kidney injury. Through the endothelin A receptor (ETA), endothelin mediates vasoconstriction of vascular smooth muscle. Through the endothelin B receptor (ETB), it causes vasodilation. While blockade of ETA receptors has been shown to improve renal blood flow, ETB receptor inhibition has been associated with renal vasoconstriction [34, 35].
The administration of non-selective ET receptor antagonists (such as tezosentan and bosentan) before or after IR injury has been shown to protect and optimize kidney function [36, 37]. They have also been found to protect the kidney from renal damage induced by cisplatin and from cardiopulmonary bypass [38, 39]. Although endothelin has been implicated in the progression of AKI to CKD, selective blockade of the ETA receptor did little to prevent the progression of renal injury in mice exposed to unilateral IR injury [92]. Phase 1 and 2 trials looking at the effects of ETA receptor antagonists in chronic kidney disease are underway. Based on experimental studies, the ETA receptor seems to be a likely target for the prevention and early treatment of AKI.
5.3 Toll-Like Receptor 2 Antagonists
Toll-like receptors (TLRs) are a family of transmembrane receptors that play a pivotal role in initiating innate immune responses. TLR2 is widely expressed in the kidney and has been implicated in the pathogenesis of AKI. It has been shown to initiate inflammatory responses after kidney injury and is upregulated in renal tubular cells in IR-induced AKI [40, 41]. Blockade of the TLR2 signalling has been shown to reduce neutrophil infiltration and renal damage in an experimental model of IR injury of the kidney [42]. In murine models, the administration of a mouse anti-TLR2 antibody protected transplanted kidneys from IR injury [43].
A humanized monoclonal antibody (OPN-305) that blocks TLR2 signalling has been developed. A phase 2 trial of its efficacy for the prevention of delayed graft function (DGF) in kidney transplant recipients is underway (NCT01794663). There is optimism that it may also prove to be a novel and effective therapy for the prevention of AKI.
6 The Endothelium
Renal endothelial cell dysfunction is prominent in the pathogenesis of AKI. IR injury disrupts endothelial cell integrity which consequently influences vascular tone and inflammatory responses. Endothelial cells play a key role in the initiation, progression and recovery phases of renal IR injury. By targeting endothelial cell damage, kidney injury can be partially prevented.
6.1 Angiopoietin-1 and TIE2 Agonists
Angiopoietin-1 (Ang1) is an angiogenic factor that acts on endothelial cells through the tyrosine kinase receptor, TIE2. Through its interaction with TIE2, Ang1 plays a role in inflammation and vascular growth and development. It has anti-inflammatory properties and enhances endothelial cell survival.
Ang1 has beneficial effects in AKI. A stable, potent variant of Ang1, COMP-Ang1, has been shown to have a protective effect in lipopolysaccharide-induced AKI [44]. In a model of cyclosporine-induced renal injury, it was shown to protect peritubular capillaries and reduce inflammation [45]. However, in a model of folic acid-induced AKI, although Ang1 was found to stabilize peritubular capillaries, it also demonstrated pro-fibrotic and pro-inflammatory effects [46]. This contrasts with evidence provided by Jung et al., who observed that COMP-Ang1 reduced interstitial fibrosis 30 days after IR injury [47]. It has been postulated that the effects of Ang1 may depend on the disease model tested. The varying effects may also be explained by differences in the potency of Ang1 and COMP-Ang1. Clinical trials in humans are awaited.
6.2 Endothelial Progenitor Cells
Endothelial dysfunction and disruption of the vascular barrier integrity are pivotal steps in the pathogenesis of multiorgan failure in septic shock. Endothelial progenitor cells (EPCs) originate in the bone marrow but migrate to the peripheral circulation, where they play a role in endothelial repair and regeneration. In septic shock, the number of EPCs increases, and there is an inverse relationship between EPC numbers and the extent of organ dysfunction. The number of circulating EPCs correlates with survival. In renal ischaemia, EPCs migrate to the renal parenchyma, where they offer partial protection from injury [48]. EPCs may have an important role in ameliorating the effects of AKI. It has been suggested that the renoprotective effects of ischaemic preconditioning may be partially mediated by enhancing the recruitment of EPCs to the renal parenchyma [49].
Stromal cell-derived factor-1 (SDF-1) is a chemokine with regulatory effects on inflammation and cell migration. Through its interaction with the CXCR4 receptor, SDF-1 plays a major role in the recruitment of EPCs to the injured kidney [50]. Theoretically, agonists of SDF-1-CXCR4 should promote the migration of EPCs to the kidney and repair of endothelial cell damage. A high-affinity CXCR4 agonist has been developed, but its effects on kidney injury have not yet been investigated. EPCs and CXCR4 agonists have the potential to play a role in early renal recovery.
7 Gene Silencing Therapy
QPI-1002
Apoptosis triggered by p53 activation has been shown to play an important role in the pathogenesis of AKI [51]. QPI-1002 (also called I5NP) is a synthetic small interfering RNA , designed to temporarily suppress expression of the p53 gene. It inhibits p53-mediated apoptosis after kidney injury, allowing kidney cells to repair and regenerate following injury. After administration, QPI-1002 rapidly accumulates within the kidney, with the main site of uptake being the proximal tubular cell [52]. Once the effect of QPI-1002 has subsided, the irreversibly damaged cells undergo apoptosis. A large phase 1/2 trial in kidney transplant patients suggested it could prevent or attenuate delayed graft function in recipients of deceased donor transplants (NCT00802347). An acceptable safety and toxicity profile was reported. Results of a phase 1 study (NCT00554359) in patients undergoing major cardiovascular surgery are awaited. This novel agent is showing promise as a preventative therapy for AKI and DGF in high-risk patients.
8 Antisepsis
8.1 HMGB1 Antagonists
High-mobility group box 1 protein (HMGB1) is a nuclear protein that bends DNA and acts as a cofactor for gene transcription . It also acts as an extracellular signalling molecule during inflammation. After release from cells undergoing necrosis, HMGB1 activates and propagates the inflammatory response. It has been implicated as an inflammatory mediator in sepsis and IR injury [53, 54]. Through its high affinity binding to the toll-like receptor 4 (TLR4), HMGB1 is also implicated in the pathogenesis of AKI [54, 55].
The blockade of HMGB1 using a neutralizing antibody has been demonstrated to attenuate neutrophil infiltration, tubular necrosis and renal dysfunction in IR injury [55]. Additionally, preconditioning with recombinant HMGB1 has been shown to downregulate the TLR4 signalling in IR injury and protect the kidney [56]. In mouse models of severe sepsis, monoclonal HMGB1 antibodies improved survival from sepsis and reduced circulating levels of pro-inflammatory cytokines [57]. However, the implications of altering the levels of other important cytokines have not yet been explored.
Ethyl pyruvate (EP) , an aliphatic ester, has been found to inhibit HMGB1 release. It has anti-inflammatory and antioxidant effects and has been shown to decrease injury in many organs, including the liver, heart and pancreas. In animal models of sepsis, EP reduced circulating levels of HMGB1, attenuated organ dysfunction and improved survival [58]. In IR injury, EP has been shown to have a nephroprotective effect [59]. Pretreatment of mice with EP resulted in improved short- and long-term kidney function [60]. Phase 1 studies have reported an adequate safety profile. EP and HMGB1 antagonists are potential therapies for sepsis and may have a role in preventing AKI.
8.2 Alkaline Phosphatase
Alkaline phosphatase (AP) is showing considerable promise as a treatment for sepsis-associated AKI. There are two proposed mechanisms for this protective effect. AP is an endogenous enzyme that catalyses the conversion of ATP to adenosine, a factor with potent anti-inflammatory and tissue protective effects. It also phosphorylates endotoxins, rendering them non-toxic. When compared with placebo, treatment with bovine AP improved renal function and reduced RRT requirement in patients with severe sepsis [61]. Human recombinant AP (recAP) has been developed, and in animal models of renal IR injury and lipopolysaccharide-induced AKI, it has been shown to exert a renal protective anti-inflammatory effect. A phase 2 trial investigating its efficacy in sepsis-associated AKI has commenced (NCT02182440), and the results will help elucidate if recAP can improve the outcomes of patients with sepsis-associated AKI. This agent may also have protective effects in other forms of AKI.
8.3 Caspase Inhibitors
Caspases are a family of intracellular proteases that promote apoptotic cell death and activate pro-inflammatory cytokines. Their ability to trigger, execute and regulate cell death has prompted investigators to explore the potential for caspase inhibition to attenuate organ injury. Caspase inhibitors have been shown to reduce renal damage in animal models of septic, drug-induced and IR-induced AKI [62,63,64,65]. The breadth of caspase functionality is being increasingly recognized and so too are the varying roles of individual members of this family. The therapeutic capability of both selective and pancaspase inhibitors is being explored. Pharmacological pancaspase inhibition was well-tolerated by patients with chronic hepatitis C and was shown to reduce markers of hepatocellular injury in a phase 2 trial reported by Shiffman et al. [66]. It seems likely that selective caspase inhibition will yield more predictable clinical effects, compared to pancaspase inhibitors. Human trials of the tolerability and efficacy of selective caspase inhibitors in AKI are awaited. Given the key role these proteases play in programmed cell death, there are grounds for cautious optimism that caspases are novel therapeutic targets for the treatment of AKI.
8.4 EA-230
Peptides derived from human chorionic gonadotrophin (hCG) are drawing attention due to the discovery of their potent anti-inflammatory effects. One such peptide is EA-230 (also known as AQGV), a tetrapeptide derived from β[beta]hCG lysates. It has been shown to attenuate multiorgan failure in sepsis and also ameliorates IR-induced kidney injury [67, 68]. In animal models of IR injury, EA-230 was also associated with a substantial survival advantage.
The exact mechanisms of action of EA-230 have not been fully elucidated. It appears to exert an early anti-inflammatory effect on the kidney, thereby preventing organ dysfunction. EA-230 has been shown to reduce neutrophil influx and the release of pro-inflammatory cytokines. It may also improve renal blood flow [69]. Phase 1 trials have been successfully completed and have reported a good safety profile.
8.5 Bone Morphogenetic Protein 7 (BMP-7) and THR-184
Bone morphogenetic protein 7 , a member of the TGFβ superfamily, plays an important role in nephrogenesis . It may play a role in repair and regeneration in the adult kidney and has been shown to ameliorate kidney damage through its anti-inflammatory and anti-fibrotic properties. In ischaemic injury to the kidney, BMP-7 inhibits neutrophil accumulation by downregulating the expression of intercellular adhesion molecule (ICAM-1) [70]. In animal models of both obstructive and ischaemic AKI, BMP-7 has been shown to reduce kidney injury and inhibit tubular atrophy [70, 71]. It represents a potential target for the treatment of both AKI and chronic kidney disease.
Due to the high doses required and the expense of production of recombinant BMP, BMP-7 agonists may be a more viable clinical option. Small peptide agonists that bind selectively to BMP receptors have been developed. THR-184 is one such agonist, which activates the BMP signalling pathway. It had a good safety profile in early clinical testing, and a phase 2 clinical trial of THR-184 in AKI has commenced.
9 Negative Trials
There is currently no specific therapy approved for the treatment of AKI. Despite optimistic preclinical results, only a small number of drugs have progressed to clinical trials. There are several potential reasons for this (see Table 21.2). AKI is a complex and multisystem disease with a multifaceted pathogenesis. The AKI patient is frequently critically ill with multiple comorbidities. Consequently, a multifaceted approach to treatment is often required. It seems likely that there is a narrow therapeutic window for the treatment of AKI, and an early diagnosis is crucial. The ability to demonstrate the success of therapy partly relies on the early detection of kidney injury. If a rise in creatinine is the trigger for treatment initiation, therapy has probably been commenced too late to show a benefit. Biomarkers of AKI are showing promise and are likely to facilitate the earlier diagnosis of AKI and the development of drugs that can be initiated in a timely fashion.
Trial design has also acted as a limitation to success in this field. Many studies, particularly those of preventative strategies, have been underpowered. To demonstrate the efficacy of preventative therapy, large numbers of patients are required to ensure a sufficient number of AKI cases. The selection and definition of endpoints can also be problematic. The Acute Dialysis Quality Initiative has endeavoured to reach a consensus regarding AKI staging and diagnosis, which has improved trial design. However, other frequently utilized endpoints such as RRT initiation and mortality also present challenges. In practice, there are substantial differences in the utilization of RRT. A consistent approach is lacking. Trials that select mortality as an endpoint may not be powered to detect small alterations in renal function. We have outlined some of the notable agents that showed initial promise but failed to demonstrate a benefit in clinical trials (see Table 21.3).
9.1 Fenoldopam
Fenoldopam is a short-acting α1-selective dopamine agonist that increases renal blood flow even at doses that lower systemic blood pressure. A meta-analysis conducted by Gillies et al. reported that perioperative fenoldopam administration may have the potential to prevent AKI after major surgery [72]. However, mortality and the need for RRT were not attenuated. More recently, Bove et al. reported a decisively negative result [73]. Fenoldopam did not reduce the need for RRT or the 30-day mortality when compared with placebo, but those treated with fenoldopam had a higher rate of hypotension. The trial was terminated for futility.
9.2 Alpha Melanocyte-Stimulating Hormone (Α[Alpha]MSH)
α[Alpha]MSH is an endogenous anti-inflammatory cytokine that may exert a nephroprotective effect by inhibiting apoptotic and inflammatory pathways [74, 75]. Animal studies of the α[alpha]MSH analogue, AP214 acetate (now known as ABT-719), demonstrated a protective effect in AKI induced by IR injury, sepsis and cisplatin [76, 77]. Unfortunately, this benefit has not been substantiated in human trials. McCullough et al. recently reported on the results of their phase 2b clinical trial of ABT-719 [78]. This agent failed to lower the incidence of AKI in high-risk cardiac surgery patients. Furthermore, it did not attenuate increments in novel biomarkers, nor did it improve clinical outcomes at 90 days.
9.3 Minocycline
Minocycline is a second-generation tetracycline antibiotic that has anti-inflammatory and anti-apoptotic effects. The mitochondria appear to be an important site for its protective property. In animal models of IR injury, minocycline was shown to reduce renal inflammation and attenuate renal injury [79, 80]. However, in a clinical trial, minocycline did not protect cardiac surgery patients against AKI [81].
9.4 Statins
Statins have been found to have many beneficial effects in addition to their lipid-lowering properties. Amongst these was the implication that they may have a role in preventing AKI by inhibiting inflammatory responses. A recent meta-analysis concluded that the data reviewed do not support the ability of statins to prevent AKI in patients undergoing cardiac surgery [82].
9.5 Insulin-Like Growth Factor 1
Insulin-like growth factor-1 (IGF-1) is a polypeptide growth factor with a similar molecular structure to insulin. It has been advocated as an important mediator of renal regeneration in models of AKI. Several preclinical studies have indicated that IGF-1 enhances renal recovery in animals with AKI [83,84,85]. However, the administration of recombinant human IGF-1 to patients with AKI in a multicentre clinical trial did not accelerate the recovery of renal function [86].
9.6 Sodium Bicarbonate
The perioperative administration of sodium bicarbonate initially showed promise as a preventative strategy for AKI post-cardiac surgery. The proposed mechanism of protection was by reducing oxidant damage in the kidney. However, a series of randomized controlled trials have failed to substantiate this [87,88,89], and in fact, this strategy may increase mortality.
9.7 Balanced Crystalloid Solutions
There has been considerable debate regarding the optimal choice of intravenous fluid in critical illness. It has been hypothesized that saline solutions contribute to the development of AKI. Evidence emerged in support of the use of chloride restrictive or “balanced” salt solutions in the prevention of AKI [90]. However, a large randomized trial found that a balanced crystalloid solution did not reduce the risk of AKI when compared with saline [91].
9.8 Mesenchymal Stem Cells
Preclinical studies suggested that mesenchymal stem cells (MSCs) had the potential to protect against AKI. Amongst the proposed mechanisms were anti-inflammatory and anti-apoptotic effects. Unfortunately, the beneficial effects of MSC in animal models of AKI have not been replicated in human trials of the disease. A phase 2 trial of mesenchymal stem cell therapy for the prevention of AKI in cardiac surgery patients did not speed up renal recovery or reduce the need for RRT [93].
References
Wen X, Peng Z, Li Y, Wang H, Bishop JV, Chedwick LR, et al. One dose of cyclosporine A is protective at initiation of folic acid-induced acute kidney injury in mice. Nephrol Dial Transplant. 2012;27(8):3100–9.
Cour M, Abrial M, Jahandiez V, Loufouat J, Belaïdi E, Gharib A, et al. Ubiquitous protective effects of cyclosporine A in preventing cardiac arrest-induced multiple organ failure. J Appl Physiol. 2014;117(8):930–6.
Lemoine S, Pillot B, Augeul L, Rabeyrin M, Varennes A, Normand G, et al. Dose and timing of injections for effective cyclosporine A pretreatment before renal ischemia reperfusion in mice. PLoS One. 2017;12(8):e0182358.
Parajuli N, Campbell L, Marine A, Brockbank K, MacMillan-Crow L. MitoQ blunts mitochondrial and renal damage during cold preservation of porcine kidneys. PLoS One. 2012;7(11):e48590.
Dare A, Bolton E, Pettigrew G, Bradley J, Saeb-Parsy K, Murphy M. Protection against renal ischemia–reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol. 2015;5:163–8.
Gane E, Weilert F, Orr D, Keogh G, Gibson M, Lockhart M, et al. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010;30(7):1019–26.
Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, et al. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord. 2010;25(11):1670–4.
Mukhopadhyay P, Horváth B, Zsengellér Z, Zielonka J, Tanchian G, Holovac E, et al. Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic Biol Med. 2012;52(2):497–506.
Birk A, Liu S, Soong Y, Mills W, Singh P, Warren D, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24(8):1250–61.
Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng F-YY, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22(6):1041–52.
Whitaker R, Wills L, Stallons J, Schnellmann R. cGMP-selective phosphodiesterase inhibitors stimulate mitochondrial biogenesis and promote recovery from acute kidney injury. J Pharmacol Exp Ther. 2013;347(3):626–34.
Lledo-Garcia E, Subira-Rios D, Ogaya-Pinies G, Tejedor-Jorge A, Cañizo-Lopez J, Hernandez-Fernandez C. Intravenous sildenafil as a preconditioning drug against hemodynamic consequences of warm ischemia-reperfusion on the kidney. J Urol. 2011;186(1):331–3.
Lauver DA, Carey EG, Bergin IL, Lucchesi BR, Gurm HS. Sildenafil citrate for prophylaxis of nephropathy in an animal model of contrast-induced acute kidney injury. PLoS One. 2014;9(11):e113598.
De Almeida LS, Barboza JR, Freitas FP, Porto ML, Vasquez EC, Meyrelles SS, et al. Sildenafil prevents renal dysfunction in contrast media-induced nephropathy in Wistar rats. Hum Exp Toxicol. 2016;35(11):1194–1202.
Mohey V, Singh M, Puri N, Kaur T, Pathak D, Singh AP. Sildenafil obviates ischemia-reperfusion injury-induced acute kidney injury through peroxisome proliferator-activated receptor γ agonism in rats. J Surg Res. 2016;201(1):69–75.
Ring A, Morris T, Wozniak M, Sullo N, Dott W, Verheyden V, et al. A phase I study to determine the pharmacokinetic profile, safety and tolerability of sildenafil (Revatio® ) in cardiac surgery: the REVAKI-1 study. Br J Clin Pharmacol. 2017;83(4):709–20.
Krane LS, Peyton CC, Olympio MA, Hemal AK. A randomized double blinded placebo controlled trial of sildenafil for renoprotection prior to hilar clamping in patients undergoing robotic assisted laparoscopic partial nephrectomy. J Surg Oncol. 2016;114(7):785–8.
Cassidy-Stone A, Chipuk J, Ingerman E, Song C, Yoo C, Kuwana T, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14(2):193–204.
Brooks C, Wei Q, Cho S-G, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119(5):1275–85.
Sumida M, Doi K, Ogasawara E, Yamashita T, Hamasaki Y, Kariya T, et al. Regulation of mitochondrial dynamics by dynamin-related protein-1 in acute cardiorenal syndrome. J Am Soc Nephrol. 2015;26(10):2378–87.
Hodeify R, Megyesi J, Tarcsafalvi A, Mustafa H, Seng N, Price P. Gender differences control the susceptibility to ER stress-induced acute kidney injury. Am J Physiol Renal Physiol. 2013;304(7):F875–82.
Carlisle R, Brimble E, Werner K, Cruz G, Ask K, Ingram A, et al. 4-phenylbutyrate inhibits tunicamycin-induced acute kidney injury via CHOP/GADD153 repression. PLoS One. 2014;9(1):e84663.
Peng P, Ma Q, Wang L, Zhang O, Han H, Liu X, et al. Preconditioning with tauroursodeoxycholic acid protects against contrast-induced HK-2 cell apoptosis by inhibiting endoplasmic reticulum stress. Angiology. 2015;66(10):941–9.
Khan MA, Liu J, Kumar G, Skapek SX, Falck JR, Imig JD. Novel orally active epoxyeicosatrienoic acid (EET) analogs attenuate cisplatin nephrotoxicity. FASEB J. 2013;27(8):2946–56.
Jiang J-G, Chen C-L, Card J, Yang S, Chen J-X, Fu X-N, et al. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65(11):4707–15.
Jiang J-G, Ning Y-G, Chen C, Ma D, Liu Z-J, Yang S, et al. Cytochrome P450 epoxygenase promotes human cancer metastasis. Cancer Res. 2007;67(14):6665–74.
Bajwa A, Jo S-KK, Ye H, Huang L, Dondeti KR, Rosin DL, et al. Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol. 2010;21(6):955–65.
Bajwa A, Rosin DL, Chroscicki P, Lee S, Dondeti K, Ye H, et al. Sphingosine 1-phosphate receptor-1 enhances mitochondrial function and reduces cisplatin-induced tubule injury. J Am Soc Nephrol. 2015;26(4):908–25.
Park SW, Kim M, Kim M, D’Agati VD, Lee HT. Sphingosine kinase 1 protects against renal ischemia-reperfusion injury in mice by sphingosine-1-phosphate1 receptor activation. Kidney Int. 2011;80(12):1315–27.
Yeboah MM, Xue X, Javdan M, Susin M, Metz CN. Nicotinic acetylcholine receptor expression and regulation in the rat kidney after ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2008;295(3):F654–61.
Sadis C, Teske G, Stokman G, Kubjak C, Claessen N, Moore F, et al. Nicotine protects kidney from renal ischemia/reperfusion injury through the cholinergic anti-inflammatory pathway. PLoS One. 2007;2(5):e469.
Chatterjee PK, Yeboah MM, Dowling O, Xue X, Powell SR, Al-Abed Y, et al. Nicotinic acetylcholine receptor agonists attenuate septic acute kidney injury in mice by suppressing inflammation and proteasome activity. PLoS One. 2012;7(5):e35361.
Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S, et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol. 2013;24(9):1451–60.
Matsumura Y, Taira S, Kitano R, Hashimoto N, Kuro T. Selective antagonism of endothelin ET(A) or ET(B) receptor in renal hemodynamics and function of deoxycorticosterone acetate-salt-induced hypertensive rats. Biol Pharm Bull. 1999;22(8):858–62.
Chade AR, Krier JD, Textor SC, Lerman A, Lerman LO. Endothelin-a receptor blockade improves renal microvascular architecture and function in experimental hypercholesterolemia. J Am Soc Nephrol. 2006;17(12):3394–403.
Herrero I, Torras J, Riera M, Condom E, Coll O, Cruzado JM, et al. Prevention of cold ischaemia-reperfusion injury by an endothelin receptor antagonist in experimental renal transplantation. Nephrol Dial Transplant. 1999;14(4):872–80.
Wilhelm SM, Stowe NT, Robinson AV, Schulak JA. The use of the endothelin receptor antagonist, tezosentan, before or after renal ischemia protects renal function. Transplantation. 2001;71(2):211–6.
Helmy MM, Helmy MW, Abd Allah DM, Abo Zaid AM, Mohy El-Din MM. Selective ET(A) receptor blockade protects against cisplatin-induced acute renal failure in male rats. Eur J Pharmacol. 2014;730:133–9.
Patel NN, Toth T, Jones C, Lin H, Ray P, George SJ, et al. Prevention of post-cardiopulmonary bypass acute kidney injury by endothelin A receptor blockade. Crit Care Med. 2011;39(4):793–802.
Kim B, Lim S, Li C, Kim J, Sun B, Ahn K, et al. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation. 2005;79(10):1370–7.
Wolfs T, Buurman W, van Schadewijk A, de Vries B, Daemen M, Hiemstra P, et al. In vivo expression of toll-like receptor 2 and 4 by renal epithelial cells: IFN-γ and TNF-α mediated up-regulation during inflammation. J Immunol. 2002;168(3):1286–93.
Kim H, Park S, Koo S, Cha H, Lee J, Kwon B, et al. Inhibition of kidney ischemia–reperfusion injury through local infusion of a TLR2 blocker. J Immunol Methods. 2014;407:146–50.
Farrar C, Keogh B, McCormack W, O’Shaughnessy A, Parker A, Reilly M, et al. Inhibition of TLR2 promotes graft function in a murine model of renal transplant ischemia-reperfusion injury. FASEB J. 2012;26(2):799–807.
Kim DH, Jung YJ, Lee AS, Lee S, Kang KP, Lee TH, et al. COMP-angiopoietin-1 decreases lipopolysaccharide-induced acute kidney injury. Kidney Int. 2009;76(11):1180–91.
Lee S, Kim W, Kim DH, Moon S-OO, Jung YJ, Lee AS, et al. Protective effect of COMP-angiopoietin-1 on cyclosporine-induced renal injury in mice. Nephrol Dial Transplant. 2008;23(9):2784–94.
Long DA, Price KL, Ioffe E, Gannon CM, Gnudi L, White KE, et al. Angiopoietin-1 therapy enhances fibrosis and inflammation following folic acid-induced acute renal injury. Kidney Int. 2008;74(3):300–9.
Jung YJ, Kim DH, Lee AS, Lee S, Kang KP, Lee SY, et al. Peritubular capillary preservation with COMP-angiopoietin-1 decreases ischemia-reperfusion-induced acute kidney injury. Am J Physiol Renal Physiol. 2009;297(4):F952–60.
Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol Renal Physiol. 2006;291(1):F176–85.
Bo C-JJ, Chen B, Jia R-PP, Zhu J-GG, Cao P, Liu H, et al. Effects of ischemic preconditioning in the late phase on homing of endothelial progenitor cells in renal ischemia/reperfusion injury. Transplant Proc. 2013;45(2):511–6.
Togel FE, Westenfelder C. Role of SDF-1 as a regulatory chemokine in renal regeneration after acute kidney injury. Kidney Int Suppl (2011). 2011;1(3):87–9.
Kelly KJ, Plotkin Z, Vulgamott SL, Dagher PC. P53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: protective role of a p53 inhibitor. J Am Soc Nephrol. 2003;14(1):128–38.
Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2009;20(8):1754–64.
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–51.
Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, et al. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. 2010;21(11):1878–90.
Li J, Gong Q, Zhong S, Wang L, Guo H, Xiang Y, et al. Neutralization of the extracellular HMGB1 released by ischaemic damaged renal cells protects against renal ischaemia-reperfusion injury. Nephrol Dial Transplant. 2011;26(2):469–78.
Wu H, Steenstra R, de Boer EC, Zhao CY, Ma J, van der Stelt JM, et al. Preconditioning with recombinant high-mobility group box 1 protein protects the kidney against ischemia-reperfusion injury in mice. Kidney Int. 2014;85(4):824–32.
Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203(7):1637–42.
Fink MP. Ethyl pyruvate: a novel treatment for sepsis. Curr Drug Targets. 2007;8(4):515–8.
Chung K-YY, Park J-JJ, Kim YS. The role of high-mobility group box-1 in renal ischemia and reperfusion injury and the effect of ethyl pyruvate. Transplant Proc. 2008;40(7):2136–8.
Rabadi MM, Ghaly T, Goligorksy MS, Ratliff BB. HMGB1 in renal ischemic injury. Am J Physiol Renal Physiol. 2012;303(6):F873–85.
Pickkers P, Heemskerk S, Schouten J, Laterre P-FF, Vincent J-LL, Beishuizen A, et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care. 2012;16(1):R14.
Servais H, Ortiz A, Devuyst O, Denamur S, Tulkens PM, Mingeot-Leclercq M-PP. Renal cell apoptosis induced by nephrotoxic drugs: cellular and molecular mechanisms and potential approaches to modulation. Apoptosis. 2008;13(1):11–32.
Daemen MA, van ‘t Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, et al. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104(5):541–9.
Guo R, Wang Y, Minto AW, Quigg RJ, Cunningham PN. Acute renal failure in endotoxemia is dependent on caspase activation. J Am Soc Nephrol. 2004;15(12):3093–102.
Homsi E, Janino P, de Faria JB. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int. 2006;69(8):1385–92.
Shiffman ML, Pockros P, McHutchison JG, Schiff ER, Morris M, Burgess G. Clinical trial: the efficacy and safety of oral PF-03491390, a pancaspase inhibitor—a randomized placebo-controlled study in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2010;31(9):969–78.
Khan NA, Khan A, Savelkoul HF, Benner R. Inhibition of septic shock in mice by an oligopeptide from the beta-chain of human chorionic gonadotrophin hormone. Hum Immunol. 2002;63(1):1–7.
Khan NA, Susa D, van den Berg JW, Huisman M, Ameling MH, van den Engel S, et al. Amelioration of renal ischaemia-reperfusion injury by synthetic oligopeptides related to human chorionic gonadotropin. Nephrol Dial Transplant. 2009;24(9):2701–8.
Gueler F, Shushakova N, Mengel M, Hueper K, Chen R, Liu X, et al. A novel therapy to attenuate acute kidney injury and ischemic allograft damage after allogenic kidney transplantation in mice. PLoS One. 2015;10(1):e0115709.
Vukicevic S, Basic V, Rogic D, Basic N, Shih MS, Shepard A, et al. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest. 1998;102(1):202–14.
Hruska KA, Guo G, Wozniak M, Martin D, Miller S, Liapis H, et al. Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am J Physiol Renal Physiol. 2000;279(1):F130–43.
Gillies MA, Kakar V, Parker RJ, Honoré PM, Ostermann M. Fenoldopam to prevent acute kidney injury after major surgery—a systematic review and meta-analysis. Crit Care. 2015;19(1):449.
Bove T, Zangrillo A, Guarracino F, Alvaro G, Persi B, Maglioni E, et al. Effect of fenoldopam on use of renal replacement therapy among patients with acute kidney injury after cardiac surgery: a randomized clinical trial. JAMA. 2014;312(21):2244–53.
Chiao H, Kohda Y, McLeroy P, Craig L, Linas S, Star RA. Alpha-melanocyte-stimulating hormone inhibits renal injury in the absence of neutrophils. Kidney Int. 1998;54(3):765–74.
Jo SK, Yun SY, Chang KH, Cha DR, Cho WY, Kim HK, et al. alpha-MSH decreases apoptosis in ischaemic acute renal failure in rats: possible mechanism of this beneficial effect. Nephrol Dial Transplant. 2001;16(8):1583–91.
Doi K, Hu X, Yuen PS, Leelahavanichkul A, Yasuda H, Kim SM, et al. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 2008;73(11):1266–74.
Simmons MN, Subramanian V, Crouzet S, Haber G-PP, Colombo JR, Ukimura O, et al. Alpha-melanocyte stimulating hormone analogue AP214 protects against ischemia induced acute kidney injury in a porcine surgical model. J Urol. 2010;183(4):1625–9.
McCullough PA, Bennett-Guerrero E, Chawla LS, Beaver T, Mehta RL, Molitoris BA, et al. ABT-719 for the Prevention of Acute Kidney Injury in Patients Undergoing High-Risk Cardiac Surgery: A Randomized Phase 2b Clinical Trial. Am Heart Assoc. 2016;5(8):e003549.
Kelly KJ, Sutton TA, Weathered N, Ray N, Caldwell EJ, Plotkin Z, et al. Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am J Physiol Renal Physiol. 2004;287(4):F760.
Wang J, Wei Q, Wang C-Y, Hill W, Hess D, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem. 2004;279(19):19948–54.
Golestaneh L, Lindsey K, Malhotra P, Kargoli F, Farkas E, Barner H, et al. Acute kidney injury after cardiac surgery: is minocycline protective? J Nephrol. 2015;28(2):193–9.
Lewicki M, Ng I, Schneider AG. HMG CoA reductase inhibitors (statins) for preventing acute kidney injury after surgical procedures requiring cardiac bypass. Cochrane Database Syst Rev 2015;(3):CD010480.
Ding H, Kopple JD, Cohen A, Hirschberg R. Recombinant human insulin-like growth factor-I accelerates recovery and reduces catabolism in rats with ischemic acute renal failure. J Clin Invest. 1993;91(5):2281–7.
Friedlaender M, Popovtzer MM, Weiss O, Nefesh I, Kopolovic J, Raz I. Insulin-like growth factor-1 (IGF-1) enhances recovery from HgCl2-induced acute renal failure: the effects on renal IGF-1, IGF-1 receptor, and IGF-binding protein-1 mRNA. J Am Soc Nephrol. 1995;5(10):1782–91.
Miller SB, Martin DR, Kissane J, Hammerman MR. Insulin-like growth factor I accelerates recovery from ischemic acute tubular necrosis in the rat. Proc Natl Acad Sci U S A. 1992;89(24):11876–80.
Hirschberg R, Kopple J, Lipsett P, Benjamin E, Minei J, Albertson T, et al. Multicenter clinical trial of recombinant human insulin-like growth factor I in patients with acute renal failure. Kidney Int. 1999;55(6):2423–32.
Haase M, Haase-Fielitz A, Plass M, Kuppe H, Hetzer R, Hannon C, et al. Prophylactic perioperative sodium bicarbonate to prevent acute kidney injury following open heart surgery: a multicenter double-blinded randomized controlled trial. PLoS Med. 2013;10(4):e1001426.
McGuinness SP, Parke RL, Bellomo R, Van Haren FM, Bailey M. Sodium bicarbonate infusion to reduce cardiac surgery-associated acute kidney injury: a phase II multicenter double-blind randomized controlled trial. Crit Care Med. 2013;41(7):1599–607.
Kristeller JL, Zavorsky GS, Prior JE, Keating DA, Brady MA, Romaldini TA, et al. Lack of effectiveness of sodium bicarbonate in preventing kidney injury in patients undergoing cardiac surgery: a randomized controlled trial. Pharmacotherapy. 2013;33(7):710–7.
Yunos N, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–72.
Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the Intensive Care Unit: the SPLIT randomized clinical trial. JAMA. 2015;314(16):1701–10.
Erika I. Boesen, (2016) Lack of an apparent role for endothelin-1 in the prolonged reduction in renal perfusion following severe unilateral ischemia-reperfusion injury in the mouse. Physiological Reports 4 (21):e13027.
Madhav Swaminathan, Mark Stafford-Smith, Glenn M. Chertow, David G. Warnock, Viken Paragamian, Robert M. Brenner, François Lellouche, Alison Fox-Robichaud, Mohamed G. Atta, Spencer Melby, Ravindra L. Mehta, Ron Wald, Subodh Verma, C. David Mazer, Allogeneic Mesenchymal Stem Cells for Treatment of AKI after Cardiac Surgery. Journal of the American Society of Nephrology:ASN.2016101150.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media, LLC, part of Springer Nature
About this chapter
Cite this chapter
Redahan, L., Murray, P.T. (2018). Emerging Therapies: What’s on the Horizon?. In: Waikar, S., Murray, P., Singh, A. (eds) Core Concepts in Acute Kidney Injury. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-8628-6_21
Download citation
DOI: https://doi.org/10.1007/978-1-4939-8628-6_21
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-8626-2
Online ISBN: 978-1-4939-8628-6
eBook Packages: MedicineMedicine (R0)