Abstract
Background
Determination of resting energy expenditure (REE) is an important step for the nutritional and medical care of patients with chronic kidney disease (CKD). Methods such as indirect calorimetry or traditional predictive equations are costly or inaccurate to estimate REE of CKD patients. We aimed to develop and validate predictive equations to estimate the REE of non-dialysis dependent-CKD patients.
Methods
A database comprising REE measured by indirect calorimetry (mREE) of 170 non-dialysis dependent-CKD patients was used to develop (n = 119) and validate (n = 51) a new REE-predictive equation. Fat free mass (FFM) was assessed by anthropometry and by bioelectrical impedance (BIA).
Results
The multiple regression analysis generated three equations: (1) REE (kcal/day) = 854 + 7.4*Weight + 179*Sex – 3.3*Age + 2.1 *eGFR + 26 (if DM) (R2 = 0.424); (2) REE (kcal/day) = 678.3 + 14.07*FFM.ant + 54.8*Sex – 2*Age + 2.5*eGFR + 140.7* (if DM) (R2 = 0.449); (3) REE (kcal/day) = 668 + 17.1*FFM.BIA – 2.7*Age − 92.7*Sex + 1.3*eGFR − 152.3 (if DM) (R2 = 0.45). The estimated REE (eREE) was not different from the mREE (P = 0.181), a high ICC was found and the mean difference between mREE and eREE was not different from zero for the three equations in the validation group. eREE accuracy between 90 and 110% was observed in 55.3%, 62.5% and 61% of the patients for Eqs. (1), (2) and (3), respectively.
Conclusion
The equations showed acceptable accuracy for REE prediction making them a valuable tool to support practitioners to provide more reliable energy recommendations for this group of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease is a widespread health condition with an estimated global prevalence of 9.1% in 2017 [1]. This outlines that a significant number of individuals require special medical nutrition therapy to control the metabolic derangements coming from CKD. This effort aims to slow the disease progression, to ameliorate the symptoms related to uremia and ultimately to maintain adequate nutritional status by avoiding the development of protein energy wasting (PEW), sarcopenia and obesity [2, 3]. According to a meta-analysis recently published, the occurrence of PEW is present in 11% to 54% of non-dialysis dependent CKD patients (NDD-CKD) and is a well-known risk factor for increased morbidity and mortality [4].
In this context, determination of energy expenditure of CKD patients is crucial to provide adequate energy supply and nutritional care [3]. Indirect calorimetry is a reference method for the determination of resting energy expenditure (REE), which is the main component of total energy expenditure, but its execution is compromised by its high cost and the need for trained professionals [5].
Therefore, the use of REE predictive equations, developed from healthy subjects is widely disseminated in the routine care of hospitals and outpatient clinics. However, low accuracy of such equations has been found when applied to CKD [6], especially in NDD-CKD patients in whom the REE has been shown to be lower in comparison to healthy individuals [7]. Thus, studies seeking to develop equations to estimate REE of this particular group of patients would add valuable information for the routine in nutritional care.
In search for more accurate tools, our group and others have developed specific equations with good accuracy to estimate the REE of patients undergoing hemodialysis [8,9,10]. But, to date, there are no equations developed specifically for NDD-CKD populations.
The aim of this study was to develop and validate an equation to estimate the REE of NDD-CKD patients, using body composition variables easily obtained by clinicians, to provide a more reliable estimation of energy requirement directed to this group of patients.
Methodology
Subjects
This study was developed using a database derived from previous cross-sectional studies [7, 11, 12] from our research group, which aimed to evaluate the REE measured by indirect calorimetry from NDD-CKD patients recruited from the renal outpatient clinic of the Federal University of Sao Paulo and Oswaldo Ramos Foundation (São Paulo, SP, Brazil). The inclusion criteria were age over 18 years, stable clinical conditions and normal thyroid function. Patients were excluded when on therapy with corticosteroid or immunosuppressive medication, had malignant diseases, human immunodeficiency virus (HIV) and acute illnesses. Most patients were following a diet prescribed by a renal dietitian with 30 to 35 kcal/kg/day and 0.6 to 0.8 g of protein/kg/day. The cohort comprised 170 patients. Written and informed consent was collected from each patient. The studies were approved by the Ethics and Research Committee of the Federal University of São Paulo.
Study protocol
All subjects were initially interviewed to ensure the eligibility criteria, to explain the study protocol and those that accepted to participate in the study, signed the informed consent and received information regarding procedures for the REE measurement. After 1 to 2 weeks, in a pre-scheduled day, the patients arrived at the outpatient CKD clinic early in the morning, in fasting conditions, for the measurement of REE and for blood drawn. The patients were then served with a light breakfast and the same trained dietitian that performed the REE measurement, performed the body composition assessment.
Resting energy expenditure
REE was measured by indirect calorimetry using an open-circuit, ventilated, computerized, metabolic system (Vmax® 29n series Sensor Medic Corp, Yorba Linda, CA, USA). The flow sensor was calibrated with a syringe piston permitting high and low inspiratory and expiratory flows’ measurements. Before each REE analysis, oxygen and carbon dioxide sensors were calibrated using a reference mix of gases of known composition.
Patients were given instructions to maintain their regular medication, to abstain from physical activity for 24 h before the test and to keep their usual sleep routine. Before the initiation of REE measurement, it was checked if patients had followed the instructions, in particular in regards to hours of fasting and physical exercise.
Patients attended the clinic at 8:00 AM after a 12 h overnight fast. Before initiating the test, they rested for 30 min in a recumbent position and, afterward, they were instructed to breathe for 30 min through a transparent plastic canopy placed over their heads in a quiet, dimly lit, thermoneutral room.
Energy expenditure was calculated from measured VO2 and VCO2 using Weir formula [13] and REE was expressed as kcal/day.
The respiratory quotient was calculated as the ratio between the VCO2 exhaled and that of the VO2 consumed.
Intraindividual variation was assessed to quantify the magnitude of error between measurements of the same individual. Nine individuals had their REE measured on two consecutive occasions and a 5% variation was found.
Body composition
Body composition was measured by bioelectrical impedance analysis using a single frequency tetrapolar technique with an electrical current of 800 µA at 50 kHz (BIA 101 Quantum, RJL Systems, USA). Subjects were instructed to lay in a supine position and two electrodes were placed on their hand and wrist and another two were positioned on the foot and ankle on the right side of their body. The software Fluids & Nutrition (Version 3.0) provided by the manufacturer was used to estimate body composition.
Anthropometric measurements
Measurements were performed by the same observer and consisted of body weight, height, and skinfold thickness. Subjects’ weight and height were measured in a platform manual scale balance equipped with a stadiometer (Filizola®, São Paulo, Brazil) wearing only light clothes and no shoes. Height was measured with patients standing in an upright position with chin aligned with feet. Body fat was determined as described by Durnin and Wormesley [14] by the sum of skinfolds performed at four sites (biceps, triceps, subscapular, and suprailiac) on the non-dominant arm using a Lange Caliper® (Cambridge Instrument, Cambridge, Maryland, USA). The average of three sets of measurements was performed for each site. Fat- free mass (kg) was calculated by the subtraction of fat mass (kg) from total body weight (kg).
Laboratory data
Blood samples were drawn just before the indirect calorimetry test and after an overnight fast of 12 h. Serum creatinine, urea, and glucose were determined by a standard autoanalyzer. Intact parathyroid hormone (PTH) and C-reactive protein (CRP—high sensitivity assay) were determined by immunochemiluminescence. Glomerular filtration rate was calculated by the CKD-EPI equations as described by Levey et al. [15].
Statistical analysis
Shapiro–Wilk test was applied to investigate normality. Results are expressed as means ± standard deviation (SD), median and interquartile ranges, or proportions. Comparisons between groups were performed using Student’s t-test, Mann–Whitney’s test or chi-squared test, as appropriate. From the entire cohort of 170 patients, 30% (n = 51) were randomly selected according to age and gender and allocated in the group used for the validation [16]. The remaining 119 patients were used to derive predictive models. Simple correlation was applied to screen for potential REE predictors, using REE as the dependent variable. The selected variables were then applied to multiple linear regression analysis to construct models using the enter method. Collinearity among independent variables was evaluated and when two variables showed high collinearity, the one with the strongest correlation coefficient with measured REE was used in the models. Equations that showed the highest determination coefficient using variables easily obtained in the clinical practice were selected to be tested with data from the validation group. The accuracy of equations was evaluated through Bland–Altman graphical analysis, systematic bias was assessed using Student's t-test for a single sample to test whether the mean differences were equal to zero. The proportional bias was evaluated by linear regression analysis to determine if the difference was influenced by the magnitude of measures, considering the difference of the values as the dependent variable and the average between them as the independent variable. Positive bias was considered when p < 0.05. Intraclass correlation (ICC) test and P10 test (percentage of patients whose estimated REE was accurately predicted within 90 to 110% range, in relation to measured REE) were also performed. Statistical analysis was performed using SPSS for Windows (Version 18, 2002, SPSS Inc., Chicago, IL, USA). Statistical significance was considered at p < 0.05.
Results
Characteristics of patients
Table 1 shows the demographic, clinical and laboratory characteristics of patients in the total cohort (n = 170) and according to the equation (n = 119) and validation (n = 51) groups. As can be seen, no differences were observed between the equation and validation groups for all characteristics. The majority of patients were men, the mean age was around 50 years and over 20% of the participants had diabetes. Mean BMI of patients in the total sample was of 26.7 ± 5 kg/m2, 3% of patients had BMI under 18.4 kg/m2, in 34.7% of the patients BMI was between 18.5 and 24.9 kg/m2. BMI between 25 and 29.9 kg/m2 was found in 41.2% of the patients and in 21.2% BMI was over 30 kg/m2.
Regarding age, 15.9% of patients were aged from 18 to 40 years, 44.1% between 41 and 59 years and age over 60 years comprised 40% of the cohort.
Patients had been on conservative treatment for 24 (9–48) months. Moreover, CKD stages 3b (27.6%) and 4 (38.8%) prevailed among the groups. No patient was classified on stage 1, 12.4% of the patients were on stage 3a, 2.4% were on stage 2 and only 1.8% were on stage 5 of CKD.
Development of REE predictive equations
In the sample of 119 patients, the variables with the highest correlation coefficient with REE in the univariate analysis were body weight (R = 0.458; p < 0.001), height (R = 0.446; p < 0.001), FFM measured by anthropometry (R = 0.587; p < 0.001) and FFM measured by BIA (R = 0.590; p < 0.001). FFM measured by anthropometry was 49.2 ± 9.2 kg and by BIA 50.2 ± 10.5 kg (p = 0.04). Among the laboratory measurements, the only REE significant correlation coefficient was with CRP (R = 0.253; p = 0.01). REE was also correlated with eGFR (R = 0.213; p = 0.02).
Since REE differed between men and women (1446 ± 220 kcal/day and 1232 ± 177.0 kcal/day, p < 0.001 respectively), and between patients with and without DM (1434 ± 246.5 kcal/day and 1345.5 ± 222.3 kcal/day, p = 0.03, respectively), both variables were tested in the REE regression models showing an overall fit improvement in the equations, especially the presence of DM, that improved the fit by close to 10% of equations based on FFM, assessed by BIA and anthropometry, and contributed to fit improvement in the body weight-based equation.
Although age was not correlated with REE (R = − 0.067; p = 0.467), a 7% increase in the regression coefficient was verified after the inclusion of this variable in the models. Both CRP and PTH variables presented a low but significant correlation with REE, however they did not improve the model´s fit when added separately or combined.
The models were constructed and those with the best determination coefficient (R2) using variables usually available in clinical practice were selected for validation. Finally, the multiple regression analysis resulted in three equations based on weight, FFM assessed by anthropometry and FFM by BIA.
The development of the equations is described as follows in Tables 2, 3 and 4:
Equation validation
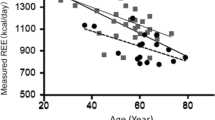
In the validation group (n = 51), REE estimated by the Eqs. (1), (2), and (3) (1396.8 ± 179.1 kcal/day, 1403.02 ± 180.1 kcal/day, and 1357.3 ± 177.8 kcal/day, respectively) were not different from the measured REE (1356.3 ± 233.9 kcal/day) and showed no differences when compared among each other (p = 0.181) (Fig. 1).
The models showed good reliability as evaluated by ICC (CI 95%). Values were of 0.761 (0.581; 0.863) for Eq. (1), 0.841 (0.711; 0.913) for Eq. (2) and 0.791 (0.62; 0.885) for Eq. (3). REE accuracy (90 to 110%) was observed in 55.3% of patients for Eq. (1), 62.5% for Eq. (2) and 61% for Eq. (3). Bland and Altman analysis plot (Figs. 2 and 3) showed mean biases of 40.5 ± 183.05 kcal/day for Eq. (1) and of 37.3 ± 155.6 kcal/day and 43.5 ± 161.6 kcal/day for Eqs. (2) and (3), respectively and were not different from zero (p = 0.121, p = 0.115, and p = 0.077, respectively). Equation (3) was the only one with no proportional bias (p = 0.154). Moreover, Eqs. (1), (2), and (3) presented SEE of 178, 177, and 168 kcal/day, respectively, which means that actual eREE falls from the regression line within and error of 12.7%, 12.6% and 12.4%, respectively. Standardized regression coefficient showed that the variables weight (0.431), FFM.Ant (0.526) and FFM.BIA (0.812) presented higher strength toward mREE, than all other variables included in each equation.
Bland–Altman analysis of measured (mREE) and estimated REE (eREE) by Eq. 3. P-value below the mean ± SD refers to systematic bias, while P-value on the bottom left of each plot refers to the proportionality bias
Discussion
In the present study, we developed equations to estimate the REE of NDD-CKD patients. To our knowledge, this is the first study to develop such equations for this patient group. In the clinical practice, the commonly used equations such as Harris-Benedict [17] and Schofield [18], both developed based on healthy individuals, are often used to estimate REE, also named as basal metabolic rate. These equations are usually applied for patients with chronic diseases such as CKD. However, the adequacy of these equations for this group of patients is questionable as clearly previously demonstrated. Kamimura et al. [6] and Sridharan et al. [19] observed that the Harris-Benedict and Schofield equations overestimated the REE of NDD-CKD.
Although differences in demographic and anthropometric characteristics of healthy populations from the previous predictive equations might explain to a certain extent the overestimation found, the disease-related features of NDD-CKD patients might also impact the results. Studies have shown that the REE measured by indirect calorimetry of clinically stable NDD-CKD patients was 8.5% [7] and 15% [20] lower than that of pair-matched healthy subjects. Even adjusting for FFM, Avesani et al. [7] found the REE of NDD-CKD patients was 7.1% lower. They also observed values of respiratory quotient (RQ) similar to the observed in this study which was higher than the RQ of nomal controls. It is possible that NDD-CKD patients have a different pattern of fuel utilization in fasting state in comparison to healthy individuals, however, this question warrants further investigation. Since the kidneys account for approximately 7% of the REE [21] and the tissue injuries present in CKD may lead to a diminished influx of blood to the organ, what may explain, at least in part, the lower energy expenditure observed [22]. Moreover, the reduction of food consumption is commonly observed in CKD patients, which in turn leads the body to reduced REE as an adaptive response in an attempt to spare the body from major weight loss [23]. In this context, the development of specific CKD-REE equations is of importance to provide a more reliable estimation of REE and to replace the use of traditional equations, which may result in unreliable dietary energy prescription.
In the present study, we sought to develop equations based on variables easily obtained in clinical practice that could better predict the REE. Besides body weight, we also investigated whether FFM obtained either by anthropometry or by BIA would provide better results.
Despite the differences in the methods used to estimate FFM in the current study, the relationship of this body compartment with measured REE was quite similar considering both methods. In the univariate analysis, FFM by anthropometry and by BIA explained around 35% the REE variation. Moreover, comparable results of validation were obtained with both FFM equations which means that both can provide an acceptable range of prediction of REE. Our results also showed that, if FFM by BIA or by anthropometry is not available, body weight may be an option to be applied in the equation to estimate REE, even though the accuracy of the prediction equation was somewhat lower than those of FFM.
It is noteworthy, though, that all three equations explained not more than 45% of variations in REE, presented a mean bias around 3% and an average standard error of estimation of 12.6%. These results are slightly lower than those observed in a previous study from our group [10], in which we developed and validated REE specific equations from hemodialysis patients based on body weight and FFM by anthropometry. Both equations predicted about 50% of REE’s variations. Conversely, equations based on healthy individuals reached a higher coefficient of determination values. Indeed, models based on body weight and FFM in young adults and overweight individuals predicted 77% and 70.6% respectively of the variations in the REE [24, 25].
It is noteworthy that equations traditionally used did not reach such high values of the coefficient of determination. For instance, equations developed by Schofield for men and women with age ranging from 30–60 years presented a coefficient of 0.36 and 0.46, respectively [18]. The coefficient achieved by the Harris and Benedict equation was 0.75 and 0.53 for men and women aged between 15 to 74 years of age, respectively [17]. To explore the reasons for these discrepancies is out of the scope of the present study, but it is important to point out that in CKD metabolic derangements and the accumulation of uremic toxins may interfere in energy metabolism in the whole body and, specifically, of FFM, through mechanisms not identified, what may affect REE.
There is evidence of a reduction in muscle mass and strength in CKD mainly due to the activation of complex catabolic pathways. In comparison to healthy controls, a reduction of mitochondrial oxidative enzymes and related proteins in the synthesis of muscle contractile proteins was found in the muscle biopsy of NDD-CKD patients [26]. Thome et al. [27] obtained similar results and pointed out that uremic metabolites might interfere negatively in skeletal muscle energetics, leading to a reduction in energy transfer to muscle cells.
It is important to point out, however, that even though some factors contribute to decreasing the REE of patients that appear to be clinically stable, there is a myriad of chronic conditions prevalent in CKD which may lead to increased REE, such as poorly controlled diabetes and inflammation.
In a previous study from our group, the REE of NDD-CKD patients with poorly controlled diabetes was 12.5% higher in comparison to those without DM [28]. In the present study, the REE of patients with diabetes was 6.6% higher than that of non-diabetic patients and the addition of DM in the model improved the general fit of the equations. Events that are common to DM such as hyperglucagonemia and insulin deficiency may lead to increased muscle catabolism and gluconeogenesis, providing cells with noncarbohydrate substrates such as amino acids or fatty acids for energy metabolism [29]. These metabolic pathways take place at high energy cost [30] and might be responsible for the higher REE.
Inflammation has also been shown to increase REE of CKD patients [11]. Besides, a 13% reduction of REE was found after treating the inflammatory condition of NDD-CKD patients [12]. In the present study, the addition of CRP in the equations did not provide improvement in the models fit, probably due to the relatively low serum levels of CRP of our patients.
Some limitations of the present study should be mentioned. The studied cohort comprised relatively clinically stable patients with CKD in stages 3b and 4, therefore, patients in the early or more advanced stages of CKD were underrepresented. Potential disease-related conditions were not identified, especially the ones related to muscle energy metabolism, which would provide a better determination coefficient of models. Studies are still needed to understand the role of muscle metabolism of CKD on energy expenditure. The strength of this study relies on the use of simple body composition variables that can be easily obtained in clinical practice in the development of the predictive equations. Besides, the large number of patients provided a sufficient sample size to validate the equations, yielding more reliable results.
In conclusion, our results showed that the three equations derived from variables easily obtainable from clinical practice can predict REE of NDD-CKD patients with an acceptable range of accuracy. These equations can be a valuable tool to be used in clinical practice to support practitioners to provide more reliable energy recommendations for this group of patients.
References
Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M et al (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet
Avesani CM, Kamimura MA, Cuppari L (2011) Energy expenditure in chronic kidney disease patients. J Ren Nutr 21(1):27–30
Ortiz A, Sanchez-ni MD (2019) Sarcopenia in CKD : a roadmap from basic pathogenetic mechanisms to clinical trials ~ o. Clin Kidney J 12(1):110–112
Kopple JD, Feroze U (2011) The effect of obesity on chronic kidney disease. J Ren Nutr (Internet) 21(1):66–71
Oshima T, Berger MM, De Waele E, Guttormsen AB, Heidegger CP, Hiesmayr M et al (2017) Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin Nutr. 36(3):651–662
Kamimura MA, Avesani CM, Bazanelli AP, Baria F, Draibe SA, Cuppari L (2011) Are prediction equations reliable for estimating resting energy expenditure in chronic kidney disease patients? Nephrol Dial Transplant 26(2):544–550
Avesani CM, Draibe SA, Kamimura MA, Dalboni MA, Basile Colugnati FA, Cuppari L (2004) Decreased resting energy expenditure in non-dialysed chronic kidney disease patients. Nephrol Dial Transplant 19(12):3091–3097
Vilar E, Machado A, Garrett A, Kozarski R, Wellsted D, Farrington K (2014) Disease-specific predictive formulas for energy expenditure in the dialysis population. J Ren Nutr 24(4):243–251
Byham-Gray LD, Parrott JS, Peters EN, Fogerite SG, Hand RK, Ahrens S et al (2018) Modeling a predictive energy equation specific for maintenance hemodialysis. J Parenter Enter Nutr 42(3):587–596
Fernandes TO, Avesani CM, Kamimura MA, Aoike DT, Cuppari L (2019) Estimating resting energy expenditure of patients on dialysis: development and validation of a predictive equation. Nutrition 67–68:1–8
Avesani CM, Draibe SA, Kamimura MA, Basile Colugnati FA, Cuppari L (2004) Resting energy expenditure of chronic kidney disease patients: influence of renal function and subclinical inflammation. Am J Kidney Dis 44(6):1008–1016
Utaka S, Cm A, Sa D, Ma K, Andreoni S, Cuppari L (2005) Inflammation is associated with increased energy expenditure in patients with chronic kidney disease. Am J Clin Nutr 82(4):801–805
Weir JdV (1948) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 109(5):1–9
Durnin JVGA, Womersley J (1974) Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 32(01):77–97
Levey AS, Stevens LA, Schmid CH, Zhang YL, Iii AFC, Feldman HI et al (2006) Article annals of internal medicine a new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Hair Jr JF, Black WC, Barry JB, Anderson RE (2014) Multivariate data analysis, 7th edn
Harris JA, Benedict FG (1918) A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci 4(12):370–373
Schofield W (1985) Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39(Suppl 1):5–41
Sridharan S, Wong J, Vilar E, Farrington K (2016) Comparison of energy estimates in chronic kidney disease using doubly-labelled water. J Hum Nutr Diet 29(1):59–66
Sullivan AJO, Lawson JA, Chan M, Kelly JJ, Al OSET (2002) Body composition and energy metabolism in chronic renal insufficiency. Am J Kidney Dis 39(2):369–375
Kuhlmann U, Schwickardi M, Trebst R, Lange H (2001) Resting metabolic rate in chronic renal failure. J Ren Nutr 11(4):202–206
Wang Z, Ying Z, Bosy-westphal A, Zhang J, Schautz B, Later W et al (2010) Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr 1–4:4
Slee AD (2012) Exploring metabolic dysfunction in chronic kidney disease. Nutr Metab 9(36):1–16
Willis EA, Herrmann SD, Ptomey LT, Honas JJ, Bessmer CT, Donnelly JE et al (2016) Predicting resting energy expenditure in young adults. Obes Res Clin Pract 10(3):304–314
Almajwal AM, Abulmeaty MMA (2019) New predictive equations for resting energy expenditure in normal to overweight and obese population. Int J Endocrinol 1–15
Adey D, Kumar R, McCarthy JT, Sreekumaran NK (2000) Reduced synthesis of muscle proteins in chronic renal failure. Am J Physiol Endocrinol Metab 278(2412):219–225
Thome T, Salyers ZR, Kumar RA, Hahn D, Berru FN, Ferreira LF et al (2019) Uremic metabolites impair skeletal muscle mitochondrial energetics through disruption of the electron transport system and matrix dehydrogenase activity 4. Am J Physiol Cell Physiol 317(4):C701–C713
Avesani CM, Cuppari L, Silvaa C, Sigulem DM, Cendoroglo M, Sesso R et al (2001) Resting energy expenditure in pre-dialysis diabetic patients. Nephrol Dial Transplant 16(960):556–565
Caron N, Peyrot N, Caderby T, Verkindt C, Dalleau G (2016) Energy expenditure in people with diabetes mellitus: a review. Front Nutr 3:1–10
Piaggi P, Thearle MS, Bogardus C, Krakoff J (2015) Fasting hyperglycemia predicts lower rates of weight gain by increased energy expenditure and fat oxidation rate. J Clin Endocrinol Metab 100(3):1078–1087
Funding
This study was supported by Fundação Oswaldo Ramos. Lilian Cuppari receives a scholarship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) 302765/2017-4.
Author information
Authors and Affiliations
Contributions
TOF and LC conceptualized the study design and wrote the manuscript; CMA conceptualized the study and performed data collection; TOF and DAT performed statistical analysis and data interpretation. All authors revised the manuscript and provided important intellectual considerations.
Corresponding author
Ethics declarations
Conflict of interest
We herein state that none of the information and material in this manuscript is included in another manuscript, has been published previously, or is currently under consideration for publication elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Oliveira Fernandes, T., Avesani, C.M., Aoike, D.T. et al. New predictive equations to estimate resting energy expenditure of non-dialysis dependent chronic kidney disease patients. J Nephrol 34, 1235–1242 (2021). https://doi.org/10.1007/s40620-020-00899-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00899-7