Abstract
Background
The presence of clinically significant portal hypertension (CSPH) remains a relative contraindication to liver resection for patients with resectable hepatocellular carcinoma (HCC). The goal of this study was to explore whether a laparoscopic approach could extend the indications for hepatectomy to patients with PH.
Method
Patients who underwent laparoscopic liver resection (LLR) from February 2016 to September 2019 performed by a single medical team were included in this study. We analyzed the surgical and oncological outcomes between groups with and without CSPH before and after propensity score matching (PSM).
Result
We enrolled 156 patients divided into two groups according to the presence (CSPH, n = 26) or absence (non-CSPH, n = 130) of CSPH. CSPH group was associated with more clinical signs of liver dysfunction (p < 0.05). After PSM (n = 48 patients), the CSPH group tended to have a longer postoperative hospital stay (p = 0.054); however, there was no difference in operation time (p = 0.329), blood loss volume (p = 0.392), transfusion rates (p = 0.701), rate of conversion to open surgery (p = 0.666), surgical margin (p = 0.306), surgical mortality (n = 0), or comprehensive complication index (p = 0.844) between the two groups. The median follow-up time for the entire cohort was 19.6 months (range 0.2–40.6 months). The 3-year overall survival rate was 62.9% in the CSPH group and 84.3% in the non-CSPH group (p = 0.1090), and results were similar after PSM (p = 0.5734).
Conclusions
LLR is safe and feasible for HCC with PH. The introduction of minimally invasive surgery, represented by LLR, can appropriately expand the indications for hepatectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC) is the sixth most common malignant neoplasm and consistently the third leading cause of cancer-related death in the world, accounting for nearly 90% of all cases of primary liver cancer [1]. Liver resection is widely considered the most curative treatment for HCC in patients with preserved liver function [2, 3].
The Asia–Pacific region, and particularly China, has a high incidence of HCC, which accounts for more than half of liver cancer cases worldwide [4]. Approximately 70–90% of patients develop HCC secondary to chronic hepatitis B virus (HBV)-related cirrhosis [4], and patients often develop clinically significant portal hypertension (CSPH) [5]. However, the majority of the guidelines for liver cancer, such as the Barcelona Clinic Liver Cancer (BCLC), European Association for the Study of the Liver, and American Association for the Study of Liver Diseases guidelines consider HCC with CSPH a contraindication for liver resection [3, 6, 7]. Liver transplantation is recommended as a potentially curative treatment for HCC with PH because transplantation removes both lesions and underlying liver diseases. However, few patients with HCC with CSPH undergo liver transplantation because of the high financial burden and the shortage of liver donors. Therefore, according to the current guidelines, patients with HCC and PH are recommended to undergo percutaneous ethanol injection or radiofrequency ablation (RFA), both of which are inferior to hepatectomy regarding recurrence-free survival (RFS) and overall survival (OS) [8].
Currently, whether liver resection is feasible in patients with HCC and CSPH remains controversial. Several institutions have reported that partial liver resection is safe and feasible for HCC with PH, with careful patient selection and a skilled surgeon [9,10,11]. However, current studies constitute small retrospective studies, and large randomized controlled trials are required to confirm the feasibility of partial liver resection in HCC with PH.
When laparoscopic liver resection (LLR) technology was introduced in 1991, the technology was limited to small wedge resection for some benign lesions [12]. With advances in surgical techniques and equipment, major LLR for malignant disease is no longer rare. LLR has many advantages such as minimizing surgical trauma, less bleeding, shorter hospital stay, and lower postoperative morbidity, and does not compromise oncological outcomes. However, it is unclear whether these benefits apply to patients with CSPH, and whether LLR achieves the same curative effect as in patients without CSPH.
The objective of this retrospective, case-matched study was to compare the surgical and oncological outcomes in HCC patients with or without clinically significant portal hypertension underwent laparoscopic liver resection, and to provide evidence that minimally invasive surgery could expand surgical indications to patients with clinically significant portal hypertension.
Materials and methods
Patients
From February 2016 to September 2019, 195 consecutive patients underwent LLR performed by a single medical team of the Hepato-Pancreatic-Biliary Surgery Department at Zhejiang University, School of Medicine, Sir Run Run Shaw Hospital. We retrospectively identified and reviewed patients’ data from a prospectively maintained LLR database.
All patients from the database provided signed consent using a form that anonymized their clinical data and which can be used for review and research. This retrospective study was approved by the Ethics committee of Sir Run Run Shaw Hospital.
Inclusion criteria
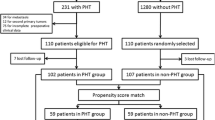
The inclusion criteria were (1) preoperative diagnosis of primary liver cancer according to imaging findings, (2) the lesion could be excised surgically, (3) liver resection was initially performed laparoscopically, and (4) the mass was identified as HCC histologically. Patients with a history of other cancers, histologically diagnosed as intrahepatic cholangiocarcinoma, and those with incomplete clinical data were excluded (Fig. 1). From these patients, we finally enrolled 26 patients in CSPH group and 130 patients in non-CSPH group.
Diagnosis and classification
Clinically significant portal hypertension (CSPH), which is considered a strong predictor of the presence of perioperative mortality and liver-related complication, was defined as a hepatic venous pressure gradient (HVPG) ≥ 10 mmHg as a gold standard [13,14,15]. However, HVPG measurement is a relatively invasive test and impractical to perform routinely in all patients with HCC; therefore, patients diagnosed as CSPH meet the surrogate criteria of the presence of gastroesophageal varices or platelet count < 100,000 cells/mL with splenomegaly (largest diameter > 12 cm) [16, 17].
We defined LLR according to the 2016 LLR experience-based guidelines [12]. Unlike open surgery, laparoscopic major resection was defined as hemihepatectomy and trisectionectomy, and as “difficult resections” (segments 1, 4a, 7, and 8) [18]. Anatomic liver resection was defined as segmentectomy and subsegmentectomy [19] according to the Couinaud segment classification [20].
Postoperative liver failure was identified using the “50–50 criteria” (the association between prothrombin time < 50% and serum bilirubin > 50 mmol/L) [21]. Liver-specific complications constituted liver failure, hypoalbuminemia requiring albumin transfusion, ascites, and bile leakage requiring abdominal drainage. We classified postoperative complications according to the Clavien–Dindo classification system and scored the complications using the Comprehensive Complication Index [22, 23]. Major complication was defined as Clavien–Dindo grade III or IV. Surgery margins were assessed for all cases and were classified into R0 (microscopically negative), R1 (microscopically positive), and R2 (gross residual disease).
Indications and surgical procedures
Indications for LLR were based mainly on patients’ preoperative Child–Pugh grade (A or B), remnant liver volume compared with the standard total volume (remnant liver volume > 40%), as well as liver reserve function (ICG-R15 ≤ 45%) [24]. During this study period, we did not consider nodule size and number, and PH as absolute exclusion criteria for surgical treatment for any resectable tumor. Each patient was assessed by a multidisciplinary team consisting of surgeons, radiologists, oncologists, pathologists, and anesthesiologists to evaluate the indications, surgical approach, and feasibility.
All operations involved a similar technique and were performed by the same experienced surgeon (Dr. Liang) and his teammates. Pure laparoscopic resection was performed in all cases without using of hand-assisted devices. The patient was placed in a supine position with the upper body rotated to the left. The primary surgeon and an assistant holding the scope stood on the left side with another assistant on the right. Intra-abdominal pressure was maintained between 10 and 14 mmHg. We used four or five ports for operation. Intraoperative ultrasonography was routinely used to confirm the location of the tumor as well as to identify the path of the hepatic vein. Central venous pressure was maintained at a low level using a restrictive intravenous fluid approach during parenchymal transection. Anatomic liver resections were performed whenever possible for lesions < 5 cm in diameter, using the Glissonean pedicles transection method advocated by Takasaki et al. [19, 25]. A novel method of Pringle maneuver was intermittently used when transecting the liver parenchyma [26]. The transection of liver parenchymal was performed with laparoscopic Peng’s multifunctional operative dissector and Ultrasonic-Harmonic Scalpel. Small vessels and bile ducts less than 2 mm were sealed by electric or ultrasonic coagulation. Massive bleeding and bile leakage were clipped or sutured. Glissonean pedicles or hepatic veins larger than 10 mm were transected by laparoscopic linear staples.
Analyzed variables and specimens
We reviewed and analyzed the following demographic and laboratory variables for both groups: age; gender; body mass index (BMI); American Society of Anesthesiologists (ASA) score; liver function tests; blood routine examination; coagulation function and liver reserve function tests (indocyanine green retention rate at 15 min (ICG-R15) and serum α-fetoprotein level; preoperative imaging findings; operation record; surgical outcomes; oncological efficacy; and survival data. A precise case-matching process was implemented before comparative analysis by considering the major confounding factors such as age, sex, BMI, ASA score, serum α-fetoprotein level, HBV infection, previous hepatectomy, tumor size and number, type of resection (anatomic or non-anatomic resection), and extent of resection.
The surgical specimens were routinely sent to department of pathology and examined by at least two experienced pathologists. Information about tumor size and number was retrieved on gross inspection. The presence of vascular invasion, satellite nodules, differentiated degree, and the status of surgical margin were confirmed by microscopy analysis. Tumors were classified as well, moderately, or poorly differentiated according to modified Edmondson and Steiner criteria. Following the pathological criteria, the patients were categorized into two groups: high recurrent risk for those who exhibited vascular invasion and/or additional nodules and/or satellites and low recurrent risk for who did not exhibit these parameters [27].
Follow-up
Follow-up data were registered on November 22, 2019. After discharge from hospital, we followed each patient as an outpatient with abdominal imaging tests and serum tumor marker measurement every 3 months for the first 2 years and every 6 months thereafter, postoperatively. Surgical mortality was regarded as any operation-related death in the first 30 days after surgery. We evaluated oncological efficiency according to OS, defined as the time from LLR to the date of death, and RFS, defined as the time from LLR to the date of the first recurrence confirmed by imaging examination.
Statistical analysis
Continuous variables are presented as means ± standard deviations or medians with ranges. Categorical variables are presented as numbers and percentages. To offset potential confounding factors, the CSPH group was matched to the non-CSPH group using a propensity score-matched (PSM) analysis by considering patients’ demographic and immunological factors as well as tumor-related variables and the surgical procedure. We used a logistic regression model and the nearest-neighbor algorithm in the matching process. Continuous variables were also analyzed by one-way analysis of variance for normally distributed variables between staging groups. Student’s t test or the Mann–Whitney U test was used to comparatively analyze continuous variables, while the chi-square or Fisher’s exact test was used to compare categorical variables between the groups. Survival and recurrence analyses were performed using the Kaplan–Meier method and are displayed with survival curves. OS and RFS comparisons between the two groups were performed using the log-rank test. A p value of less than 0.05 was considered statistically significant, and all statistical analyses were performed using IBM SPSS Statistics, (version 26.0; IBM Corp., Armonk, NY) and R software (version 3.5.3; Vienna Austria; https://www.R-project.org/).
Results
From February 2016 to September 2019, 193 patients underwent LLR for primary liver malignancies in our institution. We excluded 37 patients with intrahepatic cholangiocarcinoma, mixed hepatocarcinoma, double primary cancer, and incomplete clinical data; 156 patients were finally included in the study (Fig. 1). Among the 156 patients, 26 patients (16.7%) met the surrogate criteria for CSPH and constituted the CSPH group, and the remaining 130 (83.3%) patients constituted the non-CSPH group. After matching at a 1:1 ratio, 24 patients in each group were enrolled for further analysis.
Patients’ baseline characteristics
The baseline characteristics of the two groups are summarized in Table 1. Overall, 3 women and 23 men (median age 58 years, range 53.5–65 years) constituted the CSPH group, and 22 women and 108 men constituted the non-CSPH group (median age 61.5 years, range 51–68.2 years). The CSPH group had a higher rate of HBV infection (p = 0.018) vs the non-CSPH group, while other factors (age, sex, ASA score, BMI, previous hepatectomy, tumor size and count, and extent of resection) did not differ between the groups. After PSM, the groups were adequately balanced for all selected baseline variables.
Liver function-related characteristics
Liver function-related characteristics in overall and PSM cohorts are shown in Table 2. The CSPH group had more clinical signs of liver dysfunction, seen as a higher rate of liver cirrhosis (p < 0.001), higher international normalized ratio (p = 0.01), longer prothrombin time (p = 0.002), lower albumin level (p = 0.002) and platelet counts (p < 0.001), as well as poorer Child–Pugh grade (p < 0.001) and ICG-R15 value (p = 0.001). After PSM, the cirrhosis rate (p = 0.002), prothrombin time (p = 0.021), international normalized ratio (p = 0.015), and platelet count (p = 0.015) remained statistically significantly different between the two groups.
Surgical outcomes and complications
All procedures were performed as planned. The operative data are summarized in Tables 1, 3. A similar distribution of the type of surgical procedure was noted between the CSPH and non-CSPH groups in both the overall and PSM cohorts. In the overall cohort, anatomic resection was performed in 9/26 patients (34.6%) in the CSPH group and in 69/130 patients (53.1%) in the non-CSPH group (p = 0.086), and major resection was performed in 12 (46.2%) patients in the CSPH group and 74 (55.4%) patients in the non-CSPH group (p = 0.389). In the PSM cohort, anatomic resection was performed in 9/24 patients (37.5%) in the CSPH group and in 8/24 patients (33.3%) in the non-CSPH group (p = 0.763), and major resection was performed in 12 (50%) patients in the CSPH group and in 14 (58.3%) (p = 0.562) patients in the non-CSPH group. There was no significant difference regarding the duration of surgery, estimated blood loss, use of Pringle’s maneuver, clamping time, blood transfusion rates, and the rate of conversion before and after PSM (Table 3).
Postoperative outcomes and surgical morbidity data are shown in Table 3. There was no postoperative mortality within 30 days, and 39 patients suffered postoperative complications in the overall cohort. Among the 39 patients, 30 patients developed minor complications (Clavien–Dindo grade I/II) constituting 5 (19.2%) patients in the CSPH group and 25 (19.2%) patients in the non-CSPH group (p = 1.00), and 9 patients developed major complications (Clavien–Dindo grade III/IV) constituting 3 (11.5%) patients in the CSPH group and 6 (4.6%) patients in the non-CSPH group (p = 0.173). The most frequent minor complications were ascites treated with diuretics: 1 (4%) patient in the CSPH group and 9 (6.9%) patients in the non-CSPH group (p = 0.580) and hypoalbuminemia requiring albumin infusion in 4 (15.4%) patients in the CSPH group and in 10 (7.7%) patients in the non-CSPH group (p = 0.254). The most frequent major complication was pleural effusion requiring drainage, which occurred in 2 (7.7%) patients in the CSPH group and in 3 (2.3%) patients in the non-CSPH group (p = 0.194). Overall, only one patient in the CSPH group suffered Clavien–Dindo grade IV complication because of multiple organ failure secondary to severe allergic reactions. This patient recovered after ECMO (extracorporeal membrane oxygenation) therapy and other organ support treatments. Similarly, we found no difference in the comprehensive complication index (CCI) for complications between the two groups (p = 0.357). After matching, postoperative mortality, surgical complications (33.3% vs 33.3%, p = 1), minor complications (20.5% vs 29.2%, p = 0.505), major complications (12.5% vs 4.2%, p = 0.609), and liver-specific complications (29.2% vs 25%, p = 0.745) were similar between the CSPH group and non-CSPH group, respectively. In the overall cohort, we found no significant difference for postoperative hospital length of stay of 6 (5–7.25) days vs 6 (4–8) days, in the CSPH group vs non-CSPH group, respectively (p = 0.252). The CSPH group showed a trend toward a longer hospital stay compared with the non-CSPH group (6.5 (5–7.8) days vs 5 (4–7) days, respectively; p = 0.054) after PSM.
Long-term outcomes
The long-term outcomes of the two groups in the overall and the PSM cohorts are shown in Fig. 2. The median follow-up duration was 19.6 months (range 0.2–40.6 months) in the overall cohort and 24.2 months (range 0.2–39.5 months) in the PSM cohort.
Comparison of overall survival (OS) and recurrence-free (RFS) rates between the clinically significant portal hypertension (CSPH) and non-CSPH groups. A Kaplan–Meier curve for OS in the entire cohort (n = 156). The 3-year OS rates were 62.9% and 84.3% for the CSPH and non-CSPH groups, respectively. B Kaplan–Meier curve for RFS in the entire cohort (n = 156). The 3-year RFS rates were 57.7% and 63.4% for the CSPH and non-CSPH groups, respectively. C Kaplan–Meier curve for OS in the propensity score-matched cohort (n = 48). The 3-year OS rates were 66.5% and 78.4% for the CSPH and non-CSPH groups, respectively. D Kaplan–Meier curve for RFS in the propensity score-matched cohort (n = 48). The 3-year RFS rates were 60.0% and 78.3% for the CSPH and non-CSPH groups, respectively
During follow-up, 5 (19.2%) patients and 10 (7.7%) patients with HCC with or without CSPH died, respectively. The 1-, 2-, and 3-year OS rates were 83.0%, 75.4%, and 62.9% in the CSPH group and 94.6%, 91.3%, and 84.3%, in the non-CSPH group, respectively. Kaplan–Meier analysis revealed no significant statistical difference for OS between the two groups (p = 0.1090), and results were similar after PSM (p = 0.5506).
Seven (26.9%) patients (CSPH group) and 27 (20.8%) patients (non-CSPH group) experienced recurrence. Among these patients, 7 (26.9%) patients (CSPH) and 25 (19.3%) patients (non-CSPH1) developed intrahepatic recurrence, and the remaining two patients developed extrahepatic recurrence. The 1-, 2-, and 3-year RFS rates were 76.4%, 64.2%, and 57.7% in the CSPH group and 85.0%, 71.1%, and 63.4% in the non-CSPH group, respectively. Kaplan–Meier analysis revealed no significant statistical difference for RFS between the two groups (p = 0.3006), and results were similar after PSM (p = 0.3143).
Discussion
Liver surgery has made great progress recently because of the improvements in laparoscopic technology, and as a result, LLR has been proven safe in patients with cirrhosis [28]. In the current study, we aimed to evaluate the efficiency of LLR in patients with HCC and CSPH. Our study was currently the largest sample size study of patients with HCC with CSPH undergoing LLR [29, 30]. Furthermore, to address confounding effects and technical bias as much as possible, we performed PSM to balanced cofounding factors (non-liver function-related factors) [31]. Meanwhile, all surgeries were performed by the same experienced surgical team using a similar technique. We analyzed the CSPH and non-CSPH groups and found no difference in patients’ demographic characteristics and non-liver function-related variables after PSM (Table 1). To further evaluate any differences between the two groups, we compared the liver function-related factors and found that the CSPH group had more clinical signs of liver dysfunction both before and after PSM (Table 2). Our results confirmed that the presence of CSPH and a relatively poor liver function did not impact surgical and oncological outcomes in HCC patients undergoing LLR. Patients in both groups had similar blood loss volumes (200 ml vs 100 ml; CSPH vs non-CSPH, respectively). And the blood loss in this trial was less than levels in previously published series of LLR in patients with cirrhosis or with CSPH [32, 33]. We also saw a relatively short operation time in both groups (180 min vs 175 min (CSPH vs non-CSPH, respectively), which was even shorter than some open surgical series for cirrhotic liver [10, 34], and similar conversion rates (7.7% vs 13%, respectively) consistent with the previous studies [29, 30]. In addition, there was no mortality cases, and no difference in complications in patients with or without CSPH (32% vs 24%, respectively), including minor, major, and liver-specific complications. CSPH patients tended to have longer postoperative hospital stays, possibly because of slower recovery of liver function or a more cautious attitude of the surgeon toward patients with relatively worse liver function. Our study demonstrated that nearly half (46.2%) of patients in the CSPH group underwent major resection. Despite the fact that none of the tumors were located in Segment I, however, increased laparoscopic surgical capacities including maturation of surgical techniques and accumulation of more experiences will promote the broader application of LLR for patients with CSPH. We believe that LLR is indicated for all HCCs with CSPH, regardless of their locations in the liver.
The oncological outcome is the most important aspect of evaluating the operative technique. Almost all patients (except two cases) in this study got oncological radical resection, and there was no difference on the surgical margins between two groups. Although the outcomes of survival in the CSPH group appeared to be worse than those in the non-CSPH group, the difference was not statistically significantly different (p = 0.109), and after performing PSM to balance patients’ baseline characteristics, RFS and OS in both groups were still similar. These significant results indicated that minimally invasive surgery provided similar and acceptable oncological outcomes in HCC patients with or without CSPH.
According to the BCLC and American Association for the Study of Liver Diseases practice guidelines for the management of HCC, the presence of CSPH was considered a contraindication for liver resection because of the high mortality of 9% with minor resection and 25% with major resection [3, 35, 36]. This recommendation is based primarily on a study published in 1996 that evaluated 29 Child–Pugh class A patients with HCC and cirrhosis who underwent liver resection, and which demonstrated that CSPH was the most powerful predictor of postoperative liver decompensation. The study recommended CSPH as a contraindication for liver resection [37]; however, this small sample study without reporting long-term outcomes may not reflect clinical reality. Except for a small number of articles [38, 39], the many studies confirmed that CSPH has few impacts on surgical results [10, 15, 29, 30, 32, 33, 40, 41]. In 2009, Cucchetti and colleagues retrospectively analyzed 241 cirrhotic patients who underwent open liver resection for HCC and divided patients into two groups according to the BCLC surrogate criteria of CSPH [6]. The authors reported that CSPH was not associated with surgical outcomes both after PSM and in the multivariate analysis [10]. In 2018, another PSM analysis of 45 patients who underwent laparoscopic resection with confirmed CSPH graded as HVPG > 10 mmHg, concluded that there were no differences in perioperative results and survival between patients with and without CSPH. However, when performing PSM, both of these two studies enrolled liver function-related variables such as Child–Pugh class, total bilirubin, AST, ALT, and other variables that are strongly associated with the degree of portal hypertension. As a result, although the studies found no difference in patients’ baseline characteristics after PSM, patients in the CSPH group tended to be a mild degree of PH and the non-CSPH group tended to have a relatively poor liver function. To overcome this bias, we did not include any liver function-related variables into PSM analysis.
Without doubt, the most curative strategy for HCC with CSPH is liver transplantation because resection relieves PH and completely removes the malignancy [36]. However, donor shortage, high cost, and age restrictions limit the feasibility of transplantation [42]. Some studies suggested that proper candidate selection for LR may provide better outcomes vs transplantation [43, 44]. Adam et al. also argued that LR before transplantation is not associated with worse long-term survival after transplantation [45]. RFA, as an alternative treatment for HCC with CSPH, was also recommended in the BCLC guidelines [35]. The advantages of LLR compared with RFA are that for patients meeting the indications for liver transplantation, the pathological samples obtained by LLR can determine the recurrence risk of HCC by the degree of microvascular invasion, which better allocates the limited donor livers [46]. Furthermore, for tumors larger than 2 cm or tumors located in the periphery, resection can provide better and safer results than RFA [47]. In the current study, 108 (69%) patients whose tumors were larger than 2 cm were unsuitable for treatment of RFA, and 47 (30%) patients were a relatively advanced age (> 65 years) who may not be suitable for liver transplantation.
As a minimally invasive procedure, LLR appears to be more advantageous in patients with cirrhosis [48]. The large wound in open surgery is replaced by several small incisions for the trocars, resulting in a significant reduction in abdominal wall complications such as bleeding, infection, and hernia, especially in patients with cirrhosis with PH. Fewer disturbances to the collateral vessels and less compressive liver manipulations result in a significantly lower risk of intractable ascites, which is the main cause of liver failure and surgical infection in patients with cirrhosis [49]. Additionally, PH, coagulation dysfunction, and thrombocytopenia are conducive to hemorrhage both intraoperatively and postoperatively. Unprecedented clarity, pneumoperitoneum, and modern dissecting tools allow for more confident bleeding control in laparoscopy [50].
We identified serval important technical issues in our experience performing LLR in patients with CSPH. Preoperative liver function assessment should be performed routinely and evaluated in all patients indicated for LLR, namely, Child–Pugh grade, Model for End-stage Liver Disease (MELD) score, expected volume of the future liver remnant, and ICG-R15. In our institution, an ICG-R15 result of 15% was considered favorable for major resection, and 25% was the cutoff for minor resection. Previous hepatectomy was carefully considered as a significant factor in deciding whether to perform hepatectomy in laparoscopy, although recurrent liver cancer also has a high success rate with repeat laparoscopic hepatectomy [51]. For lesions located deep in the liver, changing body position instead of hard compression is helpful. When trocars are punched into the abdominal wall, attention should be paid to avoid dilated collateral veins because injured veins could lead to massive bleeding in patients with CSPH. Bleeding control plays an extraordinarily crucial role during transection of the liver parenchyma. Using preoperative three-dimensional models of portal pedicles and intraoperative ultrasonographic exploration identifies the path of vessels in the parenchyma, which also allows for better implementation of anatomic hepatectomy. Pringle’s maneuver is another effective measure to control intraoperative bleeding laparoscopically. Although in theory, Pringle’s maneuver may cause ischemia–reperfusion injury in cirrhotic livers, which increases the incidence of postoperative liver failure, our experience and current opinions indicate no evidence supporting that the Pringle maneuver is an independent risk factor for patients undergoing hepatectomy [32, 52, 53].
The retrospective design and relatively small sample sizes are limitations in the current study, although we collected the data from a prospective database. These limitations are why we used PSM to reduce the different distribution of patients’ baseline characteristics between the two groups as much as possible. There are many definitions of PH, and the gold standard is HVPG ≥ 10 mmHg. However, because of the invasiveness, high cost, and impracticality of this method for frequent follow‐up, most hepatobiliary centers do not perform this measurement. Therefore, we defined CSPH using the standard surrogate criteria widely accepted in clinical practice [6, 32, 34]. Because of the unique technical characteristics in our institution, our results may not apply to other centers or to surgeons using other laparoscopic techniques for LR. Performing LLR in patients with CSPH is a major challenge, particularly for lesions in difficult sites, and the procedure requires experience and skill. We are looking forward to the development of more practical laparoscopic methods and devices to increase the feasibility and effectiveness of LLR in patients with CSPH.
In conclusion, our findings demonstrated that LLR as a radical treatment provided acceptable perioperative and oncological outcomes in patients with resectable HCC and CSPH, especially for those diagnosed according to the BCLC surrogate criteria. Our results also provided evidence that the LLR indications should be appropriately expanded to patients with CSPH, even in major liver resections.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- CSPH:
-
Clinically significant portal hypertension
- LLR:
-
Laparoscopic liver resection
- PSM:
-
Propensity score matched
- HBV:
-
Hepatitis B virus
- BCLC:
-
Barcelona Clinic Liver Cancer
- RFA:
-
Radiofrequency ablation
- RFS:
-
Recurrence-free survival
- OS:
-
Overall survival
- BMI:
-
Body mass index
- ASA:
-
American Society of Anesthesiologists
- ICG-R15:
-
Indocyanine green retention rate at 15 min
- HVPG:
-
Hepatic venous pressure gradient
- CCI:
-
Comprehensive complication index
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- INR:
-
International normalized ratio
- IQR:
-
Interquartile range
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Grazi GL, Ercolani G, Pierangeli F, Del Gaudio M, Cescon M, Cavallari A, Mazziotti A (2001) Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg 234:71–78
EASL Clinical Practice Guidelines (2018) Management of hepatocellular carcinoma. J Hepatol 69:182–236
Zhu RX, Seto WK, Lai CL, Yuen MF (2016) Epidemiology of hepatocellular carcinoma in the Asia-pacific region. Gut Liver 10:332–339
Hackl C, Schlitt HJ, Renner P, Lang SA (2016) Liver surgery in cirrhosis and portal hypertension. World J Gastroenterol 22:2725–2735
Llovet JM, Bru C, Bruix J (1999) Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 19:329–338
Forner A, Reig ME, de Lope CR, Bruix J (2010) Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 30:61–74
Tian G, Yang S, Yuan J, Threapleton D, Zhao Q, Chen F, Cao H, Jiang T, Li L (2018) Comparative efficacy of treatment strategies for hepatocellular carcinoma: systematic review and network meta-analysis. BMJ Open 8:e021269
Kawano Y, Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Shibata K, Ohta M, Kitano S (2008) Short- and long-term outcomes after hepatic resection for hepatocellular carcinoma with concomitant esophageal varices in patients with cirrhosis. Ann Surg Oncol 15:1670–1676
Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, Grazi GL, Pinna AD (2009) Is portal hypertension a contraindication to hepatic resection? Ann Surg 250:922–928
Giannini EG, Savarino V, Farinati F, Ciccarese F, Rapaccini G, Marco MD, Benvegnu L, Zoli M, Borzio F, Caturelli E, Chiaramonte M, Trevisani F (2013) Influence of clinically significant portal hypertension on survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Liver Int 33:1594–1600
Coelho FF, Kruger JA, Fonseca GM, Araujo RL, Jeismann VB, Perini MV, Lupinacci RM, Cecconello I, Herman P (2016) Laparoscopic liver resection: experience based guidelines. World J Gastrointest Surg 8:5–26
Abraldes JG, Araujo IK, Turon F, Berzigotti A (2012) Diagnosing and monitoring cirrhosis: liver biopsy, hepatic venous pressure gradient and elastography. Gastroenterol Hepatol 35:488–495
Bosch J, Garcia-Pagan JC, Berzigotti A, Abraldes JG (2006) Measurement of portal pressure and its role in the management of chronic liver disease. Semin Liver Dis 26:348–362
Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J (2015) Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology 61:526–536
Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology 42:1208–1236
de Franchis R, Dell'Era A, Primignani M (2008) Diagnosis and monitoring of portal hypertension. Dig Liver Dis 40:312–317
Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D'Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS (2009) The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg 250:825–830
Makuuchi M, Hasegawa H, Yamazaki S (1985) Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 161:346–350
Couinaud C (1954) Anatomic principles of left and right regulated hepatectomy: technics. J Chir (Paris) 70:933–966
Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F (2005) The, "50–50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 242:824–828 discussion 828–829
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Clavien PA, Vetter D, Staiger RD, Slankamenac K, Mehra T, Graf R, Puhan MA (2017) The comprehensive complication index (CCI(R)): added value and clinical perspectives 3 years "down the line". Ann Surg 265:1045–1050
Kawamura H, Kamiyama T, Nakagawa T, Nakanishi K, Yokoo H, Tahara M, Kamachi H, Toi H, Matsushita M, Todo S (2008) Preoperative evaluation of hepatic functional reserve by converted ICGR15 calculated from Tc-GSA scintigraphy. J Gastroenterol Hepatol 23:1235–1241
Takasaki K, Kobayashi S, Tanaka S, Saito A, Yamamoto M, Hanyu F (1990) Highly anatomically systematized hepatic resection with Glissonean sheath code transection at the hepatic hilus. Int Surg 75:73–77
Cai J, Zheng J, Xie Y, Kirih MA, Jiang G, Liang Y, Liang X (2020) A novel simple intra-corporeal Pringle maneuver for laparoscopic hemihepatectomy: how we do it. Surg Endosc 34:2807
Sala M, Fuster J, Llovet JM, Navasa M, Solé M, Varela M, Pons F, Rimola A, García-Valdecasas JC, Brú C, Bruix J (2004) High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl 10:1294–1300
Worhunsky DJ, Dua MM, Tran TB, Siu B, Poultsides GA, Norton JA, Visser BC (2016) Laparoscopic hepatectomy in cirrhotics: safe if you adjust technique. Surg Endosc 30:4307–4314
Molina V, Sampson-Davila J, Ferrer J, Fondevila C, Diaz Del Gobbo R, Calatayud D, Bruix J, Garcia-Valdecasas JC, Fuster J (2018) Benefits of laparoscopic liver resection in patients with hepatocellular carcinoma and portal hypertension: a case-matched study. Surg Endosc 32:2345–2354
Lim C, Osseis M, Lahat E, Doussot A, Sotirov D, Hemery F, Lanteri-Minet M, Feray C, Salloum C, Azoulay D (2019) Safety of laparoscopic hepatectomy in patients with hepatocellular carcinoma and portal hypertension: interim analysis of an open prospective study. Surg Endosc 33:811–820
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46:399–424
Cipriani F, Fantini C, Ratti F, Lauro R, Tranchart H, Halls M, Scuderi V, Barkhatov L, Edwin B, Troisi RI, Dagher I, Reggiani P, Belli G, Aldrighetti L, Abu HM (2018) Laparoscopic liver resections for hepatocellular carcinoma. Can we extend the surgical indication in cirrhotic patients? Surg Endosc 32:617–626
Harada N, Maeda T, Yoshizumi T, Ikeda T, Kayashima H, Ikegami T, Harimoto N, Takaki S, Maehara Y (2016) Laparoscopic liver resection is a feasible treatment for patients with hepatocellular carcinoma and portal hypertension. Anticancer Res 36:3489–3497
Santambrogio R, Kluger MD, Costa M, Belli A, Barabino M, Laurent A, Opocher E, Azoulay D, Cherqui D (2013) Hepatic resection for hepatocellular carcinoma in patients with Child-Pugh's A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB (Oxford) 15:78–84
EASL-EORTC clinical practice guidelines (2012) Management of hepatocellular carcinoma. J Hepatol 56:908–943
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodes J (1996) Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 111:1018–1022
Jaeck D, Bachellier P, Oussoultzoglou E, Weber JC, Wolf P (2004) Surgical resection of hepatocellular carcinoma. Post-operative outcome and long-term results in Europe: an overview. Liver Transpl 10:S58–S63
Shen YN, Tang TY, Yao WY, Guo CX, Yi Z, Song W, Liang TB, Bai XL (2019) A nomogram for prediction of posthepatectomy liver failure in patients with hepatocellular carcinoma: a retrospective study. Medicine (Baltimore) 98:e18490
Capussotti L, Ferrero A, Vigano L, Muratore A, Polastri R, Bouzari H (2006) Portal hypertension: contraindication to liver surgery? World J Surg 30:992–999
Peng W, Li JW, Zhang XY, Li C, Wen TF, Yan LN, Yang JY (2019) A novel model for predicting posthepatectomy liver failure in patients with hepatocellular carcinoma. PLoS ONE 14:e0219219
Gaillard M, Tranchart H, Dagher I (2014) Laparoscopic liver resections for hepatocellular carcinoma: current role and limitations. World J Gastroenterol 20:4892–4899
Llovet JM, Fuster J, Bruix J (1999) Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 30:1434–1440
Koniaris LG, Levi DM, Pedroso FE, Franceschi D, Tzakis AG, Santamaria-Barria JA, Tang J, Anderson M, Misra S, Solomon NL, Jin X, DiPasco PJ, Byrne MM, Zimmers TA (2011) Is surgical resection superior to transplantation in the treatment of hepatocellular carcinoma? Ann Surg 254:527–537 discussion 53–528
Adam R, Bhangui P, Vibert E, Azoulay D, Pelletier G, Duclos-Vallee JC, Samuel D, Guettier C, Castaing D (2012) Resection or transplantation for early hepatocellular carcinoma in a cirrhotic liver: does size define the best oncological strategy? Ann Surg 256:883–891
Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M (2003) Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 38:200–207
Vigano L, Laurenzi A, Solbiati L, Procopio F, Cherqui D, Torzilli G (2018) Open liver resection, laparoscopic liver resection, and percutaneous thermal ablation for patients with solitary small hepatocellular carcinoma (≤30 mm): review of the literature and proposal for a therapeutic strategy. Dig Surg 35:359–371
Belli A, Cioffi L, Russo G, Belli G (2015) Liver resection for hepatocellular carcinoma in patients with portal hypertension: the role of laparoscopy. Hepatobil Surg Nutr 4:417–421
Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamazoe S, Yamamoto S, Kubo S (2013) Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg Endosc 27:2592–2597
Cheung TT, Dai WC, Tsang SH, Chan AC, Chok KS, Chan SC, Lo CM (2016) Pure laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma in 110 patients with liver cirrhosis: a propensity analysis at a single center. Ann Surg 264:612–620
Cai J, Zheng J, Xie Y, Kirih MA, Tao L, Liang X (2019) Laparoscopic repeat hepatectomy for treating recurrent liver cancer. J Minim Access Surg. https://doi.org/10.4103/jmas.JMAS_187_19
Lee KF, Wong J, Cheung SYS, Chong CCN, Hui JWY, Leung VYF, Yu SCH, Lai PBS (2018) Does intermittent pringle maneuver increase postoperative complications after hepatectomy for hepatocellular carcinoma? A randomized controlled trial. World J Surg 42:3302–3311
Zhang F, Yan J, Feng XB, Xia F, Li XW, Ma KS, Bie P (2015) Efficiency and safety of radiofrequency-assisted hepatectomy for hepatocellular carcinoma with cirrhosis: a single-center retrospective cohort study. World J Gastroenterol 21:10159–10165
Acknowledgement
We thank Jane Charbonneau, DVM, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81827804) and the Zhejiang Major Medical Science and Technology Plan supported by National Health Commission of China (Grant No. WKJ-ZJ-2030).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Xiao Liang, Junhao Zheng, Xu Feng, Yuelong Liang, Jingwei Cai, Zhaoqi Shi, Mubarak Ali Kirih, and Liye Tao have no conflict of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, J., Feng, X., Liang, Y. et al. Safety and feasibility of laparoscopic liver resection for hepatocellular carcinoma with clinically significant portal hypertension: a propensity score-matched study. Surg Endosc 35, 3267–3278 (2021). https://doi.org/10.1007/s00464-020-07763-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07763-6