Abstract
Background
Proper traction allows safer and easier endoscopic submucosal dissection; however, single-point traction may not be sufficient. In this study we assessed the safety, efficacy, and feasibility of our newly developed multipoint traction device.
Methods
During an ex vivo study using a Konjac training model, two experts and two trainees resected 80 mock lesions of 20-mm diameter by performing endoscopic submucosal dissection with and without multipoint traction. The primary outcome was the success rate of the procedure involving traction. The secondary outcomes were the submucosal dissection time, dissection speed, and perforation during endoscopic submucosal dissection. During the in vivo study, to clarify the initial clinical outcomes, we used data from the electronic medical record of patients at our institution who underwent gastric and colorectal endoscopic submucosal dissection, which was performed by experts with our newly developed multipoint traction device, from March to December 2022.
Results
The ex vivo study indicated that all traction procedures were successful. Higher resection speeds were observed with endoscopic submucosal dissection with traction than without traction (P < 0.001). Perforations were not observed. During the first in vivo clinical study, traction was feasible during 20 gastric and colorectal endoscopic submucosal dissection procedures. No adverse events occurred.
Conclusions
Our multitraction device can increase the submucosal dissection speed and simplify endoscopic submucosal dissection techniques, thus safely reducing technical challenges. The application of this device for endoscopic submucosal dissection could lead to safer and more efficient procedures.
Clinical registration UMIN Clinical Trials Registry, Japan (registration number UMIN000053384).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Endoscopic submucosal dissection (ESD), which was initially developed in Japan for the treatment of superficial gastric neoplasms [1], allows en bloc resection of superficial gastrointestinal tumors regardless of the lesion size and location. For early gastrointestinal neoplasms with a negligible risk of lymph node metastasis, ESD has been widely accepted as the standard of care in many countries, as favorable short-term and long-term outcomes have been reported [2,3,4]. However, ESD remains a difficult and time-consuming procedure because of intestinal motility, thin submucosa, and intrinsic muscles, poor scope maneuverability, frequent fibrosis, and anatomy of the folds [5]. Therefore, extensive training is required to master this technique [6, 7].
A lack of traction is one factor that contributes to the technical difficulty of ESD. During ESD with a single endoscope, direct traction cannot be applied to the tissue. Gravity and endoscopic caps are helpful tools that are routinely used to provide some traction during ESD; however, these tools are not always adequate. To compensate for the lack of traction, various methods have been developed to improve ESD in the gastrointestinal tract [8]. The devices that are currently available provide only single-point traction; therefore, an additional device may be required to provide sufficient traction or change the direction of traction for large lesions and lesions with scarring [9, 10]. Although methods involving multipoint traction have been explored, none have involved multitraction with the use of a single device. During this study, we developed a novel multipoint traction device consisting of a clip, intermaxillary rubber, and nylon thread. This device can be placed at three points on the lesion instead of at just one point. If the direction of traction needs to be changed after application, then further traction can be applied by pulling the intermaxillary rubber in a different direction. We performed ex vivo and in vivo studies to assess the feasibility, efficacy, and safety of our device.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee of Nagasaki Harbor Medical Center, Nagasaki, Japan (no. R04-019), and it conformed to the provisions of the Declaration of Helsinki.
Ex vivo model
We used an innovative training model of the colon comprising Konjac flour to simulate tissue (VTT-MCS; Kotobuki Medical, Inc., Saitama, Japan) (Fig. 1a). This model consisted of a 0.5-mm mucosal layer as the first layer, 1-mm submucosal layer as the second layer, and 2-mm muscular layer as the third layer (Fig. 1b). Each layer was manufactured using different formulations according to the desired strength and properties. To create this model, a plastic tube with a hole (similar to an overtube) was attached to one side. A plastic tube without a hole was attached to the other side and to a metal butt with an electrode (Fig. 1a). A simulated lesion was created by using a knife to make a circle with a 20-mm diameter.
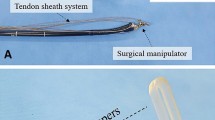
Experimental model and multipoint traction device and attachment procedure. a Colon model comprising Konjac used for endoscopic submucosal dissection. b Three layers of the model. The first layer is the mucous membrane (thickness 0.5 mm). The second layer is the submucosa (thickness 1 mm). The third layer is the muscle layer (thickness 2 mm). c Multipoint traction device comprising a clip, intermaxillary rubber, and nylon thread. d After making a full circumferential incision, attachment to the center of the lesion is performed. e Next, a ring of nylon thread is attached to the sides of the lesion. f If the lesion is large and traction is insufficient, then an additional nylon thread can be attached to the lesion as an anchor (red clip). g Finally, a ring of nylon thread is placed slightly in front of the mucosa contralateral to the lesion
Multipoint traction device
We created our multipoint traction device using a clip (Zeo clip; Zeon Medical, Inc., Tokyo, Japan), intermaxillary rubber (Elastics; Tomy International, Inc., Tokyo, Japan), and nylon thread (Fig. 1c). We cut a piece of nylon thread to approximately half the length of the intermaxillary elastic, threaded it through the wing hole of the clip, and tied the two ends of the nylon thread to the intermaxillary elastic on both sides to form a ring that could be moved along the intermaxillary elastic. We created a ring so the clip could be fixed when it was attached to the contralateral mucosa. This device can be stored in a Zeoclip system (Zeon Medical, Inc.) and delivered to the lesion through the scope.
Traction method
Before starting the ESD procedure, we prepared the multipoint traction device by placing it on a reusable catheter (Zeo clip; Zeon Medical, Inc.) that was designed to deliver and deploy the device. When performing ESD with the multipoint traction device (ESD-MT), we followed a specific process after creating the initial mucosal incision and applying the device directly to the lesion in the following order (Fig. 1d–g):
-
(1)
Front edge of the lesion,
-
(2)
Right edge of the lesion,
-
(3)
Left edge of the lesion,
-
(4)
A nylon thread ring that was connected to the intermaxillary elastic was secured to the mucosa on the opposite side of the tumor.
Trainees and experts
Two trainees and two experts participated in this study. The trainees’ experience involved performing fewer than 20 gastrointestinal ESD procedures, whereas the experts’ experience involved performing more than 200 gastrointestinal ESD procedures. Both the trainees and experts were physicians at our hospital.
ESD procedure
Conventional ESD techniques have been described in detail [11, 12]. Briefly, ESD was performed using a single-channel endoscope (GIF-Q260J; Olympus, Tokyo, Japan) with a transparent hood (D-201-11804; Olympus) attached to the tip and a dual knife (Olympus) with the VIO300D electrosurgical unit (ERBE Elektromedizin GmbH, Tübingen, Germany). A hyaluronic acid solution (MucoUp®; Boston Scientific Co., Ltd., Marlborough, MA, USA) with a small amount of indigo carmine stain was injected (01841; Top Corporation, Tokyo, Japan) in the submucosal layer of the area surrounding each mock lesion. After injection, a circumferential mucosal incision was created around each lesion using a dual knife in the EndoCut I mode (effect, 3; interval, 3; duration, 3). Submucosal dissection was performed using a dual knife in the forced coagulation mode (effect, 3; 50 W). Either ESD-MT or ESD without the multipoint traction device (ESD-C) was performed. Traction was applied to the lesion after completing the mucosal incision around the marked area when ESD-MT was performed (Fig. 2a–f).
Endoscopic submucosal dissection with multipoint traction. a A circular lesion with a 2-cm diameter was created. b A full circumferential incision is made. c Attachment at three places (center, left, and right) on the lesion and attachment of the elastic on the contralateral side. d–f Traction allows an expanded field of view of the submucosa, and the sides of the lesion can be moved outward to provide a view of the sides as well as the center of the lesion. Traction is effective throughout the procedure and the lesion is resected without perforation
Four endoscopists (expert A, expert B, trainee A, and trainee B) performed the ESD procedures for 20 simulated colon lesions; 10 lesions were resected using ESD-C and 10 lesions were resected using ESD-MT. The order in which the different types of procedures was performed was standardized to minimize bias (Fig. 3). The data of 80 lesions resected using ESD (40 using ESD-MT and 40 using ESD-C) were included in our analysis.
Outcome measurements
The efficacy and safety of the traction method were determined by comparing the ESD-C and ESD-MT groups. The primary outcome was the success rate of the procedure. Secondary outcomes were the en bloc resection rate, submucosal dissection time (measured from the start to the completion of the submucosal dissection procedure), submucosal dissection speed, and perforation rate during the procedure. En bloc resection was defined as the removal of the entire lesion as one piece. A perforation was defined as any hole created in the muscle layer during ESD. The technical results of ESD-C and ESD-MT based on the experience of the endoscopist were compared during a sub-analysis.
Statistical analysis
Because this was a pilot study, there were no previous data regarding the clinical outcomes of ESD using a multitraction device. Therefore, the sample size could not be calculated a priori. Continuous data are expressed as the median and interquartile range (IQR). Between-group comparisons (ESD-MT and ESD-C) were performed using the Mann–Whitney U test for continuous variables with non-normal distribution. All analyses were performed using Bell Curve for Excel (Microsoft, Redmond, WA, USA), and P < 0.05 was considered significant.
Evaluation of the clinical feasibility of gastric and colorectal ESD using a multipoint traction device
In vivo studies were also conducted for the purpose of confirming the performance of our traction devices in vivo with peristalsis and bleeding and the performance of insertion and deployment of the traction devices in the in vivo scope geometry.
To clarify the initial clinical outcomes of gastric and colorectal ESD using a multipoint traction device performed only by experts, the data of 20 consecutive patients who underwent gastric and colorectal ESD using our novel multipoint traction device performed only by experts between March and December 2022 at our institution were collected from the electronic medical records.
Results
Technical outcomes of ESD according to the traction method
Eighty colorectal ESD procedures were performed (Table 1). All procedures involving the multipoint traction device were successful. The median submucosal dissection time was significantly shorter for the ESD-MT group (median, 5.9 min; IQR, 4.5–8.7 min) than for the ESD-C group (median, 10.6 min; IQR, 7.8–16 min, P < 0.001). The en bloc resection rate was 100% for both groups. Using elliptical approximation, the area of the excised mucosa was larger in ESD-MT group than in the ESD-C group. Furthermore, the dissection speed of the ESD-MT group (median, 75 mm2/min; IQR, 42.9–104.5 mm2/min) was faster than that of the ESD-C group (median, 41.6 mm2/min; IQR, 28.8–64.8 mm2/min, P < 0.001). Perforations were not observed in either group.
Comparison of technical outcomes based on the experience of the endoscopist
Both the experts and trainees had shorter submucosal dissection times and faster dissection speeds when performing ESD-MT, and these results were similar to the overall results. However, the experts had significantly longer procedure times when performing ESD-MT compared to those when performing ESD-C (Table 2).
Evaluation of the clinical feasibility of gastric and colorectal ESD using a novel multipoint traction device
A total of 20 lesions were included in the evaluation of the clinical outcomes of gastric and colorectal ESD using a multipoint traction device: 10 lesions were resected using gastric ESD-MT and 10 were resected using colorectal ESD-MT. The dissection speed during gastric ESD-MT was 37.5 mm2/min (IQR 23.3–59.5), and that during colorectal ESD-MT was 23.5 mm2/min (IQR 20.8–45.8). The procedure completion, en bloc resection, and R0 resection rates were 100%. The successful multipoint traction device attachment rate was 100%. The median multipoint traction device attachment times for gastric and colorectal ESD when performing ESD-MT were 3 min (IQR 4.2–5 min) and 3.7 min (IQR 4–6.9 min), respectively, and the median multipoint traction device retrieval times were 0.5 min (IQR 0.4–0.7 min) and 1.6 min (IQR 0.6–1.6 min), respectively (Table 3).
Discussion
Using a versatile training model of the colon, our findings indicated that our novel traction device reduced the ESD duration without causing any adverse events, thus benefiting the experts and trainees.
Recently, various traction methods have been introduced for technically challenging ESD procedures. A comprehensive review of these methods for different organs (5 methods for the esophagus, 13 for the stomach, and 12 for the colon and rectum) revealed that although traction devices did not significantly reduce the procedure time during gastric ESD, they effectively reduced both time and adverse events associated with colorectal ESD [13]. For both gastric and colorectal ESD, several traction methods, such as the S–O clip [14] and multi-loop traction devices, have been used; these methods provided traction without affecting endoscopic manipulation and allowed direct application of traction to the lesion independently of the endoscope [15,16,17,18]. Our traction device provided several advantages. First, the use of intermaxillary rubber at the center of the device enabled stronger traction, clearer exposure of the submucosa and dissection line, and more precise incision and coagulation, and it reduced the risks of bleeding and perforation [19, 20]. Additionally, although single-point traction often becomes insufficient as the procedure progresses, our multipoint traction device maintained consistent force throughout the procedure and allowed a broader submucosal view and safer and quicker resection. Second, our device is a three-point traction system that attaches to the left and right edges and center edge of the area to be resected; however, additional clips can be used if needed. Therefore, if more tension or a change in direction is required, then the device can be easily adjusted. Third, the device can be delivered through an endoscope and stored within a clip system, thus eliminating the need for endoscope removal. Fourth, unlike other gravity-based traction systems [21,22,23], our device does not require repositioning of the patient to adjust the traction direction.
Using the single-point traction method, we often achieved clear visibility of the submucosal layer at the traction site. However, the visibility of the sides of the lesion was typically poor, thereby diminishing the effectiveness of traction. Although the use of multiple single-point traction devices has been reported [9], this approach may not be efficient because each device exerts traction in a different direction. In contrast, the multitraction device not only provides central traction but also applies coordinated traction to the sides of the lesion. This coordinated approach facilitates precise targeting of the lesion, enhances the visibility of its boundaries along the submucosal and muscle layers, and enables more accurate and faster ESD. Throughout our study, we did not encounter inadequate traction during dissection or damage to the specimen or mucosa caused by excessive traction.
During our study, we found that the multipoint traction method increased the overall procedure time of the experts, possibly because the lesions were small and easy to resect without traction. Furthermore, the attachment of multiple clips resulted in a longer procedure time than that of the single-point method [18, 24]. In vivo multicenter and prospective studies should be performed to further explore whether single-point or multipoint traction is more effective for different types of lesions.
Additionally, this report presents the initial clinical outcomes of gastric and colorectal ESD using a multitraction device. The results of the current study showed that ESD-MT could be performed using our device for all gastric and colorectal lesions without any adverse events. Moreover, traction appeared to reduce the stress experienced by the practitioner, improve the visibility of the dissected line, and reduce visual field disruption, even in cases of bleeding.
Our study had some limitations. This prospective study was conducted in the absence of bleeding or peristalsis. Furthermore, the number of ESD procedures performed using the multitraction device included in the clinical feasibility evaluation was limited, which might have introduced selection bias attributable to undefined inclusion criteria. Further multi-center prospective clinical studies, including single-traction, are needed to demonstrate the utility of this multitraction device.
In conclusion, using a versatile training model of the colon, we demonstrated that our multitraction device resulted in increased submucosal dissection speeds not only for experts but also for trainees. This multitraction device has the potential to significantly reduce the technical difficulty of ESD, thus benefiting both experts and trainees. Further studies involving humans are required to confirm these results.
References
Gotoda T, Yamamoto H, Soetikno RM (2006) Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol 41:929–942
Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K (2016) Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc 28:3–15
Suzuki H, Takizawa K, Hirasawa T, Takeuchi Y, Ishido K, Hoteya S, Yano T, Tanaka S, Endo M, Nakagawa M, Toyonaga T, Doyama H, Hirasawa K, Matsuda M, Yamamoto H, Fujishiro M, Hashimoto S, Maeda Y, Oyama T, Takenaka R, Yamamoto Y, Naito Y, Michida T, Kobayashi N, Kawahara Y, Hirano M, Jin M, Hori S, Niwa Y, Hikichi T, Shimazu T, Ono H, Tanabe S, Kondo H, Iishi H, Ninomiya M, Oda I for J-WEB/EGC Group (2019) Short-term outcomes of multicenter prospective cohort study of gastric endoscopic resection: ‘Real-world evidence’ in Japan. Dig Endosc 31:30–39
Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Kudo S-E, Tsuruta O, Sugihara K-I, Watanabe T, Saitoh Y, Igarashi M, Toyonaga T, Ajioka Y, Ichinose M, Matsui T, Sugita A, Sugano K, Fujimoto K, Tajiri H (2015) JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 27:417–434
Rösch T, Sarbia M, Schumacher B, Deinert K, Frimberger E, Toermer T, Stolte M, Neuhaus H (2004) Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy 36:788–801
Oda I, Odagaki T, Suzuki H, Nonaka S, Yoshinaga S (2012) Learning curve for endoscopic submucosal dissection of early gastric cancer based on trainee experience. Dig Endosc 24:129–132
Zhang X, Ly EK, Nithyanand S, Modayil RJ, Khodorskiy DO, Neppala S, Bhumi S, DeMaria M, Widmer JL, Friedel DM, Grendell JH, Stavropoulos SN (2020) Learning curve for endoscopic submucosal dissection with an untutored, prevalence-based approach in the United States. Clin Gastroenterol Hepatol 18:580-588.e1
Tsuji K, Yoshida N, Nakanishi H, Takemura K, Yamada S, Doyama H (2016) Recent traction methods for endoscopic submucosal dissection. World J Gastroenterol 22:5917–5926
Kitahara G, Kaneko T, Ishido K, Furue Y, Wada T, Watanabe A, Tanabe S, Kusano C (2023) The efficacy and safety of multi-loop traction device for gastric endoscopic submucosal dissection: a single center prospective pilot study. Sci Rep 13:20050
Okagawa Y, Abe S, Takamaru H, Sekiguchi M, Yamada M, Sakamoto T, Saito Y (2021) A novel technique for adjusting traction direction during colorectal endoscopic submucosal dissection using S-O clip. Endoscopy 53:E177–E178
Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y, Oka M, Ogura K, Kawabe T, Ichinose M, Omata M (2006) Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol 4:688–694
Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M (2009) Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 70:860–866
Abe S, Wu SYS, Ego M, Takamaru H, Sekiguchi M, Yamada M, Nonaka S, Sakamoto T, Suzuki H, Yoshinaga S, Matsuda T, Oda I, Saito Y (2020) Efficacy of current traction techniques for endoscopic submucosal dissection. Gut Liver 14:673–684
Sakamoto N, Osada T, Shibuya T, Beppu K, Matsumoto K, Mori H, Kawabe M, Nagahara A, Otaka M, Ogihara T, Watanabe S (2009) Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest Endosc 69:1370–1374
Sakamoto N, Osada T, Shibuya T, Beppu K, Matsumoto K, Shimada Y, Konno A, Kurosawa A, Nagahara A, Ohkusa T, Ogihara T, Watanabe S (2008) The facilitation of a new traction device (S–O clip) assisting endoscopic submucosal dissection for superficial colorectal neoplasms. Endoscopy 40:E94–E95
Ritsuno H, Sakamoto N, Osada T, Goto SP, Murakami T, Ueyama H, Mori H, Matsumoto K, Beppu K, Shibuya T, Nagahara A, Ogihara T, Watanabe S (2014) Prospective clinical trial of traction device-assisted endoscopic submucosal dissection of large superficial colorectal tumors using the S-O clip. Surg Endosc 28:3143–3149
Nagata M, Fujikawa T, Munakata H (2021) Comparing a conventional and a spring-and-loop with clip traction method of endoscopic submucosal dissection for superficial gastric neoplasms: a randomized controlled trial (with videos). Gastrointest Endosc 93:1097–1109
Matsui H, Tamai N, Futakuchi T, Kamba S, Dobashi A, Sumiyama K (2022) Multi-loop traction device facilitates gastric endoscopic submucosal dissection: ex vivo pilot study and an inaugural clinical experience. BMC Gastroenterol 22:10
Fukami N (2013) What we want for ESD is a second hand! Traction method. Gastrointest Endosc 78:274–276
Fujinami H, Teramoto A, Takahashi S, Ando T, Kajiura S, Yasuda I (2021) Effectiveness of S-O clip-assisted colorectal endoscopic submucosal dissection. J Clin Med 11:141
Matsuzaki I, Hattori M, Yamauchi H, Goto N, Iwata Y, Yokoi T, Tsunemi M, Kobayashi M, Yamamura T, Miyahara R (2020) Magnetic anchor-guided endoscopic submucosal dissection for colorectal tumors (with video). Surg Endosc 34:1012–1018
Kobayashi T, Gotohda T, Tamakawa K, Ueda H, Kakizoe T (2004) Magnetic anchor for more effective endoscopic mucosal resection. Jpn J Clin Oncol 34:118–123
Saito Y, Emura F, Matsuda T, Uraoka T, Nakajima T, Ikematsu H, Gotoda T, Saito D, Fujii T (2005) A new sinker-assisted endoscopic submucosal dissection for colorectal cancer. Gastrointest Endosc 62:297–301
Jinushi R, Tashima T, Terada R, Miyaguchi K, Katsuda H, Ogawa T, Nakano Y, Saito Y, Fujita A, Tanisaka Y, Mizuide M, Mashimo Y, Kawasaki T, Ryozawa S (2022) Effectiveness of a multi-loop traction device for colorectal endoscopic submucosal dissection performed by trainees: a pilot study. Sci Rep 12:10197
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Takuma Okamura, Tetsuro Honda, Tomonari Ikeda, Satoshi Ishida, Yasutaka Kuribayashi, Tatsuki Ichikawa, and Kazuhiko Nakao have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
(MP4 268257 kb)
(MP4 296310 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Okamura, T., Honda, T., Ikeda, T. et al. Development of a novel multipoint traction device for gastric and colorectal endoscopic submucosal dissection and evaluation of its efficacy and safety. Surg Endosc 38, 4704–4711 (2024). https://doi.org/10.1007/s00464-024-10987-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-024-10987-5