Abstract
Background
Postoperative pain is a common issue following laparoscopic cholecystectomy. This meta-analysis aimed to determine if active gas aspiration is more effective than passive gas aspiration in reducing postoperative pain and analgesic requirements.
Methodology
The study conducted a systematic search of various databases, including Embase, Medline, and Cochrane Central Register of Controlled Trials (CENTRAL) via Ovid. It also searched trial registries and reference lists of included studies, with no date restrictions but limited to English language, up to December 21, 2022. The study included all randomized clinical trials that had documented elective laparoscopic cholecystectomy procedure and reported at least one relevant outcome. Articles that included subdiaphragmatic drain, intraperitoneal normal saline infusion, or pulmonary recruitment maneuver were excluded from the analysis. Two reviewers independently and in duplicate assessed the eligibility of studies and extracted data. The study reported findings according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. The risk of bias of the included trials was assessed using the Revised Cochrane Risk of Bias Assessment Tool. The study used a random-effects model to pool data.
Results
This meta-analysis included 5 randomized clinical trials with 367 participants and found that active gas aspiration resulted in significantly lower residual gas volume and total analgesia requirements compared to passive gas aspiration. Active gas aspiration also led to significantly lower shoulder pain scores at 24 h postoperatively. However, no significant differences were observed in hospital stay duration or abdominal pain scores.
Conclusion
The study found that active gas aspiration can be effective in reducing postoperative shoulder pain and analgesic requirements after laparoscopic cholecystectomy, which has important implications for patient care and healthcare costs. Importantly, this intervention does not impose any additional time or financial burden. However, further research is needed to evaluate its impact on other laparoscopic procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Laparoscopic cholecystectomy is a minimally invasive surgical procedure commonly employed for the removal of the gallbladder. Pneumoperitoneum, the inflation of the peritoneal cavity with carbon dioxide (CO2), is a crucial aspect of this technique. Pneumoperitoneum serves several purposes in laparoscopic cholecystectomy, including the creation of a working space, enhanced visualization, and improved maneuverability of surgical instruments [1]. It is achieved by introducing CO2 gas into the peritoneal cavity through a Veress needle or a trocar. The insufflated CO2 gas elevates the abdominal wall, creating a sufficient distance between the gallbladder and surrounding organs, thereby facilitating a safe and efficient surgical procedure.
Despite being minimally invasive, postoperative pain remains a significant issue for patients. Pain management is a crucial aspect of laparoscopic cholecystectomy, and various techniques have been explored to improve postoperative pain outcomes. One such technique involves the aspiration of carbon dioxide gas at the end of the surgery. This method can be categorized as either passive gas aspiration, where the gas is allowed to escape naturally, or active gas aspiration, where the gas is actively removed using suction or a specialized device. The impact of these different gas aspiration techniques on postoperative pain outcomes in laparoscopic cholecystectomy remains a topic of interest and investigation. Understanding the comparative efficacy and potential benefits of passive gas aspiration versus active gas aspiration can aid in optimizing pain management strategies and improving patient satisfaction following this common surgical procedure [2,3,4].
Identifying the most effective technique for gas removal may have significant implications for patient care and healthcare costs. Furthermore, given the widespread use of laparoscopic cholecystectomy, this study may have broader implications for other laparoscopic procedures that require abdominal insufflation.
The primary objective of this study is to determine if there is a significant difference in postoperative pain scores between patients who receive active gas aspiration versus those who receive passive gas aspiration following laparoscopic cholecystectomy. Secondary objectives include evaluating the impact of each technique on the patient's recovery time, length of hospital stay, and overall satisfaction with the procedure.
Materials and methodology
This study was registered on PROSPERO under the registration ID CRD42023400033. We reported findings in this systematic review according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [5].
Eligibility criteria
We included all randomized clinical trials that had documented elective laparoscopic cholecystectomy procedure regardless the indication and the presence of relevant outcomes were deemed eligible for inclusion in this systematic review. However, the study excluded articles that included the following procedures, Sub-diaphragmatic drain, intraperitoneal normal saline infusion, and pulmonary recruitment maneuver. For a study to be included in our meat-analysis, the study had to have at least one relevant outcome to this systematic review. Our primary outcome was postoperative pain measured using visual analog scale or numeric rating scale. Our secondary outcomes were to measure need for analgesics, residual gas volume hospital stay, and abdominal muscle tension.
Search strategy
We searched the following databases; Embase, Medline, and Cochrane Central Register of Controlled Trials (CENTRAL) via Ovid, with no restriction on date; however, we restricted language to only English. Our used MESH terms and keyword were as following: “Laparoscopic Cholecystectomy,” “Gas,” “Pneumoperitoneum,” “Evacuation,” “Residual,” and “Distention.” Furthermore, we searched the following trial registries for yet to be published trials or recently completed: ClinicalTrials.gov, UMIN Clinical Trials Registry, Australian New Zealand Clinical Trials Registry, and MetaRegister of Controlled Trials. We went through the references list of the included RCTs for any possibly missed trials that fit our criteria. The last date of the systematic search was done on December 21, 2022.
Study selection and data extraction
Titles and abstracts’ eligibility screening, full text assessment, and data extraction from eligible trials were done independently and in duplicate by two reviewers. Differences were settled by consensus or the decision of a third reviewer. Data were extracted in Microsoft Office Excel using a predefined template. The extracted data contain the demographic data of the patients, perioperative variables, and outcomes.
Meta-analysis
Data analysis was performed using RevMan (Review Manager) version 5.4 (Cochrane Collaboration). All statistical analyses were performed using the random-effects model. The assessment of the statistical heterogeneity was done using I2 and p value of the Chi2 test. We used 95% confidence interval (CI) and p < 0.05 as a threshold. The standardized mean difference (SMD) was used for continuous outcomes, while the risk ratio (RR) was used for dichotomous outcomes.
Risk of bias assessment
Using the Revised Cochrane Risk of Bias Assessment Tool, two reviewers assessed the risk of bias of the included trials independently and in duplicate [6]. Differences were resolved by consensus or the decision of a third reviewer.
Results
Search
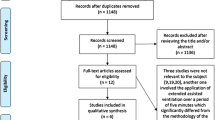
Of the 1816 articles that were screened, 1806 were excluded after duplicates removal, titles screening, and abstracts screening. Of the 10 articles remaining, five were deemed ineligible after full text assessment, leaving five eligible articles representing five RCTs [7,8,9,10,11] (Fig. 1). Four of which were included in the quantitative synthesis [7,8,9,10].
Trials characteristics
The included trials were published between 2011 and 2022 with only one study published in 1994. The total number of participants was 367 patients. Of these, 181 (49.32%) and 186 (50.68%) participants were randomly assigned to be treated by Active and Non-Active gas aspiration, respectively. The participants’ mean age ranged between 43.8 and 57.5 years. The male participants were 172 (46.87%), while the females were 195 (53.13%) (Table 1 and Supplemental Table 1). Perioperative conditions were comparable with regard to the number of trocars used, surgery duration, the presence of local inflammation, inserted gas volume, and maintained gas pressure (Supplemental Table 2).
Method of active gas aspiration
In this section, a thorough overview is provided on the approaches employed by each of the five included studies for evacuating gas in both the intervention and control groups. Notably, two of the studies explicitly indicated that there was no increase in time or cost [8, 10], while the other three studies did not mention any such increase in their findings.
Abdelsamad et al. [7]
In the intervention group, carbon dioxide was extracted through a 5-mm trocar positioned on the left side, using a negative pressure of − 50 kPa. Conversely, in the control group, gas drainage was accomplished via a 12-mm epigastric trocar, applying simultaneous pressure on the abdominal wall from the outside until no further gas could be detected escaping from the abdominal cavity.
Atak et al. [8]
Participants in the intervention group received an aspiration cannula through an accessory port, directed toward the subdiaphragmatic space. Active aspiration was then employed to remove as much residual gas as possible. In contrast, the control group underwent gas evacuation solely through the port site by opening the gas tap.
Fredman et al. [9]
In the intervention group, gas aspiration was actively and vigorously performed through suction and manual compression of the abdomen upon completion of the surgical procedure. On the other hand, the control group did not undergo any measures to evacuate gas from the abdominal cavity or reduce the volume of residual gas.
Lee et al. [10]
Within the intervention group, active suction was executed using a laparoscopic suction irrigation device inserted through a 5-mm trocar, with the trocar pointed toward the subdiaphragmatic space. Continuous suction was applied for a duration of 60 s. In comparison, the control group maintained open gas valves on all three trocars until complete deflation of the abdomen occurred, with no audible or tactile traces of gas escaping from the peritoneal cavity.
Tuvayanon et al. [11]
This study comprised one control group and two intervention groups. In the first intervention group, the subdiaphragmatic port valve was opened, and a negative pressure of 80 mmHg was applied. In the second intervention group, the port valve was opened to release residual CO2 from the abdominal cavity. In contrast, the control group experienced passive gas escape through the subumbilical wound.
Risk of bias assessment
Out of the five eligible RCTs, 2 had an overall low risk of bias, 3 had some concerns, and none had a high overall risk of bias (Fig. 2).
Outcomes
Abdominal pain
Three studies assessed the postoperative abdominal pain scores at 4 and 24 h, including a total of 93 participants in the 4-h study and 164 participants in the 24-h study [7,8,9]. The overall analysis indicates that there were no significant differences observed in the abdominal pain scores between the intervention and control groups. However, it is important to note that the analysis conducted at the 24-h mark exhibited significant heterogeneity.
Meta-analyses were conducted for both the 4-h and 24-h pain scores. The findings revealed that after 4 h, there was a trend toward lower abdominal pain scores in the non-active aspiration group compared to the active aspiration group, but this difference did not reach statistical significance (SMD = 0.21, 95% CI − 0.20–0.61; P = 0.32; I2 = 0%) (Fig. 3). Similarly, after 24 h, the active aspiration group displayed slightly lower pain scores, but again, the results were not statistically significant (SMD = − 0.77, 95% CI − 2.15–0.61; P = 0.28; I2 = 94%) (Figs. 3 and 4).
Shoulder pain after 24 h
Four studies have reported 24 h postoperative shoulder pain, but only two studies have reported the exact value using visual analog scale, while the rest only reported it using number of participants who suffered pain [7, 8, 10, 11]. Thus, our meta-analysis only included two studies with a sample size of 179 participants [8, 10]. The meta-analysis showed that lower shoulder pain scores were significantly associated with patients who underwent active gas aspiration compared to patients who did not undergo an active gas aspiration, and there was no evidence of heterogeneity (SMD = − 0.83, 95% CI − 1.14 to − 0.52; p < 0.01; I2 = 0%) (Fig. 5).
Analgesic requirement
With the exception of the study conducted by Tuvayanon et a.l [11], all the trials included in the analysis provided information on the management of analgesia [7,8,9,10]. Various analgesic approaches and drugs were used to manage pain in patients. In Abdelsamad et al. [7] and Atak et al. [8], standardized anesthesia was administered to all patients, and postoperative analgesia was achieved using medications such as piritramide, metamizol, ibuprofen, and oxycodeine. Piritramide was administered intravenously and continued as an infusion or via a patient-controlled analgesia (PCA) pump. Additional pain therapy was provided as needed, and oral analgesic therapy was initiated with metamizol, ibuprofen, or oxycodeine. Fredman et al. [9] used a similar anesthetic technique, where PCA was employed. Lee et al. [10] also followed a similar general anesthesia technique.
The similarities across these studies lie in the use of standardized anesthesia techniques and the administration of analgesic medications to manage postoperative pain. The drugs used include opioids, such as piritramide, fentanyl, and morphine, and non-opioid analgesics, such as metamizol, ibuprofen, and tramadol.
The differences among the studies include variations in the specific drugs used, dosages, and routes of administration. For example, Abdelsamad et al. [7] utilized piritramide and metamizol intravenously and initiated oral analgesic therapy with metamizol, ibuprofen, or oxycodeine. Atak et al. [8] employed fentanyl, propofol, and rocuronium intravenously for anesthesia induction and maintenance. Fredman et al. [9] used PCA with morphine. Lee et al. utilized intravenous tramadol for analgesia.
The total amount of analgesia required was reported in three studies involving a sample size of 212 participants [8,9,10]. Among these studies, two assessed the analgesic needs of patients by monitoring the frequency of PCA device usage [8, 9], while the remaining study measured the analgesic requirement based on the total amount of additional intravenous tramadol requested by the patients [10]. The findings revealed a significant decrease in the amount of analgesia needed in the active gas aspiration group compared to the non-active gas aspiration group. Notably, there was no significant heterogeneity observed in the results (SMD = − 0.38, 95% CI − 0.65 to − 0.10; p < 0.01; I2 = 0%) (Fig. 6).
Residual gas
Two studies, involving a total of 135 participants, have examined this particular outcome (Abdelsamad et al. [7] and Lee et al. [10]). In the study by Abdelsamad et al. [7], the residual gas volume was quantified in milliliters using magnetic resonance imaging (MRI). The MRI scans allowed for the measurement of the radius (r) and height (h) of the subdiaphragmatic spherical cap formed by the remaining gas. The volume (V) of the residual pneumoperitoneum was then calculated using the formula: V = (π h2/3)·(3 r − h). In the study conducted by Lee et al. [10], X-ray imaging was employed to determine the volume of residual intraabdominal gas. The methodology used for calculating this volume is explicitly outlined in the article by Jackson et al. [12].
The meta-analysis showed that lower residual gas volume was significantly associated with active gas aspiration compared to the non-active aspiration group (SMD = − 1.09, 95% CI − 2.12 to − 0.06; P = 0.04); however, there was statistically significant heterogeneity in this outcome (I2 = 87%) (Supplementary Fig. 1).
Hospital stay
Hospital stay duration has been reported by three studies with a sample size of 239 participants [7, 8, 10]. In comparison to the non-active aspiration group, the active aspiration group was associated with less duration of hospital stay; however, it was not a statistically significant result. No heterogeneity was noticed (SMD = − 0.15, 95% CI − 0.41 to 0.10; P = 0.24; I2 = 0%) (Supplementary Fig. 2).
Complications
The presence of complications was reported by three RCTs with a sample size of 239 participants [7, 8, 10]. It showed comparable results with no significant difference between the two groups. However, the provided data were not sufficient to be pooled and analyzed. Abdelsamad et al. showed two incidents of complications in each group. These included two wound infections (one in each group), a periumbilical hematoma, and a periumbilical skin reaction, while Lee et al. and Atak et al. both reported no presence of major complications (Table 2).
Abdominal muscle tension
The data of postoperative abdominal muscle tension were found only in one study with a sample size of 60 participants [7]. However, the provided data were not sufficient to be pooled and analyzed. With regard to active aspiration group, out of 30 participants, only 8 participants experienced abdominal muscle tension incidents, 7 of whom experienced minor incidents and only one participant experienced a moderate incident. On the other hand, with regard to non-active aspiration group, out of 30 participants, 11 participants experienced abdominal muscle tension incidents, 7 of whom experienced minor incidents, 3 participants experienced moderate incidents, and only one participant experienced severe incident (Supplemental Table 3).
Discussion
This systematic review and meta-analysis aim to evaluate the difference in postoperative pain scores between patients who receive active gas aspiration versus those who receive passive gas aspiration following laparoscopic cholecystectomy. Our review provided a high level of evidence since we included five well-designed randomized controlled trials (RCTs). Our primary focus was to measure postoperative pain following laparoscopic cholecystectomy, rather than any other laparoscopic surgery, using a visual analog scale or numeric rating scale. Pain following a minimally invasive procedure is a major concern for patients; furthermore, pain after these types of procedures affects the quality of life, causes prolonged hospital stay, and late return to normal activities. The pain could be divided into incisional, shoulder, and upper abdominal pain [13].
The recommendations were formulated through a meticulous assessment of evidence derived from systematic reviews and RCTs. Our study has demonstrated that the implementation of active gas aspiration yields substantial advantages, as supported by multiple studies in both our current investigation and previous reviews. Notably, employing active gas aspiration for post-surgical abdominal gas evacuation does not entail any additional costs or time requirements. It will help in reducing shoulder and abdominal pain. The cause of shoulder pain is not fully explained. One of the hypotheses is that CO2 will be converted into carbonic acid after being combined with the fluid in the abdominal cavity which will irritate the phrenic nerve at the diaphragm [11]. Our meta-analysis showed that active gas aspiration reduces shoulder pain after laparoscopic cholecystectomy. Lee et al. report the pain scale at 24 h only, showed a major statistical difference with active gas aspiration in reducing shoulder pain [10]. Moreover, Tuvayanon et al. found that active gas aspiration reduces shoulder pain [11]. Atak et al. support that active gas aspiration reduces shoulder pain using VAS at 24 h [8]. On the other hand, Abdelsamad et al. study has shown a difference in shoulder pain scale after active gas aspiration at 2 h up to 48 h post-surgery, but it was not statically significant [7].
Abdominal pain could be due to several causes, for example, the trauma caused by the entry of the trocars causes somatic pain, and the intraabdominal intervention causes visceral pain [13]. Abdelsamad et al. reported the abdominal pain score from 2 h post-surgery up to 48 h, in the 2nd and 4th hours after surgery, the active gas aspiration group reported higher intensity of abdominal pain. However, in the same study, the abdominal pain was similar in both groups after 24 and 48 h from the surgery, and the result did not show any statistical difference between patients who underwent active gas aspiration and the non-active group [7]. Our results go in accordance with the findings of Abdelsamad et al. study. Moreover, our results showed significant heterogeneity was noted in the 24-h meta-analysis study. Atak et al. reported that the abdominal pain score in 24 h post-surgery was lower in the active gas aspiration group [8]. On the other hand, Fredman et al. reported abdominal pain in the first 4 h only without any major difference between the two groups [10].
In our meta-analysis, the total required analgesia was lower in active gas aspiration than in non-active gas aspiration. In Atak et al., they have similar results to our study regarding lower required analgesia [8]. However, Lee et al. and Fredman et al. found that there was no difference in total required analgesia in both groups except for the first hour which was lower in active gas aspiration in Fredman et al. [9, 10]. The hospital stay was lower in active gas aspiration, but it is not statistically significant. Abdulsamed et al., Atak et al., and Lee et al. found that there was no difference in hospital stay in both groups [7, 8, 10]. For the residual gas volume postoperative, our study found that it was lower in active gas aspiration compared to the non-active gas aspiration group, which goes in accordance with Abdelsamad et al. and Lee et al. regarding the residual gas volume [7, 10].
It is worth noting that our study has some limitations. Firstly, the included studies have some heterogeneity in terms of the patient population, surgical technique, and outcome measures, which may affect the precision of our estimates. Secondly, the sample size of some studies is relatively small, which may limit the generalizability of our findings. Thirdly, the risk of bias assessment showed that some studies had some concerns regarding methodological quality, which may affect the validity of our conclusions. Therefore, further studies with larger sample sizes and standardized outcomes are needed to confirm these findings and to determine the optimal gas aspiration method for reducing postoperative pain.
Conclusion
In conclusion, this systematic review and meta-analysis provide evidence that there is no significant difference in postoperative pain scores between active and passive gas aspiration methods following laparoscopic cholecystectomy. However, active gas aspiration may have some benefits, including lower residual gas volume, reduced analgesic requirement, and lower shoulder pain scores after 24 h. Therefore, active gas aspiration may be considered a safe and effective method for reducing postoperative analgesic requirement and shoulder pain after laparoscopic cholecystectomy. It is worth noting that active gas aspiration does not increase the cost or time of the procedure. These findings suggest that implementing active gas aspiration as a routine practice could potentially improve patient outcomes without incurring additional resources. Further studies with larger sample sizes and standardized outcomes are needed to confirm these findings and determine the optimal gas aspiration method for reducing postoperative pain.
References
Yang X, Cheng Y, Cheng N et al (2022) Gases for establishing pneumoperitoneum during laparoscopic abdominal surgery. Cochrane Database Syst Rev 3(3):CD009569. https://doi.org/10.1002/14651858.CD009569.pub4
Bisgaard T, Kehlet H, Rosenberg J (2001) Pain and convalescence after laparoscopic cholecystectomy. Eur J Surg 167(2):84–96. https://doi.org/10.1080/110241501750070510
Abbott J, Hawe J, Srivastava P, Hunter D, Garry R (2001) Intraperitoneal gas drain to reduce pain after laparoscopy: randomized masked trial. Obstet Gynecol 98(1):97–100. https://doi.org/10.1016/s0029-7844(01)01383-7
Jorgensen JO, Gillies RB, Hunt DR, Caplehorn JR, Lumley T (1995) A simple and effective way to reduce postoperative pain after laparoscopic cholecystectomy. Aust N Z J Surg 65(7):466–469. https://doi.org/10.1111/j.1445-2197.1995.tb01787.x
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Abdelsamad A, Ruehe L, Lerch LP, Ibrahim E, Daenenfaust L, Langenbach MR (2022) Active aspiration versus simple compression to remove residual gas from the abdominal cavity after laparoscopic cholecystectomy: a randomized clinical trial. Langenbecks Arch Surg 407(5):1797–1804. https://doi.org/10.1007/s00423-022-02522-8
Atak I, Ozbagriacik M, Akinci OF et al (2011) Active gas aspiration to reduce pain after laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech 21(2):98–100. https://doi.org/10.1097/SLE.0b013e318213c301
Fredman B, Jedeikin R, Olsfanger D, Flor P, Gruzman A (1994) Residual pneumoperitoneum: a cause of postoperative pain after laparoscopic cholecystectomy. Anesth Analg 79(1):152–154
Lee JS, Kim EY, Lee SH et al (2014) A simple method of reducing residual intraperitoneal carbon dioxide after laparoscopic cholecystectomy: a prospective, randomized, controlled study. J Laparoendosc Adv Surg Tech A 24(8):563–566. https://doi.org/10.1089/lap.2014.0041
Tuvayanon W, Silchai P, Sirivatanauksorn Y et al (2018) Randomized controlled trial comparing the effects of usual gas release, active aspiration, and passive-valve release on abdominal distension in patients who have undergone laparoscopic cholecystectomy. Asian J Endosc Surg 11(3):212–219. https://doi.org/10.1111/ases.12451
Jackson SA, Laurence AS, Hill JC (1996) Does post-laparoscopy pain relate to residual carbon dioxide? Anaesthesia 51(5):485–487. https://doi.org/10.1111/j.1365-2044.1996.tb07798.x
Sözen S, Erdem H, Gençtürk M, Çetinkünar S, Şişik A (2021) General surgery: The effect of active gas aspiration to reduce pain after laparoscopic sleeve gastrectomy for morbid obesity: a randomized controlled study. Arch Med Sci Civ Dis. https://doi.org/10.5114/aic.2021.109245
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
AKH created the idea, downloaded articles for screening, extracted the data, and wrote the methodology and the results. EAA created the idea, did screening, extracted the data, and wrote the discussion. RSA created the tables of data extraction, wrote the abstract and the key points, supervised the work, and reviewed the manuscript. HMA wrote the introduction, did screening, and did risk of bias assessment. GAA did screening, wrote the methodology and the results, and did risk of bias assessment. Mohammed M. Bukhari did screening and wrote the discussion. ASA did the PROSPERO registration and did the meta-analysis. BSA downloaded articles for screening, created the tables of data extraction, and wrote the conclusion. AHA is the principal investigator and reviewed the manuscript. All authors gave final approval for the version to be published and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
Ahmed K. Haneef, Elaf A. Aljohani, Raghad S. Alzahrani, Hanin M. Alowaydhi, Ghadah A. Alarif, Mohammed M. Bukhari, Ahmed S. Abdulhamid, Bassam AlRajhi, and Amro H. Ageel have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haneef, A.K., Aljohani, E.A., Alzahrani, R.S. et al. Active gas aspiration in reducing pain after laparoscopic cholecystectomy: a systematic review and meta-analysis of randomized controlled trials. Surg Endosc 38, 597–606 (2024). https://doi.org/10.1007/s00464-023-10651-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10651-4