Abstract
Purpose
After laparoscopic surgical procedures, residual gas in the abdominal cavity can cause post-operative pain, which is commonly located in the shoulder region. Previous studies suggested that post-laparoscopy pain can be prevented by active suctioning of intraabdominal gas at the end of surgery.
Methods
This randomized controlled trial (registered at DRKS 00,023,286) compared active suctioning versus manual compression in their ability to reduce pain after laparoscopic cholecystectomy. Patients scheduled for laparoscopic cholecystectomy were eligible for trial participation. The primary outcome measure was post-operative pain intensity after 12 h. All the patients were examined by MRI scanning to quantify the intraabdominal gas volume after the intervention.
Results
As planned, 60 patients were recruited. The two groups (n = 30 each) were very similar at the end of surgery. Active suctioning reduced the amount of residual pneumoperitoneum more than simple compression (median volume 1.5 versus 3.0 ml, p = 0.002). The primary outcome measure, abdominal pain after 12 h, was slightly lower in the intervention group (− 0.5 points, 95% confidence interval + 0.5 to − 1.7), but without reaching statistical significance (p = 0.37). After 12 h, shoulder pain was present in 10 patients in each group (p = 1.0). Independent of group assignment, however, residual gas volume was significantly associated with higher pain intensity.
Conclusions
Active suctioning appears to have only a minor preventive effect on post-laparoscopy pain, probably because evacuation of the pneumoperitoneum remains incomplete in some patients. Other more effective maneuvers for gas removal should be preferred.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soon after the introduction of laparoscopic surgery, it was noted that gas that remains in the abdomen at the end of the procedure can cause relevant pain [1, 2]. This pain is most likely caused by irritation of the phrenic nerve and is primarily located at the shoulder area [3, 4]. Up to 80% of patients suffer from this post-laparoscopic shoulder pain, which may last for several days and does not respond well to general analgesics [5,6,7,8]. Furthermore, residual gas may also lead to nausea, vomiting, and prolonged hospital stay.
Many different interventions were tested as strategies to remove residual gas and thus prevent pain after laparoscopic surgery. Manual compression of the abdomen at the end of surgery is the simplest intervention [9]. Another option is the placement of a subdiaphragmatic gas drain [10,11,12,13,14], preferably with active drainage rather than passive deflation [15]. Alternatively, residual gas can be aspirated at the end of the surgical procedure [12, 16,17,18,19], thus avoiding the infection risk associated with a drain left in place for a few days. It is also possible to fill the abdominal cavity with saline at the end of laparoscopic surgery in order to wash out all residual gas [20,21,22,23]. More recently, several clinical trials in gynecologic laparoscopy examined the pulmonary recruitment maneuver, which consists of a manual lung inflation to a pressure of about 40 cmH2O [24, 25].
Evidence-based guidelines recommend some of these preventive measures, including active suctioning [26, 27], although most of these interventions were found to have only variable minor to moderate effects on pain [28, 29]. Moreover, treatment effects may differ according to type of surgical procedure and patient characteristics [30]. In clinical practice, many patients undergoing laparoscopic surgery, therefore, still receive no specific intervention to remove residual gas. Accordingly, the objective of this randomized controlled trial was to examine whether active suctioning reduces the severity of pain in the first 12 h after laparoscopic cholecystectomy.
Material and methods
Study design
This was a randomized controlled single center trial with blinded assessment of key outcomes. Before recruitment had begun, the trial received ethical approval (Vote 50/2020 by University of Witten/Herdecke). In addition, the trial was prospectively registered at the German Registry for Clinical Trials (registration number DRKS 00,023,286). Each study participant signed a consent form after being informed about the trial by a physician.
Participants
Adult, legally competent patients scheduled for elective laparoscopic cholecystectomy because of symptomatic cholecystolithiasis were eligible for trial participation. Exclusion criteria included the need for concomitant surgeries, pregnancy, and any chronic disease either interfering with pain perception or requiring permanent systemic analgesics.
Before the trial’s start, an independent researcher prepared a list of random codes in randomly permuted blocks of 4 or 6, to ensure ongoing balance between the 2 groups. After consent, just shortly before the end of the pneumoperitoneum, each patient was randomly assigned to receive either active suction or manual compression. By use of sequentially numbered, opaque, sealed envelopes, every next group allocation was concealed from the research team, until the study participant had provided consent.
Interventions
All the patients received standardized anesthesia. After premedication (midazolam 7.5 mg per os), general anesthesia was usually established. In patients with risk factors, single-shot prophylactic antibiotics were given. In the operating room, all the patients were positioned in the same way. All the operations are performed by surgical specialists. After prepping and draping, the “team time-out” procedure was carried out. Then a skin incision of about 1-cm length was made in the area of the umbilical circumference. The anterior layer of the rectus sheath was opened by pushing the muscles apart, then opening the posterior layer of the rectus sheath and the peritoneum. After placing the 11-mm optical trocar and establishing the pneumoperitoneum (15 mmHg), the camera was inserted for an abdominal inspection. The four-port approach was completed by placing two 5-mm trocars in the right mid-abdomen and one 12-mm trocar in the epigastrium. Depending on surgical expertise and anatomical situation, a three-port approach was chosen.
After clamping of the gallbladder and dissection of Calot’s triangle, the cystic artery and the cystic duct were clipped using the Lapro-Clip™ applicator (Medtronic Inc., Germany) and severed with scissors between the two clips. Then the gallbladder was dissected out of the liver bed, placed in the retrieval bag, and extracted through the umbilical port site. If necessary for extraction, the fascia was dilated using scalpel or scissors. The fascia in the area of the optical trocar was closed by single Vicryl sutures. The pneumoperitoneum was reestablished, and the surgical site was checked for bleeding or bile leakage.
In the intervention group, the patients were put in the anti-Trendelenburg position, and the carbon dioxide was aspirated via the 5-mm trocar in the left side (Fig. 1). A negative pressure of − 50 kPa was applied. In the control group, the pneumoperitoneum was drained via the 12-mm epigastric trocar under simultaneous pressure on the abdominal wall from the outside. Usually, manual compression lasted 10 to 15 s and was repeated 2 or 3 times, until no more gas could be heard escaping the abdominal cavity. The rib cage was not compressed. Finally, all the remaining trocars were removed; fascial sutures were applied to incisions measuring 10 mm or more; and skin incisions were closed using 3.0 polypropylene sutures in backstitch technique. All the wounds were dressed by sterile adhesive plasters.
Standardized analgesia was administered in all the patients. In the recovery room, the patients received 1 ampoule of piritramide (7.5 mg) or metamizol (1 g) intravenously. Piritramide was continued as an infusion or via a patient-controlled analgesia pump until the first post-operative day. Any need for additional pain therapy was recorded. Oral analgesic therapy was started with metamizol (500 mg four times a day), ibuprofen (400 mg twice a day), or oxycodone (10 mg twice a day). For persisting pain, an additional ampoule of piritramid was administered intravenously. Analgesia was quickly tapered off until discharge.
Outcome criteria
The primary outcome was the intensity of abdominal pain on the evening of the day of surgery (i.e., about 12 h post-op). Pain was measured on a numerical rating scale (NRS) ranging from 0 (no pain) to 10 (maximum pain). The NRS is a widely used, valid instrument for measuring pain in the post-operative setting [31]. Abdominal pain was defined as the average pain at rest (not while coughing), which the patient felt in the abdominal area (not necessarily at the trocar sites) since the operation or the last preceding pain measurement. Shoulder pain was defined in the same way as any pain in the shoulder area. All pain measurements were performed without the patient knowing his or her group assignment or residual gas volume. Besides the patients, however, no other group (i.e., surgeons, outcome assessors, or data analysts) was blinded.
A key secondary outcome was the amount of residual intraabdominal gas (in ml), which was quantified by MRI (magnetic resonance imaging) scanning (Fig. 2). Two hours after surgery, the patients were taken to the MRI scanner, a 1.5 Tesla Ingenia (Philips Healthcare, Germany). The examination protocol was standardized and consisted of a localizer sequence-transverse T2-TSE sequence using the old-stop technique, with 3-mm slice thickness and an examination duration of 62 s. Two measurements were required to cover the entire abdomen, which were then fused into a continuous image stack through the entire abdomen. The total examination time was approximately 5 min. In the MRI scans, the radius (r) and the height (h) of the subdiaphragmatic spherical cap caused by the residual gas were measured. The volume (V) of the residual pneumoperitoneum was calculated using the formula: V = (π h2/3)·(3 r – h).
Other secondary outcomes included pain intensity over time, length of hospital stay, severe adverse events, and return to normal activities. After discharge from hospital, the patients were interviewed again after about 1 and 6 months. The 6-month interview was done via mail or telephone.
Statistical analyses
For sample size calculation, it was assumed that 1.5 points on the NRS represent a clinically relevant difference in pain [32]. Based on other studies and own observations, the standard deviation (SD) was estimated to be 2 points. Under common statistical assumptions (i.e., alpha error 0.05, beta error 0.20, testing for superiority), a sample size of 28 per group was calculated. As some missing data were anticipated, the total sample size was set to be 60.
Data was checked for plausibility and stored in a pseudonymized format. The primary hypothesis was tested by Student’s t-test. Continuous data were tested using Student’s t-test or Mann–Whitney U test depending on data distribution. In a pre-specified subgroup analysis, the association between obesity and post-operative pain was analyzed (also using Pearson’s correlation coefficient). Data are reported as counts (with percentages) or means (± standard deviations), if not stated otherwise.
Results
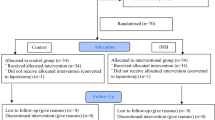
As anticipated, randomization of the 60 patients resulted in two equally sized groups, which were very similar with regard to demographic characteristics (Table 1) and surgical variables (Table 2). In none of the patients, treatment was switched. The standard pneumoperitoneum pressure of 15 mmHg was not exceeded in any patient. Furthermore, post-operative, MRI imaging and follow-up data were complete for all the patients (Fig. 3). Residual gas volume was effectively reduced by active aspiration, but the difference between the intervention and the control group was small (2.0 versus 6.1 ml, p = 0.04; Table 3). In 4 patients of the intervention group (13%), the volume of the residual pneumoperitoneum was equal to or higher than 5 ml, in spite of active aspiration.
The primary outcome, abdominal pain intensity after 12 h did not differ between the two groups, although the pain levels were slightly lower in the intervention than in the control group (3.3 versus 3.8; p = 0.37). At the other time points, pain intensity varied, with a tendency towards more pain early after active suctioning (2 h and 4 h after surgery) and very similar pain levels after 24 h and 48 h. In a similar way, the incidence of shoulder pain was equal in both groups (10 of 30, 33%, p = 1.0). Mean intensity of shoulder pain in the intervention and the control group was 1.5 ± 2.7 versus 1.2 ± 2.7 (p = 0.62) 12 h after surgery. None of the patients required additional pain therapy. Length of hospital stay was similar between the groups. In each group, two complications were seen. These included two wound infections (one in each group), a periumbilical hematoma, and a periumbilical skin reaction. There were no adverse events attributed by the investigators as being related to the study interventions.
In a post hoc analysis, which ignored group assignment, the patients with residual pneumoperitoneum (arbitrarily defined as a gas volume of 5 ml or more) were compared with the patients without relevant intraabdominal gas. This comparison showed that the patients with residual pneumoperitoneum (n = 45) had higher pain level 12 h after surgery than their counterparts without residual gas (3.2 ± 2.2 versus 4.6 ± 2.4; p = 0.04). This analysis was confirmed by assessing the correlation between residual gas volume and pain level at 12 h (Pearson r = 0.31, p = 0.02; Spearman r = 0.32, p = 0.01). Additional subgroup analysis of obesity failed to show any association between body mass index and study outcomes.
Discussion
The present data indicate that active suctioning of the residual pneumoperitoneum has only minor — if any — effect on pain levels after laparoscopic cholecystectomy. In a post hoc analysis, it appeared as if the apparent lack of analgesic effectiveness was partly caused by incomplete gas removal in the intervention group. It is nevertheless inevitable that in a few patients, some gas volume cannot be fully evacuated by active aspiration. Another issue that might have contributed to the “negative” result of the present trial is the possibility that manual compression is better suited for gas removal than previously thought. In clinical practice, manual compression is often done only gently and quickly, but the context of a clinical study might have motivated the staff in the operating theater to perform manual compression very effectively.
Even more than 30 years after the brilliant invention of laparoscopic surgery, shoulder pain still is a frequent and relevant problem [8]. The two reasons of pain, peritoneal irritation by carbon dioxide and abdominal distension, can be tackled by various interventions. Currently, the pulmonary recruitment maneuver appears to be the most promising technique for gas removal [25]. In the past, various trials have tested the use of gas drains [10,11,12,13,14], but passive drainage using limited negative pressure is likely to remove only some of the residual gas. In addition, gas drains are usually left in place for 24 or 48 h, which may entail discomfort for the patient and also may increase the risk of wound infection.
As no systematic review or meta-analysis has yet summarized the various clinical trials on active suctioning in laparoscopic surgery, it is necessary to compare the present study with individual trials. In 2011, Atak et al. described that active gas aspiration after laparoscopic cholecystectomy reduced shoulder and abdominal pain better than simple evacuation [17]. In the study reported by Salman et al. in 2013 [18], post-cholecystectomy shoulder pain was present after 12 h in 89% of the control patients, but in only 45% of the patients who had received suctioning of the pneumoperitoneum. That study was partly blinded and included 136 patients. A third study performed by Das et al. in 2013 (n = 200 patients) was able to show lower pain scores after active aspiration, but no difference in hospital stay was seen [16]. In a fourth study, based on a sample of 142 randomized patients, Tuvayanon et al. in 2018 confirmed again that active suctioning resulted in less pain as compared to passive release [12]. In summary, active suctioning of the residual gas was found to reduce pain in all four previously conducted trials.
The apparent discrepancy between the present and the previous four trials can have several reasons. First, in the previous trials, the pneumoperitoneum was obviously released only by opening the gas tap at the port site, whereas dedicated abdominal compression was applied in the control group of the present trial. Second, some of the older trials fail to record pain intensity in a blinded manner, which can have led to overestimated effects [33, 34]. Third, the present trial was smaller in sample size. As we observed some tendencies towards less pain in the intervention group, insufficient statistical power may have played a role. Fourth, gas aspiration may have been more efficacious in the previous trials. However, the amount of residual gas volume was not measured in these trials, so no data are present to confirm or refute this argument. Accordingly, one strength of the present study is the quantification of the residual gas volume and the investigation of the association between gas volume and post-operative pain.
A potential weakness of the present study might be seen in the selection of abdominal rather than periscapular pain intensity as the primary outcome. According to the data, however, both variables were well correlated, and the incidence of shoulder pain (33%) appears too low for a meaningful quantitative analysis of this outcome measure. A closely related issue is the choice of time point for measurement of the primary outcome. Pain intensity was high early after surgery (at the 2-h and 4-h time point), but these time points are probably too early to measure analgesic effects that are mediated through the evacuation of the pneumoperitoneum. Thus, the 12-h time point appears just right [5], also because pain intensity 24 h and 48 h after surgery was much lower, which renders it more difficult to detect any differences.
Conclusion
In summary, active suctioning appears to have only a minor preventive effect on post-laparoscopy pain, probably because evacuation of the pneumoperitoneum remains incomplete in some patients. Other more effective maneuvers for gas removal should be preferred in laparoscopic surgery.
References
Fredman B, Jedeikin R, Olsfanger D, Flor P, Gruzman A (1994) Residual pneumoperitoneum: a cause of postoperative pain after laparoscopic cholecystectomy. Anesth Analg 79(1):152–154
Jackson SA, Laurence AS, Hill JC (1996) Does post-laparoscopy pain relate to residual carbon dioxide? Anaesthesia 51(5):485–487
Lee DH, Song T, Kim KH, Lee KW (2018) Incidence, natural course, and characteristics of postlaparoscopic shoulder pain. Surg Endosc 32(1):160–165
Ure BM, Troidl H, Spangenberger W, Dietrich A, Lefering R, Neugebauer E (1994) Pain after laparoscopic cholecystectomy Intensity and localization of pain and analysis of predictors in preoperative symptoms and intraoperative events. Surg Endosc 8(2):90–96
Li X, Li K (2021) Time characteristics of shoulder pain after laparoscopic surgery. JSLS 25:2
Song T, Kim KH, Lee KW (2017) The intensity of postlaparoscopic shoulder pain is positively correlated with the amount of residual pneumoperitoneum. J Minim Invasive Gynecol 24(6):984-989.e981
Sabzi Sarvestani A, Zamiri M (2014) Residual pneumoperitoneum volume and postlaparoscopic cholecystectomy pain. Anesth Pain Med 4(4):e17366
McGrath B, Elgendy H, Chung F, Kamming D, Curti B, King S (2004) Thirty percent of patients have moderate to severe pain 24 hr after ambulatory surgery: a survey of 5,703 patients. Can J Anaesth 51(9):886–891
Rettenmaier MA, Micha JP, Lopez KL, Wilcox AM, Goldstein BH (2017) A prospective, observational trial assessing the efficacy of abdominal compression in reducing laparoscopic-induced shoulder pain. Surg Innov 24(6):552–556
Nursal TZ, Yildirim S, Tarim A, Noyan T, Poyraz P, Tuna N, Haberal M (2003) Effect of drainage on postoperative nausea, vomiting, and pain after laparoscopic cholecystectomy. Langenbecks Arch Surg 388(2):95–100
Haghgoo A, Chaichian S, Ghahremani M, Nooriardebili S, Akbaian A, Moazzami B (2016) The use of peritoneal suction drainage to reduce shoulder pain caused by gynecological laparoscopy. Arch Iran Med 19(3):173–178
Tuvayanon W, Silchai P, Sirivatanauksorn Y, Visavajarn P, Pungdok J, Tonklai S, Akaraviputh T (2018) Randomized controlled trial comparing the effects of usual gas release, active aspiration, and passive-valve release on abdominal distension in patients who have undergone laparoscopic cholecystectomy. Asian J Endosc Surg 11(3):212–219
Vafaei F, Kamely A, Nouri G, Teshnizi SH, Shokri A (2021) Effect of utilizing a drain on shoulder pain in laparoscopic cholecystectomy. A randomized clinical trial. Indian J Surg 83:859–864
Yang SC, Chang KY, Wei LF, Shyr YM, Ho CM (2021) To drain or not to drain: the association between residual intraperitoneal gas and post-laparoscopic shoulder pain for laparoscopic cholecystectomy. Sci Rep 11(1):7447
Jorgensen JO, Gillies RB, Hunt DR, Caplehorn JR, Lumley T (1995) A simple and effective way to reduce postoperative pain after laparoscopic cholecystectomy. Aust N Z J Surg 65(7):466–469
Das K, Karateke F, Menekse E, Ozdogan M, Aziret M, Erdem H, Cetinkunar S, Ozdogan H, Sozen S (2013) Minimizing shoulder pain following laparoscopic cholecystectomy: a prospective, randomized, controlled trial. J Laparoendosc Adv Surg Tech A 23(3):179–182
Atak I, Ozbagriacik M, Akinci OF, Bildik N, Subasi IE, Ozdemir M, Ayta NI (2011) Active gas aspiration to reduce pain after laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech 21(2):98–100
Salman JM, Merdan I, Asfar SN (2013) The effect of active gas suctioning to decrease the residual CO2 for preventing post-laparoscopic cholecystectomy shoulder pain. J Arab Board Health Specializations 14(2):2–8
Leelasuwattanakul N, Bunyavehchevin S, Sriprachittichai P (2016) Active gas aspiration versus simple gas evacuation to reduce shoulder pain after diagnostic laparoscopy: a randomized controlled trial. J Obstet Gynaecol Res 42(2):190–194
Suginami R, Taniguchi F, Suginami H (2009) Prevention of postlaparoscopic shoulder pain by forced evacuation of residual CO(2). JSLS 13(1):56–59
Tsai HW, Chen YJ, Ho CM, Hseu SS, Chao KC, Tsai SK, Wang PH (2011) Maneuvers to decrease laparoscopy-induced shoulder and upper abdominal pain: a randomized controlled study. Arch Surg 146(12):1360–1366
Ryu KH, Lee SH, Cho EA, Kim JA, Lim GE, Song T (2019) Comparison of impacts of intraperitoneal saline instillation with and without pulmonary recruitment maneuver on post-laparoscopic shoulder pain prevention: a randomized controlled trial. Surg Endosc 33(3):870–878
Barthelsson C, Sandblom G, Ljesevic-Nikoletic S, Hammarqvist F (2015) Effects of intra-abdominally instilled isotonic saline on pain, recovery, and health-related quality-of-life following laparoscopic cholecystectomy: a randomized prospective double-blind controlled study. World J Surg 39(6):1413–1420
Kihlstedt Pasquier E, Andersson E (2021) Pulmonary recruitment maneuver reduces shoulder pain and nausea after laparoscopic cholecystectomy: a randomized controlled trial. World J Surg 45(12):3575–3583
Kietpeerakool C, Rattanakanokchai S, Yantapant A, Roekyindee R, Puttasiri S, Yanaranop M, Srisomboon J (2020) Pulmonary recruitment maneuver for reducing shoulder pain after laparoscopic gynecologic surgery: a network meta-analysis of randomized controlled trials. Minim Invasive Surg 2020:7154612
Neudecker J, Sauerland S, Neugebauer E, Bergamaschi R, Bonjer HJ, Cuschieri A, Fuchs KH, Jacobi C, Jansen FW, Koivusalo AM, Lacy A, McMahon MJ, Millat B, Schwenk W (2002) The European Association for Endoscopic Surgery clinical practice guideline on the pneumoperitoneum for laparoscopic surgery. Surg Endosc 16(7):1121–1143
Barazanchi AWH, MacFater WS, Rahiri JL, Tutone S, Hill AG, Joshi GP (2018) Evidence-based management of pain after laparoscopic cholecystectomy: a PROSPECT review update. Br J Anaesth 121(4):787–803
Wood S, Lewis W, Egan R (2019) Optimising surgical technique in laparoscopic cholecystectomy: a review of intraoperative interventions. J Gastrointest Surg 23(9):1925–1932
Kaloo P, Armstrong S, Kaloo C, Jordan V (2019) Interventions to reduce shoulder pain following gynaecological laparoscopic procedures. Cochrane Database Syst Rev 1(1):Cd011101
Li XY, Tian M, Li AZ, Han CL, Li KZ (2021) The risk of shoulder pain after laparoscopic surgery for infertility is higher in thin patients. Sci Rep 11(1):13421
Bahreini M, Jalili M, Moradi-Lakeh M (2015) A comparison of three self-report pain scales in adults with acute pain. J Emerg Med 48(1):10–18
Olsen MF, Bjerre E, Hansen MD, Hilden J, Landler NE, Tendal B, Hróbjartsson A (2017) Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med 15(1):35
Probst P, Zaschke S, Heger P, Harnoss JC, Hüttner FJ, Mihaljevic AL, Knebel P, Diener MK (2019) Evidence-based recommendations for blinding in surgical trials. Langenbecks Arch Surg 404(3):273–284
Amer MA, Herbison GP, Grainger SH, Khoo CH, Smith MD, McCall JL (2021) A meta-epidemiological study of bias in randomized clinical trials of open and laparoscopic surgery. Br J Surg 108(5):477–483
Author information
Authors and Affiliations
Contributions
Abdelsamad, Ahmed: study conception and design, acquisition of data analysis and interpretation of the data, drafting of manuscript, and critical revision of the manuscript. Ruehe, Lars: study conception and design, acquisition of the data, analysis and interpretation of the data MRI analysis, and interpretation of the data. Lerch, Lutz Peter: study conception and design, acquisition of the data analysis, and interpretation of the data, and critical revision of manuscript. Ibrahim, Ehab: study conception and design, analysis and interpretation of the data, and critical revision of the manuscript. Daenenfaust, Lars: study conception and design, acquisition of the data analysis and interpretation of the data. Langenbach, Mike Ralf: study conception and design, acquisition of the data analysis and interpretation of the data, drafting of manuscript, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelsamad, A., Ruehe, L., Lerch, L.P. et al. Active aspiration versus simple compression to remove residual gas from the abdominal cavity after laparoscopic cholecystectomy: a randomized clinical trial. Langenbecks Arch Surg 407, 1797–1804 (2022). https://doi.org/10.1007/s00423-022-02522-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02522-8