Abstract
Background
Endoscopic submucosal dissection (ESD) has been accepted as a standard treatment in early gastric cancer (EGC) patients with negligible risk of lymph node metastasis. However, there are limited data regarding the long-term outcomes of ESD in comparison with surgery. This study aimed to compare the overall, recurrence-free, and metachronous cancer-free survival rates after ESD and surgery.
Methods
From May 2003 to December 2007, 391 patients with 413 EGCs and 258 patients with 276 EGCs were treated by ESD and surgery, respectively. According to inclusion criteria, 288 patients in the ESD group and 173 patients in the surgery group were eligible for this study. Using propensity score matching, 88 patients were analyzed per group.
Results
The overall survival rates were 92.0 % in the ESD group and 90.2 % in the surgery group. Local recurrence was observed in five patients (1.7 %) in the ESD group and distant recurrence in one patient (0.6 %) in the surgery group. Metachronous gastric cancers were detected in 14 patients (4.9 %) in the ESD group, whereas no patient in the surgery group. Kaplan–Meier curves exhibited no significant differences in overall or recurrence-free survival between the two groups. However, metachronous cancer-free survival of the ESD group was significantly lower than that of the surgery group (p = 0.002). In the ESD group, the late complication rate was significantly lower (0 vs. 6.8 %, p = 0.029), and the duration of hospital stay was shorter (7.3 vs. 14.2 days, p < 0.001), compared with the surgery group.
Conclusions
The overall survival was similar between the ESD and surgery groups. Compared with surgery, the benefits of ESD included fewer late complications and shorter hospital stay duration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The prevalence of gastric cancer is high in Asia, especially in Korea and Japan [1]. Early gastric cancer (EGC) is defined as mucosal or submucosal cancer, regardless of regional lymph node metastasis [2]. The presence of lymph node metastasis has been reported to range from 2 to 18 % [3]. For this reason, radical gastrectomy with lymph node dissection was considered to be the only curative treatment. In EGC patients, the surgical outcome demonstrated excellent 5-year survival rates (>90 %) [4, 5].
Instead of surgery, endoscopic resection is recommended in selected EGC patients [6]. The standard indications include differentiated, intra-mucosal, elevated cancers <20 mm in size or depressed cancers <10 mm in size, without ulceration and lymph node metastasis. In 2000, EGC subgroups with negligible risk of lymph node metastasis were newly proposed on the basis of large-scale retrospective data [7, 8]. Thereafter, the results were adopted as expanded indications: (1) differentiated-type mucosal cancer without ulceration, regardless of tumor size; (2) differentiated-type mucosal cancer with ulceration ≤3 cm in diameter; (3) superficial (SM1; tumor infiltration into the submucosal layer <500 μm from the muscularis mucosae) submucosal cancer ≤3 cm in diameter; (4) undifferentiated-type mucosal cancer without ulceration ≤2 cm in diameter.
Recently, endoscopic submucosal dissection (ESD) has resulted in high complete resection rates in EGC patients who met the expanded indications [9]. However, there are no comparative data available regarding the long-term outcomes following ESD and surgical gastrectomy. Therefore, this study aimed to evaluate the overall survival rate, tumor recurrence, and development of metachronous gastric cancers between patient groups treated with ESD versus surgery.
Methods
Patients and study design
From May 2003 to December 2007, a total of 391 patients with 413 EGCs were treated by ESD at a single, tertiary-care, academic medical center. We retrospectively reviewed the medical records and endoscopic and pathological reports of these patients. All patients underwent ESD according to the expanded indications. Of these, patients were excluded for the following reasons: EGC in a remnant stomach, additional surgery after ESD, or pathologically non-curative result based on resected ESD specimen.
During the same period, the surgery group included a total of 258 patients with 276 EGCs. For comparison with the ESD group, patients with EGC in a remnant stomach and deep submucosal cancer (SM2; tumor infiltration into the submucosal layer ≥500 μm from the muscularis mucosae) were excluded. This study was approved by the institutional review board of the hospital (SCHUH 2014-07-010) and registered in ClinicalTrials.gov (NCT02216110).
Endoscopic submucosal dissection and surgery
Before ESD, all patients underwent endoscopy, endoscopic ultrasonography (EUS), and computed tomography (CT) for assessment of tumor margin, depth of tumor invasion, and regional lymph node metastasis. ESD was performed under conscious sedation. For sedation, midazolam and/or propofol were administered intravenously, with cardiorespiratory monitoring. Initially, indigo carmine dye was sprayed onto the tumor to clarify the margin. Then, markings were made 10 mm outside the tumor margin using argon plasma coagulation (APC). After marking, a mixture of sodium hyaluronate with indigo carmine and epinephrine was injected into the submucosa outside the marking dots. Circumferential mucosal incision and submucosal dissection were performed using a Flex knife (Olympus, Tokyo, Japan) and/or IT knife (Olympus). During the procedure, immediate bleeding was treated by Coagrasper (Olympus). After ESD, chest and abdominal plain radiography were performed routinely for detection of gastric perforation. ESD-related bleeding was defined as one of the following: hematemesis, melena or bleeding proven by routine, second-look endoscopy within 24 h.
In the surgery group, distal subtotal gastrectomy, total gastrectomy, proximal gastrectomy, and segmental resection were performed according to the location and extent of the tumor. Also, D1 + β or D2 lymph node dissection was performed with gastric resection. During hospitalization, patients were managed according to a postoperative standardized protocol. Hospital discharge was determined in the absence of any complications.
Pathological evaluation
According to the guidelines issued by the Japanese Gastric Cancer Association, an experienced gastrointestinal pathologist performed pathologic examination [10]. All resected specimens were immersed in 10 % formalin. Resected ESD specimens were fixed with pins, then sliced serially at 2-mm intervals, and embedded in a paraffin block. If the piecemeal resection was performed, all pieces were reconstructed carefully. Resected surgical specimens were prepared in the same manner and sliced serially at 4-mm intervals. The gross morphology was classified as elevated, flat, or depressed. Tumor size, histologic type, depth of invasion, resection margin involvement, and lymphovascular invasion were evaluated. Histology was categorized as either differentiated type (well-differentiated or moderately differentiated adenocarcinoma) or undifferentiated type (poorly differentiated adenocarcinoma or signet-ring cell carcinoma). When the tumor consisted of both differentiated and undifferentiated types, the tumor was classified according to the quantitative predominance. Complete resection was defined when en bloc resection was achieved with tumor-free lateral and vertical margins. Curative resection was defined when all of the following conditions were fulfilled: en bloc resection, tumor-free lateral and vertical margins, superficial submucosal invasion of <500 μm from the muscularis mucosae, and no lymphovascular invasion.
Follow-up
After ESD or surgery, regular follow-up was recommended to all patients. In the ESD group, endoscopic examinations were scheduled at 2 months after initial ESD, then every 6 months for 2 years, and annually thereafter. During follow-up endoscopy, surveillance biopsy specimens of 1–3 pieces were routinely taken from the previous ESD scar and any suspicious lesions, to detect local recurrence or metachronous cancer. Abdominal CT and chest radiography were performed annually after ESD to evaluate lymph node or distant metastasis. In the surgery group, routine endoscopy and abdominal CT were scheduled at 6 months after gastrectomy and annually thereafter. For patients without follow-up visits, the survival data and cause of death were obtained from the National Cancer Registry database.
Outcome data
The primary outcome of this study was overall survival. Secondary outcomes included tumor recurrence and the development of metachronous cancer and treatment-related complications. During follow-up, tumor recurrence was categorized as local, regional, or distant. Local recurrence was defined as a malignant tumor pathologically diagnosed at a previous ESD scar or surgically resection margin. Regional recurrence was defined as a tumor detected in the regional lymph nodes. Distant recurrence was defined as tumor metastasis in other organ sites. All cases of tumor recurrence were confirmed pathologically and/or by radiologic imaging. Metachronous cancer was defined as a new cancer detected in a different area of the stomach 1 year following the initial ESD or gastrectomy. Complications within and beyond 30 days after treatment were defined as early and late complications, respectively.
Statistical analysis
To analyze the baseline characteristics of the patients, Student’s t test was used for continuous data, whereas the χ 2 test and Fisher’s exact test were used for categorical data. Because this study was designed retrospectively, there were potential confounding and treatment-related selection biases between the two groups. To balance the two treatment groups, we performed propensity score matching [11]. Propensity scores were calculated using a logistic regression model and the following covariates: age, sex, preexisting comorbidity, tumor location, tumor size, morphology, depth of invasion, and histology. For the assessment of bias reduction, we calculated absolute standardized differences of covariates before and after matching. An absolute standardized difference of <10 % suggests inconsequential residual bias.
The Kaplan–Meier method and log-rank test were used to analyze the long-term outcomes including overall survival, recurrence-free, and metachronous cancer-free survival. P values <0.05 were considered to be statistically significant. All statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC, USA) and SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
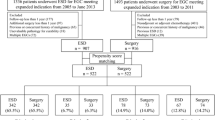
Figure 1 presents a flow chart of patient enrollment in this study. In the ESD group, 60 patients were excluded for the following reasons: cancer in a remnant stomach (n = 3), deep submucosal invasion (n = 25), predominantly undifferentiated-type cancer with submucosal invasion and/or >2 cm in diameter (n = 24), and positive lymphovascular invasion (n = 8). In the surgery group, three patients with EGC in a remnant stomach and 82 patients with deep submucosal cancer were excluded from this study. Thus, a total of 331 patients with 353 EGCs in the ESD group and 173 patients with 184 EGCs in the surgery group were eligible for analysis of initial therapeutic outcomes.
In addition, 43 patients of ESD group were excluded due to piecemeal resection (n = 6), positive lateral margin (n = 23), positive vertical margin (n = 2), and additional surgery after ESD (n = 12). In the surgery group, all patients underwent curative gastric resection, such as distal subtotal gastrectomy (n = 146, 84.4 %), total gastrectomy (n = 24, 13.9 %), proximal gastrectomy (n = 2, 1.1 %), or segmental resection (n = 1, 0.6 %). Finally, a total of 288 patients in the ESD group and 173 patients in the surgery group were eligible for the analysis of long-term outcomes. Next, using propensity scores, ESD patients were matched individually (1:1) to patients who underwent surgery, for a total of 176 patients (88 patients from each group).
The baseline characteristics of the patients are listed in Table 1. Before propensity score matching, the mean age of the ESD group was higher than that of the surgery group (62.2 ± 9.8 vs. 59.4 ± 11.5 years, p = 0.007). Among preexisting comorbidities, the surgery group was more likely to have renal dysfunction (p = 0.021). Notably, there were significant differences between the two groups in terms of tumor characteristics. In the ESD group, the tumor was more frequently located in the lower third of the stomach (p < 0.001). In the surgery group, the mean tumor size was larger, compared with the ESD group (24.9 ± 13.7 vs. 16.6 ± 8.9 mm, p < 0.001). Patients in the ESD group were more likely to have elevated type lesion, mucosal cancer, and differentiated-type cancer (p < 0.001). However, all baseline characteristics after propensity score matching were similar between groups. Except for cardiovascular diseases, absolute standardized differences of all covariates were reduced to <10 % (Fig. 2).
Therapeutic outcomes of endoscopic submucosal dissection
As given in Table 2, the rates of en bloc resection were 94.6 % (334/353) in total patients; 96.3 % (232/241) in the standard group and 91.1 % (102/112) in the expanded group (p = 0.044). Complete resection rates were 90.1 % (318/353) in total patients; 94.6 % (228/241) in the standard group and 80.4 % (90/112) in the expanded group (p < 0.001). In total, ESD-related complication rate was 5.4 % (19/353). Bleeding, intra-luminal stricture, and perforation were observed in 15 patients (4.2 %), 3 patients (0.8 %), and 1 patient (0.3 %), respectively. There was no significant difference of ESD-related complication between the standard and expanded groups (p = 0.132).
Long-term outcomes of endoscopic submucosal dissection and surgery
Long-term outcomes of ESD and surgery for EGC patients are described in Table 3. The median follow-up period was 77 months (range 18–107 months) for the ESD group and 78 months (range 1–113 months) for the surgery group. In the ESD group, 23 patients died of various causes other than gastric cancer, including cardiovascular disease (n = 4), lung cancer (n = 2), rectal cancer (n = 1), non-Hodgkin lymphoma (n = 1), and unknown (n = 15). In the surgery group, 17 patients died. Of these, one patient died of metastatic recurrence 17 months after subtotal gastrectomy, and the remaining patients died of other diseases, including cardiovascular disease (n = 2), pancreas cancer (n = 1), chronic lymphoid leukemia (n = 1), intracranial hemorrhage (n = 1), renal dysfunction (n = 1), and other causes (n = 10). The overall survival rates were 92.0 % (265/288) for the ESD group and 90.2 % (156/173) for the surgery group. In propensity score-matched patients, the overall survival rates were 89.8 % (79/88) for the ESD group and 90.9 % (80/88) for the surgery group.
Local recurrence was observed in five patients (1.7 %, 5/288) in the ESD group, all of whom showed no regional lymph node metastasis on abdominal CT. The median time until local recurrence was 24 months (range 20–30 months). One patient underwent gastrectomy for treatment of a locally recurrent tumor. The remaining four patients underwent endoscopic treatment including endoscopic mucosal resection (EMR; n = 2) or APC (n = 2). Of those who underwent repeated endoscopic treatment, no second local or regional recurrence occurred during follow-up (median 65 months; range 34–81 months). In one patient (0.6 %, 1/173) from the surgery group, tumor recurrence with para-aortic lymph node and liver metastasis was observed 13 months after subtotal gastrectomy. In propensity score-matched patients, recurrence-free survival rates were 96.6 % (85/88) for the ESD group and 100 % (88/88) for the surgery group.
Metachronous gastric cancers were detected in 14 patients (4.9 %, 14/288) in the ESD group. The median time between ESD and detection of metachronous cancer was 38 months (range 14–82 months). The mean size of metachronous gastric cancer was 15.0 mm, and all lesions were treated by EMR (n = 3) or ESD (n = 11). Of these, one patient had a third metachronous cancer that was also resected by ESD. In the surgery group, no patient had metachronous cancer of the remnant stomach. In propensity score-matched patients, metachronous cancer-free survival rates were 96.6 % (85/88) for the ESD group and 100 % (88/88) for the surgery group.
Kaplan–Meier curves were used to indicate the long-term outcomes of ESD and surgery in overall and propensity score-matched patients (Figs. 3, 4). In overall patients, there were no significant differences between the ESD and surgery groups in terms of overall survival (p = 0.565) or recurrence-free survival (p = 0.252). However, metachronous cancer-free survival was significantly lower in the ESD group compared with the surgery group (p = 0.002). In propensity score-matched patients, there was no significant difference in overall survival (p = 0.691) between the ESD and surgery groups. However, the ESD group showed a lower rate of recurrence-free (p = 0.073) and metachronous cancer-free survival (p = 0.070) compared with the surgery group.
Complications and hospital stay
Table 4 provides detailed descriptions of treatment-related complications in propensity score-matched patients. In the ESD group, a total of seven patients (five with post-ESD bleeding, one with gastric perforation, and one with pneumonia) experienced early complications. All post-ESD bleeding was treated by endoscopic hemostasis. Gastric perforation was treated by endoscopic clips and conservative management. A patient with pneumonia improved after antibiotic treatment. In the surgery group, early complications occurred in five patients. Among the surgical complications (n = 2), intra-luminal bleeding occurred in one patient, and hemostasis was achieved by surgical intervention. One patient with intra-abdominal abscess was managed by antibiotic treatment. Among the medical complications (n = 3), one patient with pleural effusion was treated by percutaneous drainage, and one with acute renal failure and one with cerebral infarction recovered after conservative management. In terms of early complication rates, there was no significant difference between the ESD and surgery groups (8.0 % [7/88] vs. 5.7 % [5/88], respectively; p = 0.550).
After hospital discharge, there were no late complications in the ESD group, whereas six patients in the surgery group experienced late complications (one with anastomotic stricture, two with intestinal obstruction, two with marginal ulcer, and one with gastric stasis). Anastomotic stricture was treated by endoscopic balloon dilation. One patient with intestinal obstruction underwent adhesiolysis after conservative management had failed. The remaining four patients were managed by conservative treatment. There was a significant difference in late complication rates between the ESD and surgery groups (0 % [0/88] vs. 6.8 % [6/88], respectively; p = 0.029). The mean hospital stay duration was significantly shorter in the ESD group, compared with the surgery group (7.3 ± 2.9 vs. 14.2 ± 8.4 days, respectively; p < 0.001).
Discussion
Before the ESD era, gastrectomy was the treatment of choice for EGC patients, and the long-term survival rate was >90 %. Recently, several studies have reported highly favorable 5-year overall and disease-specific survival rates in EGC patients who underwent curative ESD [12, 13]. However, there are limited data regarding the long-term outcomes of ESD in comparison with surgery.
In EGC, larger tumor size, deeper invasion, and undifferentiated-type histology are associated with the presence of lymph node metastasis [7]. Because the ESD technique allows removal of only the primary tumor along the submucosal layer, patients with possible lymph node metastasis were excluded from the ESD group of our study. Thus, there were significant differences in the baseline characteristics between the ESD and surgery groups. By propensity score matching, new patient groups were generated, and tumor characteristics affecting treatment outcome were balanced through bias reduction. As a result, the overall survival of the ESD group was similar to that of the surgery group.
Although ESD may achieve curative resection of EGC, the remnant background mucosa still has a high risk of multiple cancers [14–16]. During follow-up after endoscopic resection, the detection rate of metachronous gastric cancer has been reported to range from 8.2 % to 14 %. In a large-scale study, metachronous cancers were observed in 5.2 % of patients who underwent curative ESD of EGC. Almost all metachronous cancers were treated curatively with repeat endoscopic resection (96.2 %, 50/52). In our study, surveillance endoscopy was performed at least annually, and the detected lesions were appropriate for endoscopic resection. Although the ESD group showed a higher incidence of metachronous gastric cancer when compared with the surgery group (4.9 vs. 0 %), all lesions were treated completely by endoscopic resection. In Korea and Japan, annual or biannual endoscopic surveillance is recommended after endoscopic treatment for EGC [17, 18]. In patients with Helicobacter pylori-infected stomach, eradication therapy may reduce the occurrence of metachronous gastric cancers [19].
In this study, five patients in the ESD group had local tumor recurrence despite curative resection of primary EGC. At the time of initial ESD, all patients showed differentiated-type mucosal (n = 4) or superficial submucosal (n = 1) cancer. Of these, three EGCs were resected en bloc. In the remaining two EGCs, piecemeal resection (two pieces) was performed, and resected specimens were properly reconstructed by an expert endoscopist. The lateral and vertical margins showed no tumor positivity. In our endoscopy unit, routine biopsies of ESD-induced scars are performed for early detection of local recurrence. A previous study reported no local recurrence observed in completely resected EGCs during follow-up [20]. Risk factors associated with local recurrence after ESD include incomplete resection with margin positivity and/or piecemeal resection [21]. In this regard, local tumor recurrence in this study was considered to be uncommon. We suggest that the ESD-induced artificial ulcer was healed with highly inflamed mucosa, and a new malignant lesion might occur at the same scar site. Further investigation may be necessary to determine whether surveillance biopsy of ESD scars should be performed routinely.
A recent study reported the effectiveness and favorable long-term outcomes of ESD for local recurrence after previous endoscopic resection [22]. In our study, it was difficult to perform secondary ESD due to severe submucosal fibrosis caused by previous ESD. Instead, four patients underwent EMR or APC as additional treatment of local recurrence. Among them, there was no second local or regional recurrence during a median follow-up of 65 months. However, further studies are needed to confirm the efficacy and safety of EMR or APC for the treatment of local recurrence.
The major complications of ESD are bleeding and perforation [23]. In a study by Choi et al., the rates of ESD-related bleeding and perforation were 5.2 and 0.4 %, respectively [9]. All patients were treated by coagulation device and endoscopic clipping. A multicenter Korean study reported rates of delayed bleeding, significant bleeding, and perforation of 15.6, 0.6, and 1.2 %, respectively [24]. Except for two patients, most complications (98.9 %, 172/174) were managed with endoscopic treatment. In the current study, ESD-related complications (five delayed bleeding and one perforation case) were acceptable and managed successfully without surgical intervention.
EGC treatment through surgery is performed under general anesthesia, and patients experience postoperative pain and abdominal scarring. In some patients, surgical intervention can be required for management of postoperative complications. Early complications of gastrectomy for EGC treatment can include bleeding, anastomotic leakage, intra-abdominal abscess, and perioperative medical diseases [25]. Late complications include anastomotic stenosis, anastomotic ulcer, and intestinal obstruction. In the current study, two patients in the surgery group underwent surgical intervention due to intraluminal bleeding and intestinal obstruction. In contrast, patients in the ESD group showed a shorter length of hospital stay and no complications after hospital discharge.
Although treatment efficacy and safety are important, quality of life is relevant to oncologic outcomes in cancer patients. Following gastrectomy, many patients experience dyspepsia, diarrhea, and eating restriction [26]. In particular, patients who undergo total gastrectomy have functional problems, such as fatigue and weight loss [27]. In contrast, ESD has the major advantage of removing only the tumor and preserving the stomach. A recent study reported that endoscopic treatment for EGC provided a better quality of life, as compared with surgery [28].
This study has several limitations. First, the baseline patient characteristics were significantly different among overall patients. However, application of the propensity score-matching method allowed balancing of the ESD and surgery groups in this retrospective study. Second, some patients did not receive surveillance endoscopy due to loss to follow-up, although their survival was confirmed through the National Cancer Registry. Consequently, the incidence rate of metachronous cancer may be higher than that reported in this study. Third, the H. pylori infection status of enrolled patients was not evaluated, although H. pylori might influence the development of metachronous gastric cancers after ESD. Fourth, this single-center study included 20 submucosal invasive cancers and eight undifferentiated-type cancers, and the numbers of patients may have been insufficient to analyze the long-term outcomes. In the future, a multicenter large-scale study is needed to confirm our results.
In conclusion, ESD was an effective treatment with preservation of the stomach in selected EGC patients. The overall survival was similar between the ESD and surgery groups. Compared with surgery, the benefits of ESD were fewer late complications and shorter hospital stay duration. Furthermore, regular surveillance endoscopy after ESD is essential for the detection of local tumor recurrence and metachronous cancers.
References
Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, Gotoda T, Lin JT, You WC, Ng EK, Sung JJ, The Asia Pacific Working Group on Gastric Cancer (2008) Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol 9:279–287
Carter KJ, Schaffer HA, Ritchie WP Jr (1984) Early gastric cancer. Ann Surg 199:604–609
Sano T, Kobori O, Muto T (1992) Lymph node metastasis form early gastric cancer: endoscopic resection of tumour. Br J Surg 79:241–244
Okamura T, Tsujitani S, Korenaga D, Haraguchi M, Baba H, Hiramoto Y, Sugimachi K (1988) Lymphadenectomy for cure in patients with early gastric cancer and lymph node metastasis. Am J Surg 155:476–480
Itoh H, Oohata Y, Nakamura K, Nagata T, Mibu R, Nakayama F (1989) Complete ten-year postgastrectomy follow-up of early gastric cancer. Am J Surg 158:14–16
Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S (2001) Endoscopic mucosal resection for treatment of early gastric cancer. Gut 48:225–229
Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y (2000) Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 3:219–225
Gotoda T, Yamamoto H, Soetikno RM (2006) Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol 41:929–942
Choi MK, Kim GH, Park do Y, Song GA, Kim DU, Ryu DY, Lee BE, Cheong JH, Cho M (2013) Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc 27:4250–4258
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma, 3rd edn. Gastric Cancer 14:101–112
D’Agostino RB Jr (1998) Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17:2265–2281
Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S (2009) Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 58:331–336
Goto O, Fujishiro M, Kodashima S, Ono S, Omata M (2009) Outcomes of endoscopic submucosal dissection for early gastric cancer with special reference to validation for curability criteria. Endoscopy 41:118–122
Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I (2005) Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy 37:990–993
Nakajima T, Oda I, Gotoda T, Hamanaka H, Eguchi T, Yokoi C, Saito D (2006) Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance? Gastric Cancer 9:93–98
Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, Yoshio T, Nakamura T, Komori M, Kawai N, Nishihara A, Nakanishi F, Nakahara M, Ogiyama H, Kinoshita K, Yamada T, Iijima H, Tsujii M, Takehara T (2013) Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicenter retrospective cohort study by Osaka University ESD study group. Gut 62:1425–1432
Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, Kim JM, Kim YI, Ryu KW, Kong SH, Kim HI, Jung HY, Kim YS, Zang DY, Cho JY, Park JO, Lim do H, Jung ES, Ahn HS, Kim HJ (2014) Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer 14:87–104
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123
Bae SE, Jung HY, Kang J, Park YS, Baek S, Jung JH, Choi JY, Kim MY, Ahn JY, Choi KS, Kim do H, Lee JH, Choi KD, Song HJ, Lee GH, Kim JH (2014) Effect of Helicobacter pylori eradication on metachronous recurrence after endoscopic resection of gastric neoplasm. Am J Gastroenterol 109:60–67
Lee JY, Choi IJ, Cho SJ, Kim CG, Kook MC, Lee JH, Ryu KW, Kim YW (2012) Routine follow-up biopsies after complete endoscopic resection for early gastric cancer may be unnecessary. J Gastric Cancer 12:88–98
Takenaka R, Kawahara Y, Okada H, Hori K, Inoue M, Kawano S, Tanioka D, Tsuzuki T, Yagi S, Kato J, Uemura M, Ohara N, Yoshino T, Imagawa A, Fujiki S, Takata R, Yamamoto K (2008) Risk factors associated with local recurrence of early gastric cancers after endoscopic submucosal dissection. Gastrointest Endosc 68:887–894
Sekiguchi M, Suzuki H, Oda I, Abe S, Nonaka S, Yoshinaga S, Taniguchi H, Sekine S, Kushima R, Saito Y (2013) Favorable long-term outcomes of endoscopic submucosal dissection for locally recurrent early gastric cancer after endoscopic resection. Endoscopy 45:708–713
Oda I, Gotoda T, Hamanaka H, Eguchi T, Saito Y, Matsuda T, Bhandari P, Emura F, Saito D, Ono H (2005) Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc 17:54–58
Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, Kim HJ, Kim JJ, Ji SR, Seol SY (2009) Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 69:1228–1235
Ryu KW, Kim YW, Lee JH, Nam BH, Kook MC, Choi IJ, Bae JM (2008) Surgical complications and the risk factors of laparoscopy-assisted distal gastrectomy in early gastric cancer. Ann Surg Oncol 15:1625–1631
Kim AR, Cho J, Hsu YJ, Choi MG, Noh JH, Sohn TS, Bae JM, Yun YH, Kim S (2012) Changes of quality of life in gastric cancer patients after curative resection: a longitudinal cohort study in Korea. Ann Surg 256:1008–1013
Davies J, Johnston D, Sue-Ling H, Young S, May J, Griffith J, Miller G, Martin I (1998) Total or subtotal gastrectomy for gastric carcinoma? A study of quality of life. World J Surg 22:1048–1055
Choi JH, Kim ES, Lee YJ, Cho KB, Park KS, Jang BK, Chung WJ, Hwang JS, Ryu SW (2015) Comparison of quality of life and worry of cancer recurrence between endoscopic and surgical treatment for early gastric cancer. Gastrointest Endosc 82:299–307
Acknowledgments
This work was supported by the Soonchunhyang University Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jun-Hyung Cho, Sang-Woo Cha, Hyun Gun Kim, Tae Hee Lee, Joo Young Cho, Weon Jin Ko, So-Young Jin, and Suyeon Park have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Cho, JH., Cha, SW., Kim, H.G. et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a comparison study to surgery using propensity score-matched analysis. Surg Endosc 30, 3762–3773 (2016). https://doi.org/10.1007/s00464-015-4672-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4672-1