Abstract
The acronym VATER/VACTERL association (OMIM #192350) refers to the rare non-random co-occurrence of the following component features (CFs): vertebral defects (V), anorectal malformations (A), cardiac defects (C), tracheoesophageal fistula with or without esophageal atresia (TE), renal malformations (R), and limb defects (L). According to epidemiological studies, the majority of patients with VATER/VACTERL association present with a “Renal” phenotype comprising a large spectrum of congenital renal anomalies. This finding is supported by evidence linking all of the human disease genes for the VATER/VACTERL association identified to date, namely, FGF8, FOXF1, HOXD13, LPP, TRAP1, and ZIC3, with renal malformations. Here we review these genotype–phenotype correlations and suggest that the elucidation of the genetic causes of the VATER/VACTERL association will ultimately provide insights into the genetic causes of the complete spectrum of congenital renal anomalies per se.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The VATER/VACTERL association was first described as the VATER association in 1972 by Quan and Smith whereby the acronym identifies a non-random co-occurrence of vertebral anomalies (V), anal atresia (A), tracheoesophageal fistula and/or esophageal atresia (TE), and radial dysplasia (R) [1]. In 1973, only 1 year later, the same authors published an extended definition of the acronym in which they linked the letter R not only to radial dysplasia but also to renal anomalies [2]. In 1974, again only 1 year later, Temtamy and Miller [3] added cardiac (C) and limb defects (L), and the acronym was revised to the VACTERL association.
In the years following these initial descriptions of the association, several extensions of the clinical definition were proposed, including vascular anomalies “V”, auricular anomalies “A”, and rib anomalies “R” [4, 5]. Recent studies have shown that as well as presenting with the classical and the extended component features (CFs) of this rare disorder, many patients present with less frequently observed congenital anomalies that still seem to be inherent to the association [6, 7]. In addition to these clinical diagnostic criteria, several studies have reported clinically defined clusters depending on the type and spatial location of the CFs (e.g., “upper vs. lower” VATER/VACTERL phenotypes) [8]. However, in his review on the “VACTERL/VATER association” Solomon [8] pointed out that these clusters may very well reflect variable diagnostic criteria instead of true distinct phenotypes. Irrespective of these extended CFs and the rare but inherent congenital anomalies, it is now generally accepted in the field of Medical Genetics that the clinical diagnosis of the VATER/VACTERL association (OMIM %192350) requires the presence of at least three of the CFs: vertebral defects (V), anorectal malformations (A), cardiac defects (C), tracheoesophageal fistula with or without esophageal atresia (TE), renal malformations (R), and limb defects (L) [2, 9, 10].
The involvement of genetic factors in the development of this rare association is suggested by reports of familial occurrence, the increased prevalence of component features among first-degree relatives of affected individuals, high concordance rates among monozygotic twins, chromosomal (micro-) aberrations or single gene mutations in individuals with the VATER/VACTERL phenotype, as well as murine knock-out models. In this review, we summarize current knowledge on the underlying genetic factors of the VATER/VACTERL association with special emphasis on the “Renal” phenotype.
Frequency of “Renal” anomalies in patient cohorts with VATER/VACTERL association
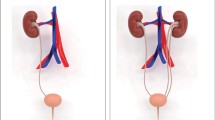
Population-based epidemiological studies in Europe and the USA have reported that the birth prevalence of this non-random association of birth defects [11–13] ranges from 1 in 10,000 to 1 in 40,000 [14]. Because of this low birth prevalence Botto and colleagues [13] studied infants with the clinical diagnosis of the VATER/VACTERL association in a combined registry of infants with multiple congenital anomalies from 17 birth defects registries worldwide that are part of the International Clearinghouse for Birth Defects Monitoring Systems. In their study, they analyzed the frequency of core CFs among patients with a clear clinical diagnosis of VATER/VACTERL association. Overall they analyzed data from approximately 10 million infants born between 1983 and 1991, ultimately identifying 286 infants who presented with the clinical diagnosis of VATER/VACTERL association, defined by the presence of at least three core CFs. Interestingly, 231 of these 286 patients (80.8 %) presented with renal anomalies (R) making this CF the second most common CF in this survey, only surpassed by anorectal malformations (A) (236/286; 82.5 %). In 2002, Cuschieri and the EUROCAT Working Group [15] carried out an extended survey, from 1980 to 1994, and re-analyzed the dataset. Probably due to better ultrasound screening due to technological improvements, they found renal anomalies (R) to be by far the most common CF among the VATER/VACTERL patients. This epidemiologically based observation suggests that disease-causing genes involved in the etiology of the VATER/VACTERL association might also be involved in the development of renal anomalies per se.
Familial VATER/VACTERL association with the “Renal” phenotype
In 2012 we reviewed the familial occurrence of the VATER/VACTERL association by performing a systematic literature search of the NCBI (www.ncbi.nlm.nih.gov/pubmed) and DIMDI (www.dimdi.de/static/de/index.html) databases and searching congress reports using the Medical Subject Headings (MeSH) of VATER, VACTERL, association, esophageal atresia, anorectal malformation, congenital heart defect, limb anomalies, renal anomalies, and vertebral anomalies [16]. We identified 12 families in total in which at least one member exhibited three or more core CFs of the VATER/VACTERL association, and at least one additional member had a minimum of one CF [16]. In some of these families, the association appeared to follow a Mendelian mode of inheritance [16–24]. In almost all of the families, at least one of the affected members presented with renal anomalies [16, 18, 20, 22–24], providing additional support for the notion that among families with a possible involvement of genetic factors in disease development, these genetic factors might also be involved in the development of the renal anomalies.
Chromosomal (micro-)aberrations in patients with VATER/VACTERL and VATER/VACTERL-like phenotypes
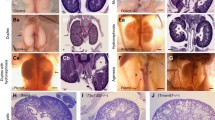
To date, cytogenetic and molecular analyses have revealed chromosomal (micro-)aberrations in at least 13 patients with the full clinical picture of the VATER/VACTERL association (see Table 1). These include: (1) deletions of 5q11.2 [25], 6q [26], 7q35-qter [27], distal 13q [28, 29], 19p13.3 [30], and 20q13.33 [31]; (2) duplications on 1q41, 2q37.3, and 8q24.3 [32], 9q [33], and at 22q11.21 [34]; (3) deletion at Yq with duplication at Yp [35], and deletion of 9p24.3-p24.1 with duplication of 18q12.3-q23 [36]; (4) supernumerary der(22) syndrome [37]; (5) mosaicism for supernumerary ring chromosome 12 [38] or 18 [39]; (6) partial monosomy 16p13.3pter/partial trisomy 16q22qter [40]. The smallest of these microaberrations was identified in the study by Hilger et al. [32], describing a de novo microduplication at 2q37.3 with an estimated size of 25 kb. The duplication comprised exons 3–4 of one splice variant of Calpain-10 (CAPN10) and exons 3–6 of GPR35 encoding the G-protein-coupled receptor 35. The index case carrying the duplication was an aborted female fetus. The pregnancy was terminated at 30 + 1 weeks of gestation because of sonographically proven severe bilateral renal dysplasia. Macroscopic and histological findings obtained at autopsy revealed a cloacal malformation with anal atresia, bilateral renal dysplasia, urethral agenesis, a secondary bell-shaped thorax, and Potter-sequence facies due to anhydramnion. Gpr35 expression studies in mice at embryonic day (E) 14.5 showed detectable transcripts in, among other tissues, the genital tubercle and rectum. However, mutation analysis of GPR35 in 192 VATER/VACTERL and VATER/VACTERL-like patients did not identify any sequence variant of likely pathological significance [32].
Genetically engineered VATER/VACTERL murine mutant models with the “Renal” phenotype
Shh, Gli2, Gli3
Mutant murine models have recently provided some clues and several candidate genes to the etiology of the VATER/VACTERL association. The vast majority of these genetically engineered mice present with congenital renal anomalies (see Table 2). Genetically engineered mice with mutations in genes of the Sonic hedgehog (SHH) signaling cascade, namely, Shh, Gli2, and Gli3, show the complete spectrum of VATER/VACTERL phenotype, including variable renal anomalies, such as unilateral renal agenesis, horseshoe, or ectopic kidney [41]. In humans, various allelic phenotypes have been associated with heterozygous SHH mutations (MIM #142945, holoprosencephaly 3; MIM #611638, microphthalmia with coloboma 5), GLI2 (MIM #610829, holoprosencephaly 9; MIM #615849, Culler–Jones syndrome), and GLI3 (MIM #175700, Greig cephalopolysyndactyly syndrome; MIM #146510, Pallister–Hall syndrome). However, none of these syndromic phenotypes in humans completely resembles the classic human VATER/VACTERL association.
Pcsk5
Szumska and colleagues [42] identified an ethylnitrosourea (ENU)-induced recessive mouse mutation (Vcc) resulting from a p.Cys470Arg exchange in the proprotein convertase subtilisin/kexin type 5 (PCSK5) that resembled all CFs of the human VATER/VACTERL association, including bilateral renal agenesis. Nevertheless, to date, all candidate gene sequencing studies in human VATER/VACTERL patients have revealed only heterozygous missense variants and one heterozygous frameshift variant [36, 42, 43]. To determine the origin of the variants, the authors of these studies sequenced the family samples that were available and found that all of the respective variants were transmitted by an unaffected parent. Based on the assumption that these PCSK5 variants have a pathogenic effect, an autosomal recessive model of inheritance would appear to be most consistent with these findings, as a model of reduced penetrance in the parents would imply that at least some of the parents are mildly affected. Hence, further exploration of the non-coding regulatory regions of PCSK5 with the aim to identify the second hit is warranted.
Ptf1a
The molecular basis of Danforth’s short tail (Sd) mouse [44] was recently elucidated by three research groups who independently reported the insertion of a retrotransposon in the 5′ regulatory domain of the murine Ptf1a gene that encodes pancreas specific transcription factor 1A [45–47]. Consequently, and contrary to their wild-type litter-mates, Sd mice showed ectopic Ptf1a expression in the notochord and hindgut at E8.5–E9.5, which extended to the cloaca and mesonephros at E10.5 and to the pancreatic bud at E10.5 and E11.5 [46]. The resultant phenotype of this Sd mutation not only causes reduction or absence of kidneys, but it mirrors the complete phenotype of the human VATER/VACTERL association. While PTF1A had been previously confirmed as an autosomal-recessive disease-causing gene for human “pancreatic and cerebellar agenesis” (MIM #609069), PTF1A sequence analysis of 103 VATER/VACTERL and VATER/VACTERL-like patients could not identify any pathogenic alterations affecting the coding region and 1.5 kb of its 5′ flanking sequence [48]. These findings suggest that mutations in PTF1A do not play a significant role in the development of human VATER/VACTERL association.
Ift172
Friedland-Little and colleagues [49] identified a recessive ENU-induced mutation, avc1 (atrioventricular canal 1), causing the VACTERL-H phenotype, including vertebral anomalies, anal atresia, cardiac defects, tracheoesophageal anomalies, renal dysplasia, limb anomalies, and hydrocephalus, as well as an avc1-specific polymorphism in Ift172, an intraflagellar transport gene. They showed that avc1 is a hypomorphic mutation of intraflagellar transport protein 172 (Ift172), which is required for ciliogenesis and Shh signaling. The authors described renal dysplasia with hypoplastic glomeruli in four of nine avc1 mutant embryos, compared with the normal phenotype in six wild-type mice [49]. In humans, homozygous and compound heterozygous mutations in IFT172 have been proven to cause the phenotypes of retinitis pigmentosa 71 (MIM #616394) or short-rib thoracic dysplasia 10 with or without polydactyly (MIM #615630).
While no human phenotype has been associated with mutations in PCSK5, various allelic human phenotypes have been detected with heterozygous mutations in SHH, GLI2, and GLI3, as well as with homozygous or compound heterozygous mutations in PTF1A and IFT172 (see above). Of these, only heterozygous mutations in GLI3 and homozygous or compound heterozygous mutations in IFT172 are associated with micro- and macroscopic renal anomalies in humans.
Human VATER/VACTERL disease genes and their contribution to the “Renal” phenotype
TRAP1
Saisawat et al. [50] very recently identified recessive mutations in the gene encoding TNF receptor-associated protein 1 (TRAP1) in two families with severe vesicoureteral reflux and in three families with VATER/VACTERL association (including right duplex kidney or right renal agenesis in two of these three cases). This is the first report of autosomal recessive inheritance in a gene causing the full clinical picture of the VATER/VACTERL association, as well the first report of such mutations being a cause for isolated congenital abnormalities of the kidneys and the urinary tract (CAKUT). TRAP1 is a heat-shock protein 90-related mitochondrial chaperone, possibly involved in antiapoptotic and endoplasmic reticulum stress signaling. Trap1 is expressed in renal epithelia of the developing mouse kidney at E13.5 and in the kidney of adult rats, most prominently in proximal tubules and in the thick medullary ascending limbs of Henle's loop [50]. Yan et al., who investigated several heat shock proteins and their embryonic function in mice, found Trap1 to be involved in forelimb development, another organ system affected by the VATER/VACTERL association [51].
ZIC3
In addition to the classic VATER/VACTERL association, an associated condition presents with hydrocephalus. In view of its X-linked transmission, it is termed X-linked VATER/VACTERL-hydrocephalus (or VACTERL-H), and it can be caused by mutations in the ZIC3 gene. According to the studies described in this section, the hydrocephalus is an optional feature that is not necessarily present in the VATER/VACTERL association caused by ZIC3 . In 2010, Wessels and colleagues [52] described a male newborn with VATER/VACTERL association, including a unilateral multicystic kidney. As the clinical picture of this patient overlapped with that of X-linked heterotaxy caused by ZIC3 mutations, the ZIC3 coding region was sequenced, revealing a 6-nucleotide insertion in a GCC repeat of the ZIC3 gene. The resultant polyalanine expansion was not found in 192 ethnically matched controls, nor was it present in the mother, suggesting that it occurred de novo in the patient. In a recently study, mutation analysis of ZIC3 identified a recurrent disease-causing mutation (c.49G > T, p.Gly17Cys) in four patients with VATER/VACTERL and VATER/VACTERL-like phenotypes [53]. The first patient was male and presented with three CFs, namely, recto-vesical fistula, atrial septal defect, and right renal agenesis with grade IV–V vesicoureteral reflux on the left side. Additional features were cryptorchidism and penoscrotal transposition. He inherited the ZIC3 p.Gly17Cys mutation from his unaffected mother. The second and third patients with the p.Gly17Cys mutation were a sister and her brother. The girl presented with vestibular fistula, high-grade vesicoureteral reflux, and 13 ribs on both sides. Her brother presented with recto-prostatic fistula and atrial septal defect. Testing for skewed X-chromosome inactivation in the family showed, that the mutant allele was predominant (only 9 % inactivated), whereas the paternal allele was nearly 91 % inactivated. The same active allele in the sister was shared by her brother, which explains why both siblings were similarly affected. The fourth p.Gly17Cys patient presented with recto-urethral fistula and right-sided ectopic kidney with grade I vesicoureteral reflux; he also displayed penoscrotal transposition and glandular hypospadias [53]. While ZIC3 is known to regulate left-right body asymmetry during embryonic development it remains unclear how ZIC3 is involved in early renal development [54]. However, several of the VATER/VACTERL patients with ZIC3 mutations showed unilateral renal anomalies, suggesting that a disturbed regulation of body asymmetry during embryonic development may be involved, pointing towards ZIC3 regulation of renal development.
Human disease genes in patients with VATER/VACTERL-like phenotypes and renal anomalies
In a few cases, distinct gene mutations have been reported, mostly in association with VATER/VACTERL-like phenotypes. Mutations in these genes were almost always found in single patients, rendering their contribution to the development of the association uncertain (see Table 2).
HOXD13
In a female with tetralogy of Fallot, bilateral hydronephrosis and hydroureters, anorectal malformation, and inter-phalangeal joints of her fourth and fifth toes, lending resemblance to a VATER/VACTERL (“ACR”) phenotype, Garcia-Barceló et al. [55] identified a heterozygous 21-bp de novo deletion in the HOXD13 gene. According to the “Mouse Genome Database (MGD)” none of the HOXD13 cre mice displays renal anomalies [56]. Accordingly, Hoxd13 is not expressed in the mouse renal progenitor structures (expression data from E11.5–E17) [57]. Hence, HOXD13 does not seem to play a major role in the development of renal anomalies in the context of the VATER/VACTERL association.
FGF8
The FGF8 gene encodes a transcription factor involved in multiple embryonic signaling cascades and also in the regulation of SHH expression. A recent FGF8 analysis by Zeidler and colleagues [58] in 78 patients with VATER/VACTERL association or VATER/VACTERL-like phenotype revealed two different mutations. While the p.Gly29_Arg34dup mutation in a patient with five CFs (“ACTERL”) including horseshoe kidney has not been reported yet, the p.Pro26Leu substitution in a patient with two CFs (“AR”), comprising right-sided renal dysplasia, had earlier been found in a patient with Kallmann syndrome (KS) who did not show any CFs of the VATER/VACTERL association [59]. Interestingly, both patients had bilateral cryptorchidism, a phenotypic feature in FGF8-associated KS. Each mutation was paternally inherited. However, apart from delayed puberty in both fathers and unilateral cryptorchidism in one father, the fathers were healthy, suggesting a variable expressivity for mutations in FGF8 in which patients with the VATER/VACTERL association may constitute the severe end [58]. According to the MGD, Fgf8 tm1.3Mrt/Fgf8 tm1.4Mrt mice display abnormal kidney development and absent renal glomerulus [56]. Furthermore, Fgf8 is expressed from E11.5 to E15 in the metanephros of the mouse, suggesting that FGF8 might play a role in the formation of renal anomalies within the VATER/VACTERL association spectrum [57].
LPP
Arrington et al. [60] detected haploinsufficiency of the LPP gene in a patient with VATER/VACTERL association including a “Renal” phenotype. This patient presented with tetralogy of Fallot, rib anomalies, hypospadias, small kidneys and esophageal atresia, yielding the phenotype “CTER”. LPP codes for the LIM domain containing preferred translocation partner in lipoma, which has been shown to bind PEA3 (polyomavirus enhancer activator 3 homolog), an ETS domain transcription factor involved in the SHH signaling pathway [61]. However, LPP gene analysis detected no deleterious sequence changes in 70 additional patients with VATER/VACTERL association [62]. None of the Lpp-associated mouse phenotypes in MGD display renal anomalies [56]. Furthermore, Lpp expression is either absent or ambiguous in the mouse metanephros substructures at E15 [57]. Hence, LPP does not seem to play a major role in the development of renal anomalies in the context of the VATER/VACTERL association.
FOXF1
The transcription factor FOXF1, a downstream target of the SHH pathway, plays an important role in epithelium–mesenchyme signaling [63]. Most recently, a heterozygous de novo FOXF1 missense mutation was found in a patient with a VATER/VACTERL-like phenotype (“AR”) who presented with an anorectal malformation, left-sided renal agenesis, and glandular hypospadias [53]. In 2009, Stankiewicz et al. [64] reported several patients with alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV) displaying different heterozygous genomic deletions and point mutations in FOXF1. All of these patients also showed additional malformations in VATER/VACTERL organ systems, and in all of these systems, except for the kidney, strong Foxf1 expression was noted in mice at E12.5 and E13.5 [53]. Moreover, Foxf1 heterozygote mutant mice displayed tracheo-esophageal fistulas [65], one hallmark of the VATER/VACTERL association. Why Foxf1 did not show significant staining in mouse kidney and why the patient reported by Hilger et al. [53] did not develop ACDMPV remains to be elucidated, but the resultant phenotype might be dependent on residual function and amount of FOXF1 protein [64].
Monogenic syndromes resembling the clinical picture of the “Renal” VATER/VACTERL phenotype
Several known monogenic syndromes resemble the full clinical picture of the “Renal” VATER/VACTERL phenotype, including Baller–Gerold syndrome (MIM #218600; autosomal recessive; RECQL4), Townes–Brocks syndrome (MIM #107480; autosomal dominant; SALL1), and Fanconi anemia (FA) complementation group (i.e., FANCB, FANCF or FANCL). With respect to the latter syndrome, Faivre et al. [66] found that in more than 200 FA patients, approximately 5 % could be judged to have a VATER/VACTERL or VATER/VACTERL-like phenotype. In a recent review, Lubinsky [67] concludes that the frequency and number of CFs of the VATER/VACTERL association with FA correlate with the severity and onset of hematopoietic and malignancy issues. Here, radial anomalies are the most common CF, interestingly followed by renal anomalies.
Summary
Although the identified genetic causes underlying the VATER/VACTERL association are heterogeneous and gene–gene interactions have yet to be elucidated, all of the identified human disease genes are associated with “Renal” phenotypes. This is in accordance with the observation that the majority of patients with VATER/VACTERL association presents with a “Renal” phenotype, suggesting that the elucidation of the genetic causes of the VATER/VACTERL association might very well provide insights into the genetic causes of isolated renal anomalies per se.
References
Quan L, Smith DW (1972) The VATER association: vertebral defects, anal atresia, tracheoesophageal fistula with esophageal atresia, radial dysplasia. Birth Defects Orig Arctic Ser 8:75–78
Quan L, Smith DW (1973) The VATER association. Vertebral defects, anal atresia, T-E fistula with esophageal atresia, radial and renal dysplasia: a spectrum of associated defects. J Pediatr 82:104–107
Temtamy SA, Miller JD (1974) Extending the scope of the VATER association: definition of the VATER syndrome. J Pediatr 85:345–349
Baumann W, Greinacher I, Emmrich P, Spranger J (1976) Vater or Vacterl syndrome. Klin Padiatr 188:328–337
Barnes JC, Smith WL (1978) The VATER association. Radiology 126:445–449
Reutter H, Gurung N, Ludwig M (2014) Evidence for annular pancreas as an associated anomaly in the VATER/VACTERL association and investigation of the gene encoding pancreas specific transcription factor 1A as a candidate gene. Am J Med Genet A 164A:1611–1613
Fritz CJ, Reutter HM, Herberg U (2015) Scimitar syndrome in a case with VACTERL association. Cardiol Young 25:606–609
Solomon BD (2011) VACTERL/VATER association. Orphanet J Rare Dis 6:56
Jenetzky E, Wijers CHW, Marcelis CM, Zwink N, Reutter H, van Rooij IALM (2011) Bias in patient series with VACTERL association. Am J Med Genet A 155A:2039–2041
Solomon BD, Bear KA, Kimonis V, de Klein A, Scott DA, Shaw-Smith C, Tibboel D, Reutter H, Giampietro PF (2012) Clinical geneticists’ views of VACTERL/VATER association. Am J Med Genet A 158A:3087–3100
Khoury MJ, Cordero JF, Greenberg F, James LM, Erickson JD (1983) A population study of the VACTERL association: evidence for its etiologic heterogeneity. Pediatrics 71:815–820
Czeizel A, Ludányi I (1985) An aetiological study of the VACTERL-association. Eur J Pediatr 144:331–337
Botto LD, Khoury MJ, Mastroiacovo P, Castilla EE, Moore CA, Skjaerven R, Mutchinick OM, Borman B, Cocchi G, Czeizel AE, Goujard J, Irgens LM, Lancaster PA, Martinez-Frias ML, Merlob P, Ruusinen A, Stoll C, Sumiyoshi Y (1997) The spectrum of congenital anomalies of the VATER association: an international study. Am J Med Genet 71:8–15
Mastroiacovo P, Bianchi F (1991) The descriptive epidemiology of congenital birth defects in Italy. Epidemiol Prev 13:94–103
Cuschieri A, EUROCAT working group (2002) Anorectal anomalies associated with or as part of other anomalies. Am J Med Genet 110:122–130
Hilger A, Schramm C, Draaken M, Mughal SS, Dworschak G, Bartels E, Hoffmann P, Nöthen MM, Reutter H, Ludwig M (2012) Familial occurrence of the VATER/VACTERL association. Pediatr Surg Int 28:725–729
Fuhrmann W, Rieger A, Vogel F (1958) Two observations on the genetics of atresia ani. Arch Kinderheilkd 158:264–270
Say B, Balci S, Pirnar T, Hiçsönmez A (1971) Imperforate anus (polydactyly) vertebral anomalies syndrome: a hereditary trait? J Pediatr 79:1033–1034
Finer NN, Bowen P, Dunbar LG (1978) Caudal regression anomalad (sacral agenesis) in siblings. Clin Genet 13:353–358
Auchterlonie IA, White MP (1982) Recurrence of the VATER association within a sibship. Clin Genet 21:122–124
McMullen KP, Karnes PS, Moir CR, Michels VV (1996) Familial recurrence of tracheoesophageal fistula and associated malformations. Am J Med Genet 63:525–528
Nezarati MM, McLeod DR (1999) VACTERL manifestations in two generations of a family. Am J Med Genet 82:40–42
Becker J, Hernandez A, Dipietro M, Coran AG (2005) Identical twins concordant for pulmonary sequestration communicating with the esophagus and discordant for the VACTERL association. Pediatr Surg Int 21:541–546
Solomon BD, Pineda-Alvarez DE, Raam MS, Cummings DA (2010) Evidence for inheritance in patients with VACTERL association. Hum Genet 127:731–733
De Jong EM, Douben H, Eussen BH, Felix JF, Wessels MW, Poddighe PJ, Nikkels PG, de Krijger RR, Tibboel D, de Klein A (2010) 5q11.2 deletion in a patient with tracheal agenesis. Eur J Hum Genet 18:1265–1268
McNeal RM, Skoglund RR, Francke U (1977) Congenital anomalies including the VATER association in a patient with del(6)q deletion. J Pediatr 91:957–960
Zen PR, Riegel M, Rosa RFM, Pinto LLC, Graziadio C, Schwartz IV, Paskulin GA (2010) Esophageal stenosis in a child presenting a de novo 7q terminal deletion. Eur J Med Genet 53:333–336
Walsh LE, Vance GH, Weaver DD (2001) Distal 13q deletion syndrome and the VACTERL association: case report, literature review, and possible implications. Am J Med Genet 98:137–144
Dworschak GC, Draaken M, Marcelis C, de Blaauw I, Pfundt R, van Rooij IALM, Bartels E, Hilger A, Jenetzky E, Schmiedeke E, Grasshoff-Derr S, Schmidt D, Märzheuser S, Hosie S, Weih S, Holland-Cunz S, Palta M, Leonhardt J, Schäfer M, Kujath C, Rißmann A, Nöthen MM, Zwink N, Ludwig M, Reutter H (2013) De novo 13q deletions in two patients with mild anorectal malformations as part of VATER/VACTERL and VATER/VACTERL-like association and analysis of EFNB2 in patients with anorectal malformations. Am J Med Genet A 161A:3035–3041
Peddibhotla S, Khalifa M, Probst FJ, Stein J, Harris LL, Kearney DL, Vance GH, Bull MJ, Grange DK, Scharer GH, Kang SH, Stankiewicz P, Bacino CA, Cheung SW, Patel A (2013) Expanding the genotype-phenotype correlation in subtelomeric 19p13.3 microdeletions using high resolution clinical chromosomal microarray analysis. Am J Med Genet A 161A:2953–2963
Solomon BD, Pineda-Alvarez DE, Hadley DW, Keaton AA, Agochukwu NB, Raam MS, Carlson-Donohoe HE, Kamat A, Chandrasekharappa SC (2011) De novo deletion of chromosome 20q13.33 in a patient with tracheo-esophageal fistula, cardiac defects and genitourinary anomalies implicates GTPBP5 as a candidate gene. Birth Defects Res A Clin Mol Teratol 91:862–865
Hilger A, Schramm C, Pennimpede T, Wittler L, Dworschak GC, Bartels E, Engels H, Zink AM, Degenhardt F, Müller AM, Schmiedeke A, Grasshoff-Derr S, Märzheuser S, Hosie S, Holland-Cunz S, Wijers CHW, Marcelis CLM, von Rooij IALM, Hildebrandt F, Herrmann BG, Nöthen MM, Ludwig M, Reutter H, Draaken M (2013) De novo microduplications at 1q41, 2q37.3, and 8q24.3 in patients with VATER/VACTERL association. Eur J Hum Genet 21:1377–1382
Aynaci FM, Celep F, Karaguzel A, Baki A, Yildiran A (1996) A case of VATER association associated with 9qh+. Genet Couns 7:321–322
Schramm C, Draaken M, Bartels E, Boemers TM, Aretz S, Brockschmidt FF, Nöthen MM, Ludwig M, Reutter H (2011) De novo microduplication at 22q11.21 in a patient with VACTERL association. Eur J Med Genet 54:9–13
Bhagat M (2015) VACTERL association-type anomalies in a male neonate with a Y-chromosome abnormality. OMCR 1:1–3
Winberg J, Gustavsson P, Papadogiannakis N, Sahlin E, Bradley F, Nordenskjöld E, Svensson PJ, Annéren G, Iwarsson E, Nordgren A, Nordenskjöld A (2014) Mutation screening and array comparative genomic hybridization using a 180K oligonucleotide array in VACTERL association. PLoS ONE 9:e85313
Prieto JC, Garcia NM, Elder FF, Zinn AR, Baker LA (2007) Phenotypic expansion of the supernumerary derivative (22) chromosome syndrome: VACTERL and Hirschsprung’s disease. J Pediatr Surg 42:1928–1932
Cinti R, Priolo M, Lerone M, Gimelli G, Seri M, Silengo M, Ravazzolo R (2001) Molecular characterisation of a supernumerary ring chromosome in a patient with VATER association. J Med Genet 38, E6
Van der Veken LT, Dieleman MMJ, Douben H, van de Brug JC, van de Graaf R, Aj H, Poddighe PJ, de Klein A (2010) Low grade mosaic for a complex supernumerary ring chromosome 18 in an adult patient with multiple congenital anomalies. Mol Cytogenet 3:13
Yamada K, Uchiyama A, Arai M, Kubodera K, Yamamoto Y, Orii KO, Nagasawa H, Masuno M, Kohno Y (2009) Severe upper airway stenosis in a boy with partial monosomy 16p13.3pter and partial trisomy 16q22qter. Congenit Anom 49:85–88
Kim JH, Kim PCW, Hui CC (2001) The VACTERL association: lessons from the Sonic hedgehog pathway. Clin Genet 59:306–315
Szumska D, Pieles G, Essalmani R, Bilski M, Mesnard D, Kaur K, Franklyn A, El Omari K, Jefferis J, Bentham J, Taylor JM, Schneider JE, Arnold SJ, Johnson P, Tymowska-Lalanne Z, Stammers D, Clarke K, Neubauer S, Morris A, Brown SD, Shaw-Smith C, Cama A, Capra V, Ragoussis J, Constam D, Seidah NG, Prat A, Bhattacharya S (2008) VACTERL/caudal regression/ Currarino syndrome-like malformations in mice with mutations in the proprotein convertase Pcsk5. Genes Dev 22:1465–1477
Nakamura Y, Kikugawa S, Seki S, Takahata M, Iwasaki N, Terai H, Matsubara M, Fujioka F, Inagaki H, Kobayashi T, Kimura T, Kurahashi H, Kato H (2015) PCSK5 mutation in a patient with the VACTERL association. BMC Res Notes 8:228
Gluecksohn-Schoenheimer S (1945) The embryonic development of mutants of the Sd-strain in mice. Genetics 30:29–38
Lugani F, Arora R, Papeta N, Patel A, Zheng Z, Sterken R, Singer RA, Caridi G, Mendelsohn C, Sussel L, Papaionnou VE, Gharavi AG (2013) A retrotransposon insertion in the 5’ regulatory domain of Ptf1a results in ectopic gene expression and multiple congenital defects in Danforth’s short tail mouse. PLoS Genet 9:e1003206
Semba K, Araki K, Matsumoto K, Suda H, Ando T, Sei A, Mizuta H, Takagi K, Nakahara M, Muta M, Yamada G, Nakagata N, Iida A, Ikegawa S, Nakamura Y, Araki M, Abe K, Yamamura K (2013) Ectopic expression of Ptf1a induces spinal defects, urogenital defects, and anorectal malformations in Danforth’s short tail mice. PLoS Genet 9:e1003204
Vlangos CN, Siuniak AN, Robinson D, Chinnaiyan AM, Lyons RH Jr, Cavalcoli JD, Keegan CE (2013) Next-generation sequencing identifies the Danforth’s short tail mouse mutation as a retrotransposon insertion affecting Ptf1a expression. PLoS Genet 9:e1003205
Gurung N, Grosse G, Draaken M, Hilger AC, Nauman N, Müller A, Gembruch U, Merz WM, Reutter H, Ludwig M (2015) Mutations in PTF1A are not a common cause for human VATER/VACTERL association or neural tube defects mirroring Danforth’s short tail mouse. Mol Med Rep 12:1579–1583
Friedland-Little JM, Hoffmann AD, Ocbina PJR, Peterson MA, Bosman JD, Chen Y, Cheng SY, Anderson KV, Moskowitz IP (2011) A novel murine allele of intraflagellar transport protein 172 causes a syndrome including VACTERL-like features with hydrocephalus. Hum Mol Genet 20:3725–3737
Saisawat P, Kohl S, Hilger AC, Hwang DY, Gee HY, Dworschak GC, Tasic V, Pennimpede T, Natarajan S, Sperry E, Matassa DS, Stajić N, Bogdanovic R, de Blaauw I, Marcelis CLM, Wijers CHW, Bartels E, Schmiedeke E, Schmidt D, Märzheuser S, Grasshoff-Derr S, Holland-Cunz S, Ludwig M, Nöthen MM, Draaken M, Brosens E, Heij H, Tibboel D, Herrmann BG, Solomon BD, de Klein A, van Rooij IALM, Esposito F, Reutter H, Hildebrandt F (2014) Whole exome resequencing reveals recessive mutations in TRAP1 in individuals with CAKUT and VACTERL association. Kidney Int 85:1310–1317
Yan Z, Wei H, Ren C, Yuan S, Fu H, Lv Y, Zhu Y, Zhang T (2015) Gene expression of Hsps in normal and abnormal embryonic development of mouse hindlimbs. Hum Exp Toxicol 34:563–574
Wessels MW, Kuchinka B, Heydanus R, Smit BJ, Dooijes D, de Krijger JJ, Lequin MH, de Jong EM, Husen M, Willems PJ, Casey B (2010) Polyalanine expansion in the ZIC3 gene leading to X-linked heterotaxy with VACTERL association: a new polyalanine disorder? J Med Genet 47:351–355
Hilger AC, Halbritter J, Pennimpede T, van der Ven A, Sarma G, Braun DA, Porath JD, Kohl S, Hwang DY, Dworschak GC, Hermann BG, Pavlova A, El-Maarri O, Nöthen MM, Ludwig M, Reutter H, Hildebrandt F (2015) Targeted resequencing of 29 candidate genes and mouse expression studies implicate ZIC3 and FOXF1 in human VATER/VACTERL association. Hum Mutat 36:1150–1154
Winata CL, Kondrychyn I, Kumar V, Srinivasan KG, Orlov Y, Ravishankar A, Prabhakar S, Stanton LW, Korzh V, Mathavan S (2013) Genome wide analysis reveals Zic3 interaction with distal regulatory elements of stage specific developmental genes in zebrafish. PLoS Genet 10, e1003852
Garcia-Barceló MM, Wong KK, Lui VC, Yuan ZW, So MT, Ngan ES, Miao XP, Chung PH, Khong PL, Tam PK (2008) Identification of a HOXD13 mutation in a VACTERL patient. Am J Med Genet A 146A:3181–3185
Eppig JT, Blake JA, Bult CJ, Kadin JA, Richardson JE, The Mouse Genome Database Group (2015) The mouse genome database (MGD): facilitating mouse as a model for human biology and disease. Nucleic Acids Res 43(Database issue):D726–D736
Smith CM, Finger JH, Hayamizu TF, McCright IJ, Xu J, Berghout J, Campbell J, Corbani LE, Forthofer KL, Frost PJ, Miers D, Shaw DR, Stone KR, Eppig JT, Kadin JA, Richardson JE, Ringwald M (2014) The mouse gene expression database (GXD): 2014 update. Nucleic Acids Res 42(D1):D818–D824
Zeidler C, Woelfle J, Draaken M, Mughal SS, Große G, Hilger AC, Dworschak GC, Boemers TM, Jenetzky E, Zwink N, Lacher M, Schmidt D, Schmiedeke E, Grasshoff-Derr S, Märzheuser S, Holland-Cunz S, Schäfer M, Bartels E, Keppler K, Palta M, Leonhardt J, Kujath C, Rißmann A, Nöthen MM, Reutter H, Ludwig M (2014) Heterozygous FGF8 mutations in patients presenting cryptorchidism and multiple VATER/VACTERL features without limb anomalies. Birth Defects Res A Clin Mol Teratol 100:750–759
Falardeau J, Chung WCJ, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SHS, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N (2008) Decreased FGF8 signaling causes deficiency of ginadotropin-releasing hormone in humans and mice. J Clin Invest 118:2822–2831
Arrington CB, Patel A, Bacino CA, Bowles NE (2010) Haploinsufficiency of the LIM domain containing preferred translocation partner in lipoma (LPP) gene in patients with tetralogy of Fallot and VACTERL association. Am J Med Genet A 152A:2919–2923
Guo B, Sallis RE, Greenall A, Petit MM, Jansen E, Young L, van de Ven WJ, Sharrocks AD (2006) The LIM domain protein LPP is a coactivator for the ETS domain transcription factor PEA3. Mol Cell Biol 26:4529–4538
Hernández-García A, Brosens E, Zaven HP, de Jong EM, Yu Z, Namwanje M, Mayle A, Fernandes CJ, Lee B, Blazo M, Lalani SR, Tinnoel D, de Klein A, Scott DA (2012) Contribution of LPP copy number and sequence changes to esophageal atresia, tracheoesophageal fistula, and VACTERL association. Am J Med Genet A 158A:1785–1787
Mahlapuu M, Enerbäck S, Carlsson P (2001) Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development 128:2397–2406
Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, Bejjani BA, Ou Z, Wiszniewska J, Driscoll DJ, Maisenbacher MK, Bolivar J, Bauer M, Zackai EH, McDonald-McGinn D, Nowaczyk MMJ, Murray M, Shaikh TH, Martin V, Tyreman M, Simonic I, Willatt L, Paterson J, Mehta S, Rajan D, Fitzgerald T, Gribble S, Prigmore E, Patel A, Shaffer LG, Carter NP, Cheung SW, Langston C, Shaw-Smith C (2009) Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet 84:780–791
Dharmadhikari AV, Szafranski P, Kalinichenko VV, Stankiewicz P (2015) Genomic and epigenetic complexity of the FOXF1 locus in 16q24.1: implications for development and disease. Curr Genomics 16:107–116
Faivre L, Portnoĩ MF, Pals G, Stoppa-Lyonnet D, Le Merrer M, Thauvin-Robinet C, Huet F, Mathew CG, Joenje H, Verloes A, Baumann C (2005) Should chromosome breakage studies be performed in patients with VACTERL association? Am J Med Genet A 137A:55–58
Lubinsky M (2015) Sonic hedgehog, VACTERL, and Fanconi anemia: pathogenetic connections and therapeutic implications. Am J Med Genet A 167A:2594–2598
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Reutter, H., Hilger, A.C., Hildebrandt, F. et al. Underlying genetic factors of the VATER/VACTERL association with special emphasis on the “Renal” phenotype. Pediatr Nephrol 31, 2025–2033 (2016). https://doi.org/10.1007/s00467-016-3335-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-016-3335-3