Abstract
Background
Meeting energy requirements of children with chronic kidney disease (CKD) is paramount to optimising growth and clinical outcome, but little information on this subject has been published. In this study, we examined basal metabolic rate (BMR; a component of energy expenditure) with the aim to determine whether it is related to kidney function independently of weight, height and lean body mass (LBM).
Methods
Twenty children with CKD and 20 healthy age- and gender-matched control children were studied on one occasion. BMR was measured by indirect open circuit calorimetry and predicted by the Schofield equation. Estimated glomerular filtration rate (eGFR) was related to BMR and adjusted for weight, height, age and LBM measured by skinfold thickness.
Results
The adjusted BMR of children with CKD did not differ significantly from that of healthy subjects (1296 ± 318 vs.1325 ± 178 kcal/day; p = 0.720). Percentage of predicted BMR also did not differ between the two groups (102 ± 12 % vs. 99 ± 14 %; p = 0.570). Within the CKD group, eGFR (mean 33.7 ± 20.5 mL/min/m2) was significantly related to BMR (β 0.3, r = 0.517, p = 0.019) independently of nutritional status and LBM.
Conclusions
It seems reasonable to use estimated average requirement as the basis of energy prescriptions for children with CKD (mean CKD stage 3 disease). However, those who were sicker had significantly lower metabolic rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nutritional care is an integral part of the management of infants, children and adolescents with chronic kidney disease (CKD). The purpose of dietetic support is to help manage phosphate, protein, electrolyte and fluid intake while also ensuring that the nutritional needs of children with CKD are met to optimise growth and reduce clinical complications. The initial point of departure in any dietetic plan is to ensure that the energy needs of the child are met before consideration is given to the adequacy of micronutrients and protein. However, the evidence base to inform likely energy requirements is insecure, leading to uncertainty when developing and prescribing dietary plans for a child, deciding when to use energy supplements and selecting pragmatic approaches based on clinical experience.
One commonly used approach is to use the dietary reference values—in particular, the estimated average requirement (EAR) by height age [1, 2]. However, the EAR was intended to meet the energy needs of 50 % of a healthy population, and not an individual’s needs in chronic disease [3, 4, 2, 5]. Therefore, a deeper appreciation of the factors that have been used to determine the EAR is required, including how these factors might alter with differences in disease pathology and treatment. Firstly, in CKD the use of energy in the basal state—captured as resting energy expenditure (REE)—may be increased in response to inflammation, infection or hyperparathyroidism [6–12] or decreased as part of a reductive adaption to undernourishment. Only three full-text studies have been published on metabolic rate and children with CKD, with the evidence suggesting that metabolic rate in children with CKD is reduced compared to that in healthy controls when expressed in absolute terms [13, 14], but unchanged when expressed per kilogram body weight or lean tissue [13] or when expressed against predictive values [15]. The discrepancy in the findings probably relates to methodological differences (no control group [15]), significantly shorter and lighter CKD children (a factor which was not adjusted for in the statistical analysis [13]), different ages, stages of disease and treatment modes (with no statistical adjustment for fat-free mass [14]) and different approaches to expressing the results [kcal/cm, kcal/kg, kcal/lean body mass (LBM)/day, kcal/day]. Furthermore, the relationship between REE and the severity of disease, as marked by decline in kidney function, was not explored in any of these studies to determine whether those with more advanced kidney disease had the greatest increase in metabolic demands. In addition, little attention has been directed towards determining the more variable component of energy needs associated with differences in the amount of energy expended in physical activity at the time of study.
Therefore, there is a need to better understand the impact of CKD on energy requirements in children in order to better inform dietetic care. The aim of this study was to determine basal metabolic rate (BMR) in children and to relate this to the severity of disease, growth status (age, weight, height) and body composition (lean body mass).
Methods
Study design
Twenty children with CKD and 20 healthy age- and sex-matched controls were recruited into this cross-sectional study. SamplePower2 (SPSS Inc., Chicago, IL) [16] was used to determine that a sample size of 20 was sufficiently large to establish a relationship with kidney function (r = 0.55 with a power calculation of 80 % and a significance of p < 0.05).
Ethical approval
The Southampton & South West Hampshire local ethics committee and University Hospitals Southampton Research and Development committee approved the project. All parents signed the consent form prior to the inclusion of their children in the study.
Inclusion and exclusion criteria
Children with CKD were included if they met the following pre-specified inclusion criteria: uncomplicated CKD with a glomerular filtration rate (GFR) of <60 ml/min/1.73 m2 (stage 3–5 CKD); age between 6 and 17 years, under conservative management (diet and medication) or dialysis (peritoneal dialysis, haemodialysis) for 3 months after diagnosis. Children with CKD were excluded if they had suffered recent significant infection in the previous 3 months.
The age- and sex-matched control children had no known underlying medical condition.
Subject selection and recruitment
The medical team and research dietitian recruited children with CKD under the care of the regional paediatric nephrology unit, University Hospital Southampton NHS Foundation Trust. Local advertisements in the hospital were used to recruit healthy controls.
Data collection and calculations
Children attended the Wellcome Trust Clinical Research Facility (WTCRF) at the University Hospital Southampton NHS Foundation Trust, where measurements of growth, body composition and energy expenditure were undertaken. Children with CKD also had blood tests (to determine kidney function) taken during their routine clinical visit, which was scheduled to coincide with the start of the study.
Weight and height were measured at the start of the study with an empty bladder, to the nearest 0.1 kg using Seca [17] floor scales and to the nearest 0.1 cm using a Leicester stadiometer, respectively [17] . Dry weight was estimated by clinical examination by the paediatric nephrologist and included an assessment of jugular ventricular pressure, blood pressure and the amount and distribution of oedema, as well as the response of blood pressure and oedema to fluid removal during dialysis. Three of the four children with oedema were on dialysis. In the only child receiving peritoneal dialysis all assessments and measurements, including weight, were taken post drain removal when the peritoneum was considered to be empty. Weight and height were converted to z scores (standard deviation scores; SDS) using the LMS software programme [18]. BMI-SDS for height age was calculated using the Manchester Children’s online package [19]. LBM was estimated by skinfold thickness measurements at four sites, measured using Harpenden callipers [20], and the equation of Lohman et al. [21], which despite its limitations was chosen here due to its clinical utility and accessibility.
BMR was measured by indirect open circuit calorimetry (Deltatrac II; Datex-Ohmeda [22]) following a 12-h fast and abstinence from strenuous physical activity, and after resting in the recumbent position for 30 min. Indirect calorimetry estimates the heat generated in the body from food or fuel oxidation by measuring oxygen (O2) consumption (difference between inspired and expired O2) and carbon dioxide (CO2) production (difference between expired and inspired CO2) under controlled conditions. The amount of energy expended (heat) per litre of oxygen used varies according to the type of nutrient (fuel) consumed. By measuring the volume of O2 (VO2) and CO2 (VCO2) exchanged every minute for 30 min, energy expenditure estimations can be made [23, 24]. In the ventilated hood system one-directional air (indoor) flows into an enclosed area (ventilated hood) and is adjusted to keep CO2 levels under a set threshold. Air is then sampled and analysed [24, 23] every minute for 30 min under standard conditions [25, 26]. The equation of Elia and Livesey [27] was used to calculate BMR [EE (kJ) = 15.818 O2 + 5.176 CO2, where O2 and CO2 are in litres] [27]. (Elia and Livesey [27] also provide the information on VO2 and CO2 constants used in this equation, which yields similar results to others). BMR was also predicted by the Schofield equation [28] (SchBMR) using age, gender, weight and height for direct comparison with measured BMR, and although this and other equations have not been validated for use in children with CKD, the Schofield equation was being used in UK clinical practice at the time of study. However, to improve its utility, estimated dry weight and actual height were used.
Measured BMR (mBMR) was expressed in kilocalorie per 24 h and as a percentage of Schofield-predicted BMR (SchBMR) [(BMR × 100)/SchBMR)].
Estimated GFR (eGFR) was calculated using the modified Schwartz formula [29] based on height and serum creatinine concentration.
Statistical analysis
The unpaired t test was used to test the difference between the CKD group and the control group. Regression analysis was used to assess the relationship between eGFR and BMR and the effect of confounding variables within the CKD group. A p value of <0.05 (2-tailed) was used to indicate significance.
All analysis was carried out using software by the Statistical Package for the Social Sciences [30] (SPSS, Chicago, IL; ver. 20).
Results
Characteristics of the study populations
Twenty children with CKD and 20 age- and gender-matched control children were studied. Fifty percent of the control children were siblings of the children with CKD. Table 1 shows the characteristics of the children with CKD, and Table 2 shows the anthropometric characteristics of both groups of children. None of the children had circulating parathyroid hormone concentrations that fell outside national recommendations.
Age, weight, height, BMI
Compared with healthy controls, children with CKD were not significantly different in age, but they were lighter (weight-SDS −0.31 ± 1.17 vs. 0.66 ± 0.99; p = 0.008) and their BMI was significantly lower (BMI-SDS: −0.13 ± 1.06 vs. 0.65 ± 1.04; p = 0.023) for actual age, but not when the BMI-SDS was adjusted according to height age (0.89 ± 0.78 vs. 0.65 ± 1.04; p = 0.085) (Table 2). Children with CKD also had a non-significant tendency to be shorter and have less LBM (Table 2).
Basal metabolic rate
Compared with healthy age- and gender-matched controls, children with CKD tended to have slightly lower values for BMR (1296 ± 318 vs. 1325 ± 178 kcal/day; p = 0.720) and for predicted BMR (1269 ± 247 vs. 1360 ± 261 kcal/day; p = 0.262), but the BMR value expressed as a percentage of predicted BMR was higher for children with CKD (102 ± 12 vs. 99 ± 14; p = 0.570). However, none of these differences reached statistical significance (linear regression adjusted for covariates) (Table 3).
Severity of disease
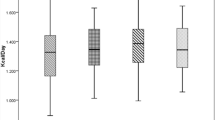
Within the CKD group, and despite the small sample size, a lower eGFR (ml/min/m2) was significantly related to a lower BMR expressed as the percentage of predicted BMR (β = 0.3, r = 0.517, p = 0.019). This relationship remained independent of nutritional status (using weight- and height-SDS: β = 0.314, r 2 = 0.41, p = 0.022) or LBM (using the equation of Lohman et al. [21]: β = 0.357, r 2 = 0.414, p = 0.014) when considered either alone or in combination (multiple regression models) (Fig. 1). The same significant effect was found for eGFR on BMR expressed in kilocalories per day adjustment for age, weight, height and sex [p = 0.020 using weight (kg) and height (m); p = 0.040 using weight- and height-SDS), which remained significant after further adjustment with LBM (kg) (p = 0.017, p = 0.025, respectively), despite the small numbers.
Discussion
Comparison with healthy children
This is the first UK study to report measured BMR (kcal/day) in children with CKD. It adds to the sparse literature available from other countries and helps clarify some conflicting and confusing information about energy expenditure in children with CKD.
Three previous studies [14, 13, 15] involving REE measurements in children with CKD differed in their reports on severity of disease and growth deficit. Because the terms REE and BMR are not necessarily synonymous, and REE measurement conditions may differ, we have kept the terminology of the original studies and state where differences are known or unclear.
The first study [14] found no significant difference in REE between CKD children on haemodialysis and controls, although the mean REE was 9 % lower in the CKD group (1386 ± 262 vs. 1524 ± 170 kcal/day), compared to 2 % in the present study. However, all of the children in the study of Tounian et al. [14] had end-stage disease and were receiving haemodialysis, compared to 15 % (3/20) in the present study. The more severe disease in the study of Tounian et al. [14] was associated with greater weight and height deficits relative to their control group [mean 10 kg (21 %) and 13 cm (8 %)] than in the present study [mean 7 kg (16 %) and 4 cm (3 %)]. When the results of Tounian et al. were expressed in kilocalories per kilogram fat-free mass (kcal/kg/fat-free mass, with fat-free mass measured by anthropometry), the discrepancy became smaller.
The second study [13] found that the REE of the CKD children was reduced by as much as 22 % compared to the control group (1067 ± 191 vs. 1372 ± 290 kcal/day). However, all of the children in this study again had end-stage renal disease and were receiving haemodialysis, which was associated with the largest weight and height deficits compared to the control group [mean 15 kg (33 %) and height 18 cm (12 %)]. When adjustments were made for fat-free mass, the differences in REE were no longer significant. Using graphical data presented in the paper we was estimated that the discrepancy between the groups after adjustment for fat-free mass (at a calculated mean value of 30 kg for the combined groups) was reduced to 7 %.
The third study [15] found no significant difference in REE (1147 ± 278 kcal/day) between CKD children and three predictive equations based on reference populations (Mayo: 1336 ± 299 kcal/day; Passmore: 1116 ± 309 kcal/day; FAO/WHO/UNU: 1125 ± kcal/day). The mean measurements of REE in children with CKD were slightly higher than values estimated by the Passmore equation (+2.8 %) and FAO/WHO/UNU equation (+1.9 %), but lower than those by the Mayo equation (–14 %). Unlike the other two studies this study lacked a control group, which meant that no comparisons could be made with a reference group of children under the same study conditions. Although the eGFR was >50 mL/min/m2, the children had a greater weight (−0.85) and height (−1.16) deficit than the children in the present study (−0.31 and −0.26, respectively) for reasons that are unclear.
The above studies also differed in other respects, such as the uncertainty of measurement conditions for energy expenditure and its duration (which ranged from 20 min to 2 h). The study of Tounian et al. [14] was also confounded by inclusion of the results on two adults. However, despite these differences between studies, they seemed to be consistent in three respects. First, children with CKD tended to be shorter and lighter and have a lower mean metabolic rate than control children, with highly significant differences reported in one study [13]. Secondly, when adjustments were made for body size (fat-free mass), the discrepancies were reduced, with no significant differences between the groups in any of the studies, including the present study. Thirdly, no significant difference was found between children with CKD and hypothetical children with the same weight, height and gender (using predictive equations). Finally, children with more severe CKD had a lower metabolic rate. Looked at in this way, despite differences in methodologies and study populations, the results of this study are consistent with those in the reviewed literature. However, any significant differences may be masked by the small sample sizes, stages of disease and modes of treatment, ages, gender mix and the wide range of eGFR.
Severity of disease
This is the first study to explore BMR in the context of kidney function (eGFR) in children with CKD, although three studies [31, 32, 6] in adults have explored this relationship.
One of these studies [31] reported an increase in REE, measured in kilocalories per kilogram per day, with decreasing creatinine clearance (r = −0.763, p < 0.001), another study [32] found a decrease in REE (r = 0.48, p = 0.009), measured in kilocalories per day, with decreasing eGFR, and the third study [6] found no relationship between the two variables. In one of the studies REE was measured in a sitting position a few hours after a light breakfast [32]. This study only involved patients with diabetes and CKD, while the two other studies involved CKD patients without comorbid conditions. It is difficult to draw consistent conclusions from these studies, which differed in population groups, methodology and indices of REE.
The reasons for the progressively lower BMR in children with more severe CKD assessed using eGFR in our study are unclear. However, the kidney is known to account for 6 % of REE [33] in healthy children. Since the majority of kidney energy consumption occurs in the kidney tubules, which reabsorb solutes from the glomerular filtrate, it is likely that, as kidney function decreases, so does kidney EE. To our knowledge, this has not been formally studied in children.
Inflammation [6–8, 34, 10], infection [11] and hyperparathyroidism [12, 8] may also affect REE. However, measures to assess inflammation and infection were not studied here, so the effect of these factors on this group of children is unknown. Furthermore, all children studied here had parathyroid hormones levels within national recommended limits at the time of study, and none had significant infections within the previous 3 months.
In conclusion, this is the first study to compare BMR (kcal/day) of children with CKD and age- and sex-matched controls across modes of treatment. Although those with CKD (mean CKD stage 3) were not found to differ significantly from healthy controls (before and after adjustment for age, gender, weight and height), there was a tendency for children with CKD to have lower values of measured BMR, which can be explained by the tendency for children with CKD to be lighter. This is also the first study to examine the relationship between BMR and kidney function (eGFR) in children with CKD. A significant relationship was found so children with a lower eGFR had a lower BMR before and after adjustment for weight, height and LBM.
These findings need careful consideration since BMR alone does not necessarily reflect total energy requirements. Physical activity needs to be taken into account as it is the most variable component of total energy expenditure. In addition, increases or decreases in energy need to be considered for malnourished, growth-retarded children who need repletion, or obese individuals who need to lose weight (or not gain it).
This study suggests that children with CKD (mean CKD stage 3 disease) have a BMR comparable to healthy children, but that it is significantly related to the severity of disease measured by eGFR: those who were sicker had significantly lower metabolic rates. It therefore seems reasonable to use EAR as the basis of energy prescriptions for children with CKD.
References
Scientific Advisory Committee on Nutrition (2011) Dietary reference values for energy. TSO, London. Available at: http://www.SACN.gov.uk
Department of Health (1991) Dietary reference values for food energy and nutrients for the United Kingdom. No.41. HMSO, London
Shaw V, Lawson M (2007) Clinical paediatric dietetics. Nutritional assessment. John Wiley & Sons, Chichester
KDOQI Work Group (2009) KDOQI clinical practice guideline for nutrition in children with CKD: 2008 update. Executive summary. Am J Kidney Dis 53[3 Suppl 2]:S11-104
Food and Agriculture Organization (2004) Human energy requirements. Report of a joint FAO/WHO/UNU Expert consultation no.1. FAO, Rome. Available at: http://www.fao.org
Avesani CM, Draibe SA, Kamimura MA, Colugnati FA, Cuppari L (2004) Resting energy expenditure of chronic kidney disease patients: influence of renal function and subclinical inflammation. Am J Kidney Dis 44:1008–1016
Utaka S, Avesani CM, Draibe SA, Kamimura MA, Andreoni S, Cuppari L (2005) Inflammation is associated with increased energy expenditure in patients with chronic kidney disease. Am J Clin Nutr 82:801–805
Kamimura MA, Draibe SA, Avesani CM, Canziani ME, Colugnati FA, Cuppari L (2007) Resting energy expenditure and its determinants in hemodialysis patients. Eur J Clin Nutr 61:362–367
Wang AY, Sea MM, Tang N, Lam CW, Chan IH, Lui SF, Sanderson JE, Woo J (2009) Energy intake and expenditure profile in chronic peritoneal dialysis patients complicated with circulatory congestion. Am J Clin Nutr 90:1179–1184
Mafra D, Deleaval P, Teta D, Cleaud C, Arkouche W, Jolivot A, Fouque D (2011) Influence of inflammation on total energy expenditure in hemodialysis patients. J Ren Nutr 21:387–393
Schneeweiss B, Graninger W, Stockenhuber F, Druml W, Ferenci P, Eichinger S, Grimm G, Laggner AN, Lenz K (1990) Energy metabolism in acute and chronic renal failure. Am J Clin Nutr 52:596–601
Cuppari L, de Carvalho AB, Avesani CM, Kamimura MA, Dos Santos Lobao RR, Draibe SA (2004) Increased resting energy expenditure in hemodialysis patients with severe hyperparathyroidism. J Am Soc Nephrol 15:2933–2939
Marques de Aquino T, Avesani CM, Brasileiro RS, de Abreu Carvalhaes JT (2008) Resting energy expenditure of children and adolescents undergoing hemodialysis. J Ren Nutr 18:312–319
Tounian P, Salaun JF, Bensman A, Melchior JC, Veinberg F, Morgant G, Fontaine JL, Girardet JP (1995) Energy-balance in children and young-adults receiving hemodialysis for chronic renal failure. Clin Nutr 14:341–347
Shapiro AC, Bandini LG, Kurtin PS (1992) Estimating energy-requirements for children with renal-disease—a comparison of methods. J Am Diet Assoc 92:571–573
IBM (2012) SPSS SamplePower. Available at: http://www.ibm.com/software/products/us/en/spss-samplepower
SECA GmbH & Co. KG (2012). Available at: http://www.seca.com/english/uk/home/. Accessed 23 Sept 2013
Child Growth Foundation (1994) LMS growth programme. Harlow Healthcare, South Shields. Available at: http://www.childgrowthfoundation.org. Accessed Jan 2007
Delderfield M (2005) SDS individual calculator for British 1990 Growth Reference Data. Public Health Informatics, University of Manchester, Manchester. Available at: http://www.phsim.man.ac.uk/SDScalculator. Accessed Dec 2014
Baty International. Available at: http://www.baty.co.uk/products/categories/170. Accessed Jan 2007
Lohman TG, Slaughter MH, Boileau RA, Bunt J, Lussier L (1984) Bone mineral measurements and their relation to body density in children, youth and adults. Hum Biol 56:667–679
GE Healthcare. Deltatrac 11. GH Healthcare UK, Little Chalfont. Available at: http://www.gehealthcare.com/usen/oximetry/docs/881439.pdf. Accessed 23 Sept 2013
Garrow JS, James WPT, Ralph A (2000) Human nutrition and dietetics, 10th edn. Churchill Livingston, Edinburgh
Medical Research Council. Physical activity assessment—indirect calorimetry. Available at http://dapa-toolkit.mrc.ac.uk/physical-activity-assessment/methods/indirect-calorimetry/index.html. Accessed 31 March 2011
University Hospital Southampton Southampton University Hospital NHS Foundation Trust UHS SUHNFT (2006/2007) Standard operating procedure: measuring resting energy expenditure using indirect calorimetry: GEM standard operating procedure: measuring resting energy expenditure using indirect calorimetry: GEM). Standard operating procedure. WTCRF, Southampton University Hospital NHS Foundation Trust, Southampton
UHS SUHNFT (2006/2007) Standard operating procedure: measuring resting energy expenditure using indirect calorimetry: Delta-trac II standard operating procedure: measuring resting energy expenditure using indirect calorimetry: Delta-trac II). Standard operating procedure. WTCRF, Southampton University Hospital NHS Foundation Trust, Southampton
Elia M, Livesey G (1992) Energy expenditure and fuel selection in biological systems: the theory and practice of calculations based on indirect calorimetry and tracer methods. World Rev Nutr Diet 70:68–131
Schofield WN (1985) Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39[Suppl 1]:5–41
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
IBM SPSS (2012) Available at: http://public.dhe.ibm.com/common/ssi/ecm/en/yts03009usen/YTS03009USEN.PDF. Accessed 23 Sept 2013
Kuhlmann U, Schwickardi M, Trebst R, Lange H (2001) Resting metabolic rate in chronic renal failure. J Ren Nutr 11:202–206
Panesar A, Agarwal R (2003) Resting energy expenditure in chronic kidney disease: relationship with glomerular filtration rate. Clin Nephrol 59:360–366
Elia M (1992) Energy expenditure in the whole body. Energy metabolism. Tissue determinants and cellular corollaries. Raven Press, New York
Wang AY-M, Sea MM-M, Tang N, Lam CW-K, Chan IH-S, Lui S-F, Sanderson JE, Woo J (2009) Energy intake and expenditure profile in chronic peritoneal dialysis patients complicated with circulatory congestion. Am J Clin Nutr 90:1179–1184
Acknowledgments
Research funding was awarded by the Health Foundation, through the ‘Leadership through clinical practice award’ (2005), for clinical research and leadership training. This study was also supported by the NIHR Southampton Clinical Research Facility.
This report is independent research by the National Institute for Health Research Biomedical Research Unit Funding Scheme. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anderson, C.E., Gilbert, R.D. & Elia, M. Basal metabolic rate in children with chronic kidney disease and healthy control children. Pediatr Nephrol 30, 1995–2001 (2015). https://doi.org/10.1007/s00467-015-3095-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3095-5