Abstract
Deposition of calcium oxalate crystals in the kidney and bone is a hallmark of primary hyperoxaluria (PH). Since the bone compartment can store massive amounts of oxalate, patients present with recurrent low-trauma fractures, bone deformations, severe bone pains, and specific oxalate osteopathy on X-ray. Bone biopsy from the iliac crest displays specific features such as oxalate crystals surrounded by a granulomatous reaction corresponding to an invasion of bone surface by macrophages. The objective of this manuscript is therefore to provide an overview of bone impairment in PH, by reviewing the current literature on bone and dental symptoms as well as imaging techniques used for assessing bone disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calcium oxalate is a highly insoluble salt, corresponding to an end-product of the metabolism of carbohydrate, vitamin C, and some amino acids [1]; it is excreted almost entirely by the kidney [2]. Primary hyperoxalurias (PH) are a group of orphan autosomal recessive diseases inducing an overproduction of oxalate in the liver, leading first to a renal deposition of insoluble oxalate crystals, and explaining the onset of an almost constant nephrocalcinosis, as recently summarized in a thorough review on the topic [2]. A global deposition of insoluble oxalate crystals (also known as systemic oxalosis) occurs when renal function declines. End-stage renal disease (ESRD) can be reached anytime between the first months and the sixth decade of life, however before 25 years of age in 50 % of cases [3]. Oxalate crystals can be found in kidneys, urinary tract, bones, heart, nerves, joints, skin, bone marrow, soft tissues, and retina [2, 3]. In the most severe cases of PH, namely in PH1 (mutations in the AGXT gene encoding the peroxisomal alanine-glyoxylate aminotransferase enzyme), the overall prognosis has been completely modified with the development of combined (synchronous or sequential) liver-kidney transplantation (CLKT); in less severe cases of PH, such as PH2 (mutations in the GRHPR gene encoding the cytosolic glyoxylate reductase-hydroxypyruvate reductase), ESRD may also occur. In the other forms/types of PH, for example in PH3 (mutations in the HOGA1 gene, encoding the mitochondrial 4-hydroxy-2-oxoglutarate aldolase), ESRD has not been described so far [4].
The bone compartment can store massive amounts of oxalate [5, 6], but the threshold of glomerular filtration rate (GFR) at which this occurs is debatable and might be as high as 30 to 45 ml/min per 1.73 m2 [3, 7, 8]. Patients with PH1, and less frequently patients with PH2, can present with severely impaired renal function, and thus systemic oxalosis and bone damage [2]. From a clinical point of view, they can experience severe bone pain, frequent pathological fractures and subsequent bone deformations.
Our objective is therefore to provide an overview of bone impairment in PH, by reviewing the current literature on bone and dental symptoms as well as imaging techniques used for assessing bone disease.
Clinical symptoms of bone impairment in PH patients
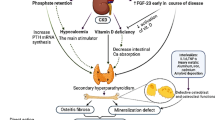
In addition to renal osteodystrophy directly related to chronic kidney disease itself [9], patients with PH1 or PH2 can experience severe bone pain, pathological fractures, subsequent bone deformations, erythropoietin-resistant anemia, and in the most severe cases, subperiosteal tophi; however, the exact epidemiology of such bone impairment is extremely difficult to assess due to the rarity of the disease and the lack of clinical data evaluating bone metabolism in the international registries. A transversal Lebanese study performed in 2008 in 12 consecutive patients with PH1, aged between 2 and 17 years, found skeletal pains in four and fractures in two patients (all of them undergoing renal replacement therapy (RRT)); it is noteworthy that radiological signs were present even in the absence of clinical bone symptoms [10]. In this study, the authors also found spondylolysis in three patients, a frequency that is greater than the expected incidence of such an abnormality in a general pediatric population [10].
From the literature review, fractures appear to occur mainly at the femoral neck; the fracture risk persists after CLKT, but, again, the relative risk in comparison to other patients undergoing pediatric renal transplantation is almost impossible to determine [10]. One can hypothesize that the excess risk is observed as long as urine oxalate excretion remains abnormal after CLKT, but no quantitative study has been performed on the topic. From our personal experience, urine oxalate excretion can persist after transplantation for weeks, months, or even years after CLKT.
In the most recent epidemiological studies on PH (OxalEurope registry), a threefold increased risk of death was reported in young patients undergoing RRT before 19 years of age in comparison to other pediatric patients undergoing RRT for another renal disease, but there were no details concerning bone disease in the two groups [11].
PH patients, and mainly those with PH1, due to their younger age at ESRD onset, also present with severe growth retardation that does not appear to truly catch-up after CLKT: in a European multi-center study involving 24 children with PH1 from nine different centers, Nissel reported a moderate catch-up after CLKT performed at a mean age of 8.9 years, with a height SDS at the time of transplantation of –1.79 and a height at the time of last follow-up (mean duration of follow-up after CLKT 5.7 years) of –1.47; at the end of growth, one-third of patients had a reduced final height [12].
From an orphan disease point of view, contiguous gene syndromes involving both the AGXT and the HDAC4 gene have been described to induce both PH1 and brachydactyly during infancy [13]. In older patients with PH, microcrystalline arthropathy has also been described [14].
Hyperoxaluria may also be “secondary” and then occur as a result of excess dietary intake or the result of enteric hyperoxaluria, such as in Crohn disease and bypass surgery; even though these forms may lead to ESRD [15], no specific data on bone impairment are available in this setting. Last, even though a murine model of PH1 has been developed [16], no data on bone metabolism are available.
Conventional radiological evaluation of patients with PH1
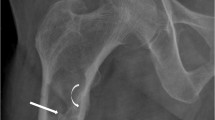
Previous description of bone damage in PH1 dates back to before CLKT was performed and new tools for managing hyperparathyroidism were made available [17]. Under X-ray, characteristics of oxalate osteopathy include dense metaphyseal bands, submarginal metaphyseal lucency, sclerosis of adjacent diaphysis, cystic bone changes, deformities, subperiosteal resorption, blurred trabecular pattern, and increased bone density in vertebra and iliac crest [17–19]. Symmetrical transverse lines of increased bone density at areas of rapid growth have been related to crystalline precipitation in cartilage calcification sites as well as in hematopoietic and other highly vascularized areas [17, 20–22].
In a previous study from our group, we have also shown an acceleration of bone maturation in the youngest patients with PH1, as well as evidence of bone damage such as cystic bone changes, deformations, dense metaphyseal bands in a symmetric manner, cortical thickening in long bones and dense rims around the ossification nucleus in the calcaneum and carpal/tarsal bones; after transplantation, hyperdensity around ossification nucleus progressively decreased [23]. We interpreted these dense circles around the calcaneum as oxalate deposition in the mineralization area during the first months of life. The presence of such abnormalities in children with severe infantile PH1 in this study proves that despite therapeutic improvement, bone involvement remains a problem as these patients had presented several bone fractures both before and after transplantation [23]. These lesions (osteopenia, symmetrical metaphyseal bands in long bones, radio-opaque rims in flat bones, and epiphyseal nuclei) were also described in three young children with infantile severe PH1 [24].
Evaluation of bone impairment in PH1 with newer bone imaging techniques
In 2001, Behnke suggested that measuring volumetric bone mineral density (vBMD) with peripheral quantitative computed tomography (pQCT) could be a valuable and non-invasive tool in determining and monitoring oxalate burden in PH1 [25]; indeed, pQCT is a bone imaging technique that distinguishes cortical vBMD from trabecular vBMD [25]. With this technique, the authors showed increased vBMD at the distal radius, attributed to oxalate deposits, in ten pediatric patients with PH1. This cohort consisted of three patients undergoing maintenance hemodialysis and seven children on conservative therapy, among them one child with pre-emptive liver transplantation. Interestingly, plasma oxalate levels directly correlated with both trabecular and cortical vBMD, but also with serum creatinine.
After pQCT, more accurate tridimensional imaging techniques such as high-resolution peripheral quantitative computed tomography (HR-pQCT) have been developed to assess not only compartmental vBMDs but also trabecular microarchitecture [23]. We previously compared six teenagers with PH1 to 22 non-PH1 CKD children and 19 healthy controls. HR-pQCT parameters did not statistically differ between PH1 patients and other non-PH1 CKD children, but a trend towards lower vBMDs in all bone compartments was observed, with more disorganized trabecular microarchitecture in PH1 children. When compared to healthy controls, PH1 children had significantly lower height and body weight, without any difference for age and gender. They also had a significantly decreased total vBMD and cortical thickness with a trend for decreased cortical and trabecular vBMDs, without significant differences for trabecular microarchitecture [23]. Our cohort consisted of two patients undergoing maintenance hemodialysis and four patients on conservative therapy. Even though the number of patients was very low, it appeared that PH1 patients undergoing hemodialysis displayed higher trabecular/total vBMDs but more disorganized trabecular microarchitecture. We therefore explained the increased vBMDs by oxalate deposits in trabecular bone, since oxalate deposits have a higher volumic mass than hydroxyapatite crystals. Notably, we did not find associations between HR-pQCT results and oxalate levels [23].
Interestingly, in this paper, we also found increased FGF23 serum levels in PH1 patients: the only biological markers that statistically differed between PH1 and non-PH1 pre-dialysis CKD patients were FGF23 and 1,25-dihydroxy (OH)2 vitamin D. Other determinants of phosphate/calcium metabolism such as PTH or 25-vitamin D, serum calcium and phosphorus, glomerular filtration rate, gender, and age were similar in both groups. Osteocalcin, crosslaps, and alkaline phosphatase are all secreted by osteoblasts whilst FGF23 is synthesized by osteocytes. Thus, an isolated increased serum FGF23 may reflect osteocyte activation by oxalate crystals in PH1 patients. If this proved to be true, the suggestion that bone oxalate deposition occurs when the serum oxalate level exceeds 30 to 50 μmol/l and the GFR is below 40 ml/min per 1.73 m2 could be revisited, as patients with subnormal GFR on conservative treatment already experienced a borderline elevated serum FGF23 level.
Considering that children with the highest FGF23 were also those with the greater oxalate load (three with CLKT, one with high urinary oxalate), this would point to FGF23 as a possible marker for bone oxalate storage and could prove very useful if confirmed. However, there was no correlation between serum oxalate level and serum FGF23, probably due to the small number of patients. To the best of our knowledge, other bone biomarkers such as markers of bone formation (e.g., osteocalcin) or bone resorption (e.g., TRACP5b) have not been evaluated in view of their potential ability to reflect oxalate burden, even though a preliminary German report recently discussed the potential relationship between TRACP5b and oxalate burden [26].
Therefore, many questions were raised with these two pilot studies using pQCT and HR-pQCT in PH patients and providing conflicting results. However, these two studies included a limited number of patients; moreover, these patients were not at the same stage of PH1 and CKD, likely explaining at least partly these discrepancies. In the future, prospective longitudinal studies including more patients worldwide would be useful to help physicians in the evaluation of oxalate burden, with both the reference standard (namely bone biopsy) and novel non-invasive bone techniques (such as pQCT, HR-pQCT, or bone MRI).
Dental abnormalities in PH patients
Although teeth are mineralized tissues, oral findings associated with PH are rarely described: less than a dozen case reports have been published since 1973 [27], including bone resorption in the jaws, aggressive external root resorption, rapidly progressive dental mobility, dental pain, deposits in the periodontium, and alveolar bone loss [28–30]. Tooth resorption may be due to chronic inflammation but also to the presence of osteoclast-like cells surrounding the oxalate crystal deposit, as described in bone biopsies from patients with PH and as detailed below [30]. Moskow described in a 29-year-old PH patient an extensive infiltration of crystals in the pulp of the teeth, in the marrow space of the alveolar bone, in the gingival corium, and in the periodontal ligament, with a granulomatous reaction under microscopy [29]. When observed in polarized light, these deposits were green and presented a birefringent aspect, corresponding to calcium oxalate crystals [30]. Since CLKT has been proven successful in patients with PH, one can imagine an increased frequency of oral problems in PH patients in the future; there are no specific guidelines for dental care in patients with PH, but in addition to periodontal therapy and maintenance of a meticulous oral hygiene, splinting of the mobile teeth can be a therapeutic option [28].
Bone biopsy in PH patients
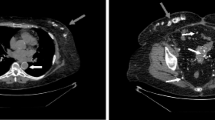
Although they are considered as the reference standard for bone evaluation, iliac bone biopsies remain invasive and cannot be performed for clinical routine follow-up [31]. If performed in patients with PH, oxalate crystals are isolated or grouped in clusters, forming star-like figures often surrounded by a granulomatous reaction due to an invasion of bone surface by macrophages, as illustrated in Fig. 1. Indeed, the presence of deposits of calcium oxalate into bone induces a granulomatous reaction in this tissue; in this setting, macrophages do not phagocytize crystals but appear involved in bone resorption [32–34]. Some reports have focused on bone histomorphometry in patients with PH [5, 6, 35]; to the best of our knowledge, the largest study of bone histomorphometry in patients with PH was published in 1991. It corresponded to the clinical, radiological, and histological study of 20 cases of PH, at a time when genetic testing was not available: 19 out of 20 patients had ESRD, either treated by maintenance hemodialysis or by renal (or liver-kidney) transplantation. After starting hemodialysis, 17 patients suffered from bone pain; three presented with vertebral crush fractures, and one with multiple spontaneous fractures. Diffuse bone sclerosis with a homogeneous pattern on the axial skeleton and a patchy appearance on the peripherical skeleton, bone translucency, and subperiosteal phalangeal resorption were the main radiological symptoms. Oxalate crystals surrounded by a giant cell granuloma were always observed on bone biopsy (16 cases). Bone resorption was observed in nine cases, hyperparathyroidism in 14 cases, and osteomalacia in seven cases. Bone resorption was not necessarily observed with hyperparathyroidism and was therefore explained (at least partly) by the granulomatous reaction around oxalate crystals [36]. In 1995, Marangella also demonstrated that the presence of calcium oxalate crystals in patients with PH affected the histomorphometric pattern on iliac bone biopsies, with increased resorptive areas and decreased bone formation rate [5]. In 1987, we already reported a case of a 50-year-old adult with a late onset of PH and bone oxalosis [32].
Conventional microscopy on iliac bone tissue from a patient with bone oxalosis. Bone tissue showed a normal lamellar texture (periphery of A). The bone marrow (center A and high magnifications in B and C) contains numerous oxalate deposits (white needle-like ghosts, with black stars in the middle). These deposits are surrounded by giant multinucleated cells (A, B, C) represented by black arrows suggesting a foreign-body granuloma. The black rectangle on Fig. 1A corresponds to the magnified zone (Fig. 1B). A, B, C: May Grünwald Giemsa staining (original magnifications × 5, × 10, × 20, respectively)
Bone is a living material that has a complex hierarchical structure [37]. Besides its mechanical and protective functions, bone mineral is also a reservoir of ions (mainly calcium and phosphorus) that can be stored or released during remodeling to maintain mineral balance. Bone tissue is composed of a mineral phase (calcium phosphate crystallized as apatite crystals) deposited in an organic matrix mainly constituted by type I collagen fibrils. The term mineralization involves not only the initial deposition of mineral in organic matrix but also its maturation until the upper mineral density in a given volume of matrix is reached. Besides physiological calcifications (apatite in bone, teeth, calcified cartilage), pathological deposits (apatite, calcium pyrophosphate, calcium oxalate) have also been identified in humans in joints, in numerous soft tissues (including kidney, skin, muscles and blood vessels), but also in bone tissue, mainly in the bone marrow in the vicinity of the trabeculae from cancellous bone tissue. Calcium oxalate deposits were exclusively reported as case reports in PH patients without a global analysis in a substantial number of patients. Indeed, scanning and transmission electron microscopy analyses have already been performed on the calcium oxalate deposits in soft tissues and X-ray diffraction studies have been essentially used in order to identify the nature of the crystals in urinary calculi, but specific data in bone are missing, with biophysical and ultrastructural methods being rarely used to study human bone lesions during oxalosis [38–40].
Conclusions and perspectives
This review illustrates the challenge of bone metabolism in patients with PH despite continuous therapeutic improvement such as earlier diagnosis and treatment, new tools for managing hyperparathyroidism, or CLKT [41]. In any case, early CLKT should be the ultimate goal in treating PH1 patients who reach ESRD, since the rate of hepatic oxalate production exceeds the clearance of any dialytic modality.
In the future, we hope that international registries, including more patients worldwide, will help physicians to more precisely define bone impairment in patients with PH on one hand, and to accurately evaluate oxalate burden to improve therapeutic adequacy on the other hand.
References
Marengo SR, Romani AM (2008) Oxalate in renal stone disease: the terminal metabolite that just won’t go away. Nat Clin Pract Nephrol 4:368–377
Cochat P, Rumsby G (2013) Primary hyperoxaluria. N Engl J Med 369:649–658
Cochat P, Liutkus A, Fargue S, Basmaison O, Ranchin B, Rolland MO (2006) Primary hyperoxaluria type 1: still challenging! Pediatr Nephrol 21:1075–1081
Belostotsky R, Seboun E, Idelson GH, Milliner DS, Becker-Cohen R, Rinat C, Monico CG, Feinstein S, Ben-Shalom E, Magen D, Weissman I, Charon C, Frishberg Y (2010) Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am J Hum Genet 87:392–399
Marangella M, Vitale C, Petrarulo M, Tricerri A, Cerelli E, Cadario A, Barbos MP, Linari F (1995) Bony content of oxalate in patients with primary hyperoxaluria or oxalosis-unrelated renal failure. Kidney Int 48:182–187
Toussaint C, Vienne A, De Pauw L, Gelin M, Janssen F, Hall M, Schurmans T, Pasteels JL (1995) Combined liver-kidney transplantation in primary hyperoxaluria type 1. Bone histopathology and oxalate body content. Transplantation 59:1700–1704
Morgan SH, Purkiss P, Watts RW, Mansell MA (1987) Oxalate dynamics in chronic renal failure. Comparison with normal subjects and patients with primary hyperoxaluria. Nephron 46:253–257
Marangella M, Cosseddu D, Petrarulo M, Vitale C, Linari F (1993) Thresholds of serum calcium oxalate supersaturation in relation to renal function in patients with or without primary hyperoxaluria. Nephrol Dial Transplant 8:1333–1337
Bacchetta J, Harambat J, Cochat P, Salusky IB, Wesseling-Perry K (2012) The consequences of chronic kidney disease on bone metabolism and growth in children. Nephrol Dial Transplant 27:3063–3071
El Hage S, Ghanem I, Baradhi A, Mourani C, Mallat S, Dagher F, Kharrat K (2008) Skeletal features of primary hyperoxaluria type 1, revisited. J Child Orthop 2:205–210
Harambat J, van Stralen KJ, Espinosa L, Groothoff JW, Hulton SA, Cerkauskiene R, Schaefer F, Verrina E, Jager KJ, Cochat P, European Society for Pediatric Nephrology/European Renal Association-European Dialysis and Transplant Association (ESPN/ERA-EDTA) Registry (2012) Characteristics and outcomes of children with primary oxalosis requiring renal replacement therapy. Clin J Am Soc Nephrol 7:458–465
Nissel R, Latta K, Gagnadoux MF, Kelly D, Hulton S, Kemper MJ, Ruder H, Soderdahl G, Otte JB, Cochat P, Roquet O, Jamieson NV, Haffner D (2006) Body growth after combined liver-kidney transplantation in children with primary hyperoxaluria type 1. Transplantation 82:48–54
Tammachote R, Kingsuwannapong N, Tongkobpetch S, Srichomthong C, Yeetong P, Kingwatanakul P, Monico CG, Suphapeetiporn K, Shotelersuk V (2012) Primary hyperoxaluria type 1 and brachydactyly mental retardation syndrome caused by a novel mutation in AGXT and a terminal deletion of chromosome 2. Am J Med Genet A 158A:2124–2130
Verbruggen LA, Bourgain C, Verbeelen D (1989) Late presentation and microcrystalline arthropathy in primary hyperoxaluria. Clin Exp Rheumatol 7:631–633
Bernhardt WM, Schefold JC, Weichert W, Rudolph B, Frei U, Groneberg DA, Schindler R (2006) Amelioration of anemia after kidney transplantation in severe secondary oxalosis. Clin Nephrol 65:216–221
Salido EC, Li XM, Lu Y, Wang X, Santana A, Roy-Chowdhury N, Torres A, Shapiro LJ, Roy-Chowdhury J (2006) Alanine-glyoxylate aminotransferase-deficient mice, a model for primary hyperoxaluria that responds to adenoviral gene transfer. Proc Natl Acad Sci U S A 103:18249–18254
Day DL, Scheinman JI, Mahan J (1986) Radiological aspects of primary hyperoxaluria. Am J Roentgenol 146:395–401
Brancaccio D, Poggi A, Ciccarelli C, Bellini F, Galmozzi C, Poletti I, Maggiore Q (1981) Bone changes in end-stage oxalosis. Am J Roentgenol 136:935–939
Kamoun A, Hammou A, Chaouachi S, Bellagha I, Lakhoua R (1995) Radiological signs of type I primary hyperoxaluria. Ann Radiol (Paris) 38:440–446
Wiggelinkhuizen J, Fisher RM (1982) Oxalosis of bone. Pediatr Radiol 12:307–309
Fisher D, Hiller N, Drukker A (1995) Oxalosis of bone: report of four cases and a new radiological staging. Pediatr Radiol 25:293–295
Ring E, Wendler H, Ratschek M, Zobel G (1989) Bone disease of primary hyperoxaluria in infancy. Pediatr Radiol 20:131–133
Bacchetta J, Fargue S, Boutroy S, Basmaison O, Vilayphiou N, Plotton I, Guebre-Egziabher F, Dohin B, Kohler R, Cochat P (2010) Bone metabolism in oxalosis: a single-center study using new imaging techniques and biomarkers. Pediatr Nephrol 25:1081–1089
Orazi C, Picca S, Schingo PM, Fassari FM, Canepa G (2009) Oxalosis in primary hyperoxaluria in infancy: Report of a case in a 3-month-old baby. Follow-up for 3 years and review of literature. Skeletal Radiol 38:387–391
Behnke B, Kemper MJ, Kruse HP, Muller-Wiefel DE (2001) Bone mineral density in children with primary hyperoxaluria type I. Nephrol Dial Transplant 16:2236–2239
Stenger KO, Sterneck M, Kromminga A (2014) TRACP5b als ein alternativer marker für schwere und fortschreiten der erkrankung an prïmarer hyperoxalurie typ 1. Nieren und Hochdruckkrankheiten:217–228
Glass RT (1973) Oral manifestations in primary hyperoxaluria and oxalosis. Report of a case. Oral Surg Oral Med Oral Pathol 35:502–509
Mitsimponas KT, Wehrhan T, Falk S, Wehrhan F, Neukam FW, Schlegel KA (2012) Oral findings associated with primary hyperoxaluria type I. J Craniomaxillofac Surg 40:e301–e306
Moskow BS (1989) Periodontal manifestations of hyperoxaluria and oxalosis. J Periodontol 60:271–278
Guerra EN, Vianna L, Sobreira MN, de Araujo FN, de Melo NS (2011) Oral manifestations of hyperoxaluria. J Craniofac Surg 22:2191–2192
Hernandez JD, Wesseling K, Pereira R, Gales B, Harrison R, Salusky IB (2008) Technical approach to iliac crest biopsy. Clin J Am Soc Nephrol 3(Suppl 3):S164–S169
Benhamou CL, Pierre D, Geslin N, Viala JF, Maitre F, Chavassieux P, Edouard C, Meunier PJ (1987) Primary bone oxalosis: the roles of oxalate deposits and renal osteodystrophy. Bone 8:59–64
Adams ND, Carrera GF, Johnson RP, Latorraca R, Lemann J Jr (1982) Calcium-oxalate-crystal-induced bone disease. Am J Kidney Dis 1:294–299
Milgram JW, Salyer WR (1974) Secondary oxalosis of bone in chronic renal failure. A histopathological study of three cases. J Bone Joint Surg Am 56:387–395
Brady HR, Fay WP, Meema HE, Rabinovich S, Rapoport A, Oreopoulos DG (1989) Oxalate bone disease–an emerging form of renal osteodystrophy. Int J Artif Organs 12:715–719
Benhamou CL, Bardin T, Tourliere D, Voisin L, Audran M, Edouard C, Lafage MH, Sebert JL, de Vernejoul MC, Wendling D (1991) Bone involvement in primary oxalosis. Study of 20 cases. Rev Rhum Mal Osteoartic 58:763–769
Bala Y, Farlay D, Boivin G (2013) Bone mineralization: from tissue to crystal in normal and pathological contexts. Osteoporos Int 24:2153–2166
Gherardi G, Poggi A, Sisca S, Calderaro V, Bonucci E (1980) Bone oxalosis and renal osteodystrophy. Arch Pathol Lab Med 104:105–111
Jahn H, Frank RM, Voegel JC, Schohn D (1980) Scanning electron microscopy and x-ray diffraction studies of human bone oxalosis. Calcif Tissue Int 30:109–119
Lagier R, Revell P, Schoenboerner A (1982) Calcium oxalate deposition in growing bone: anatomical and radiological study in a case of primary oxalosis. Metab Bone Dis Relat Res 4:49–59
Cochat P, Hulton SA, Acquaviva C, Danpure CJ, Daudon M, De Marchi M, Fargue S, Groothoff J, Harambat J, Hoppe B, Jamieson NV, Kemper MJ, Mandrile G, Marangella M, Picca S, Rumsby G, Salido E, Straub M, van Woerden CS, OxalEurope (2012) Primary hyperoxaluria Type 1: indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant 27:1729–1736
Disclosures
None of the authors has direct conflicts of interest concerning the present manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bacchetta, J., Boivin, G. & Cochat, P. Bone impairment in primary hyperoxaluria: a review. Pediatr Nephrol 31, 1–6 (2016). https://doi.org/10.1007/s00467-015-3048-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3048-z