Abstract
Background
Postoperative hypocalcemia is a common complication of thyroidectomy. This problem is most often associated with accidental devascularization or excision of the parathyroid glands (PG).
Aim
Aim was to study near-infrared (NIR) fluorescent imaging with intraoperative PG indocyanine green (ICG) angiography to help identify and preserve PG during total thyroidectomy in order to avoid postoperative hypocalcemia.
Material and methods
From 2017 to 2022, a total of 92 patients underwent total thyroidectomy at Odessa Regional Hospital. Indications for surgery were multinodular goiter (n = 42), thyroid cancer (n = 43), and Graves’ disease (n = 7). By randomization all patients were divided into two groups: in the control group, 48 patients underwent standard total thyroidectomy, and in the main group, 44 patients underwent NIR-assisted total thyroidectomy with ICG angiography. Serum calcium and parathyroid hormone levels were compared between the two groups of patients in 1, 7–15 days after surgery and then 3, 6 months later.
Results
In the control group, based on a visual assessment of the PG, autotransplantation of the PG was conducted in only five cases. In the second group, autotransplantation was performed in 16 patients. The transient postoperative hypocalcemia was observed in 8 patients of the control group (16, 70%) and in the 2 patients of ICG group (4, 50%) on 5–10 postoperative days. In the first group, 2 patients at 3 months after surgery had permanent hypocalcaemia.
Conclusion
NIR fluorescent imaging with intraoperative PG ICG angiography is a safe and an easily repeatable method. This technique provides improved detecting and assessment of the perfusion of the PG. The need for autotransplantation of the PG can be determined more objectively using ICG imaging than simple visualization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The most common complications during thyroidectomy are hypocalcemia and recurrent nerve palsy. Recent studies report postoperative hypocalcemia rates of around 15% to 30% for transient hypocalcemia and 1% to 7% for definitive cases [1, 2]. Hypocalcemia primarily occurs due to unintentional damage to the parathyroid glands (PG) or their devascularization [2, 3]. Factors such as the small size and variable location of PGs significantly complicate their visualization, potentially leading to injury even with meticulous dissection by an experienced surgeon [2,3,4,5,6]. While most authors concur that one intact gland is adequate to maintain normal serum calcium and hormone levels in the blood, preserving as many PG as possible can minimize the incidence of postoperative hypoparathyroidism [1,2,3]. Autotransplantation of devascularized PG has been shown to be an effective method of restoring their function. However, visual assessment of their blood supply is subjective and may not accurately reflect the actual situation.
Intraoperative identification of PGs is a critical concern during total thyroidectomy. The conventional identification method primarily relies on visual inspection and palpation by the surgeon, with careful preservation of the PG blood supply based on the surgeon’s experience [5]. Recently, near-infrared (NIR) fluorescence imaging with indocyanine green (ICG) angiography has emerged as one of the most popular methods for detecting PGs and assessing their vascularization [3,4,5,6,7,8,9,10,11]. Due to their higher vascularity compared to surrounding tissues, PGs exhibit strong fluorescence on ICG angiography [4].
This study aimed to determine the potential benefits of routinely using intraoperative ICG angiography with NIR fluorescent imaging in total thyroidectomy. The primary objective was to examine the efficacy of NIR fluorescent imaging with intraoperative PG ICG angiography in identifying and preserving PG during total thyroidectomy, thereby reducing the risk of postoperative hypocalcemia.
Materials and methods
From April 1, 2017 to January 1, 2022, a total of 92 patients underwent total thyroidectomy at Odessa Regional Hospital. The patients were randomly divided into two groups: the control group consisting of 48 patients who underwent standard total thyroidectomy, and the ICG group with 44 patients who underwent NIR-assisted total thyroidectomy with ICG angiography.
The indications for surgery included thyroid cancer, multinodular goiter, and Graves’ disease. Parathyroid autofluorescence was detected using a NIR/ICG endoscopic system (Karl Storz, Tuttlingen, Germany). The system consisted of a high-end full high-definition camera system (H3-Z 3-Chip Full HD camera, Karl Storz) connected to a 10-mm 0-degree ICG laparoscope (Hopkins™ II, Karl Storz).
All patients received general anesthesia with endotracheal intubation. A 4 to 5 cm Kocher transverse collar incision was made 1 cm below the cricoid cartilage. The strap muscles were divided along the midline throughout their entire length until the thyroid gland was exposed. The sternohyoid muscles were separated from the underlying sternothyroid muscle through dissection until the internal jugular vein and nerves were identified. The inferior thyroid vessels were dissected and divided as close to the thyroid gland’s surface as possible to minimize parathyroid devascularization or injury to the recurrent laryngeal nerves.
For the ICG group, ICG angiography was performed during surgery. The anesthesiologist administered a standard intravenous dose of 15 mg ICG. The PG absorbed the dye within 2 min and remained fluorescent for up to 15 min. The surgeon’s assistant recorded the video in real time after ICG injection.
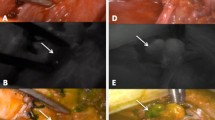
The parathyroid fluorescence intensity (FI) depended on the amount of ICG absorbed by the PG. The mechanism for parathyroid dye uptake is likely related to the abundant blood supply of endocrine organs. Consequently, the parathyroid FI reflects gland vascularization. In ICG angiography applications, different gray scale grades can indicate various levels of parathyroid function. PG were scored from 0 to 2 based on their FI after ICG angiography, with score 0 representing weak FI (Fig. 1), score 1 indicating moderate FI (Fig. 2), and score 2 signifying strong FI (Fig. 3). Surgeons performed parathyroid autotransplantation for glands that appeared well vascularized visually but were deemed devascularized in ICG angiography.
Serum calcium and parathyroid hormone (PTH) levels were compared between the two patient groups at 1, 3–10 days post surgery, and subsequently at 1, 3, and 6 months. Statistical analysis was performed using the χ2 test for discrete variables, with p < 0.05 considered statistically significant. Data for continuous variables are presented as mean (SD) values. The software Statistica version 5.0 was used for statistical analysis.
Results
The demographic profiles of patients were similar between the two groups. No significant differences were observed in comorbidities, including cardiac, pulmonary, vascular, or renal diseases (Table 1). In the control group, autotransplantation of the PG was performed in only five cases (three cases with one gland and two cases with two glands), based on visual assessment. In the ICG group, autotransplantation was conducted in 16 patients (9 cases with one gland, 6 cases with two glands, and 1 case with three glands). The table showcases the specific number of parathyroid glands that were successfully identified using ICG (Table 2).
Hypocalcaemia (calcium level below 2.25 mmol/l) was observed in either group. The transient postoperative hypocalcemia was observed in 8 patients of the control group (16.70%) and in 2 patients of ICG group (4.50%) on 5–10 postoperative days. There was no statistical difference in calcium or PTH levels between the groups on POD 30 (Table 3). But in the first group 2 patients at 3 months after surgery had hypoparathyroidism. Two patients in the control group experienced typical symptoms of hypocalcaemia but had PTH levels in the normal range on POD 30, without calcium supplementation. One had a calcium level of 2.05 mmol/l, PTH level of 3.7 pmol/l, and mild finger paraesthesia, which rapidly improved after oral calcium supplementation (dose 1 g). The other patient also experienced finger paraesthesia, with calcium and PTH values of 2.02 mmol/l and 5.2 pmol/l, respectively. The symptoms improved a few minutes after oral calcium supplementation (1 g). For patients in the control group, standard oral supplementation kept calcium levels within normal limits. In some cases, if necessary, we prescribed active vitamin D.
Discussion
Over the past century, thyroid and parathyroid surgery have become increasingly safe, with significant advancements and improvements in surgical techniques and patient care [1,2,3,4].
The literature reports that transient hypoparathyroidism occurs with a frequency of 15 to 30%, and permanent hypoparathyroidism occurs with a frequency of 3–7% [3].
Despite these advances, hypoparathyroidism remains a concern, with 15–30% rates of post-thyroidectomy hypoparathyroidism reported in the literature [1, 2].
Permanent hypoparathyroidism leads to fatal consequences despite treatment: basal ganglia calcifications, renal calcification, carpopedal spasm, cardiac issues, and psychiatric problems [12].
Currently, there is no gold standard for detecting and preserving PG and their vascularization during thyroidectomy [7, 8]. The blood supply and localization of PG are crucial factors for preservation during surgery, but anatomical variability in this region is high, demanding great experience and flexibility from the surgeon [2, 9]. Some parathyroids receive their blood supply directly from the thyroid gland (8.2%), which makes preservation difficult [2, 9].
The location of the PG in relation to the thyroid gland has a huge amount of variation. In addition, there are various options for the blood supply to the PG. In some cases, they have a separate vessel. In some cases, they receive their blood supply from the thyroid gland or are generally located within the thyroid gland. Some authors distinguish up to five different types of blood supply to the PG. [13]. And in some of them it is impossible to maintain a normal blood supply during thyroidectomy.
There are several basic techniques aimed at preventing hypoparathyroidism.
The traditional technique is capsular dissection, which allows you to gradually identify all the PG with the release of the thyroid gland. [14]. However, it is not always possible to identify all PG. Identification of two or less PG dramatically increases the risk of developing hypoparathyroidism [15]. In addition, routine dissection may be accompanied by trauma to the nerve, blood vessels, and bleeding, which further complicates the identification of the PG and significantly prolongs the operation time [16].
The problem is so controversial that some surgeons suggest looking for PG only in a typical location. If they cannot be found there, then it is better to simply continue with the capsular dissection in order to avoid trauma to the glands located in atypical places [17, 18].
Most surgeons prefer a selective approach to autotransplantation. That is, autotransplantation is performed only for those PG that appear macroscopically ischemic or have been accidentally removed. However, macroscopic evaluation is very subjective and inconclusive. A discolored gland does not necessarily indicate that it is not viable. At the same time, the absence of discoloration is not a reliable sign of a good blood supply [19, 20].
Delbridge et al. suggested routine autotransplantation of at least one PG [21]. At the same time, the frequency of temporary hypocalcemia increases, and the frequency of permanent hypocalcemia decreases. However, selective autotransplantation remains the most widely practiced technique, although it is not perfect [22].
In our research, approximately two-thirds of unintentional parathyroid removals coincided with thyroid cancer cases and central neck dissections. Accidental PG excision has been linked to factors like a low volume of surgical procedures, the PG's intrathyroidal location, and central neck dissection operations [4, 5].
New optical-based techniques without radiation exposure have been developed to aid in the detection of PG (particularly autofluorescence) and confirmation of their vascularization (specifically ICG angiography) [23, 24]. The angiography imaging system has become essential for a wide range of surgeries [10, 23, 24]. One of the initial objectives of this study was to demonstrate that ICG angiography has emerged as a powerful tool for predicting parathyroid function immediately after thyroidectomy [25]. At the same time, fluorescence imaging systems are quickly becoming key instruments in thyroid and parathyroid surgeries [25]. Combining NIRAF with ICG can highlight not only anatomy but also vascularization and, consequently, gland function [4]. In fact, injecting ICG at the end of the procedure allows verification of PG vitality and can be considered a valid predictor of postoperative calcium levels, as demonstrated by several authors [4, 11]. In particular, Vidal Fortuny et al. emphasized that at least one well-vascularized PG at the end of the surgical procedure is predictive of normal postoperative PTH levels [4].
Data from various studies suggest that ICG administration may have some complications [26]. Recent research has established those rare reactions, such as anaphylactic or urticarial reactions, may occur [27]. Over the past 34 years, only 17 adverse reactions have been reported, with no additional information available on ICG dye allergic reactions [27]. What is known about ICG dye allergic reactions is largely based on the fact that the ICG substance contains 5% sodium iodine for solubility, and patients allergic to ICG were all iodine-allergic and had renal insufficiency [27]. However, these reactions were reported to be 0.00167% and mainly occurred in patients with a history of allergy to iodides [27]. With an anesthesia team ready to treat allergic reactions, ICG angiography maintains a high degree of safety.
Regarding autotransplantation of PG, it is essential to determine which ischemic PGs require replantation. However, replantation does not guarantee that the gland will adapt to its new location and function normally. Therefore, the best use of the system to ensure safe blood flow to the PG is during dissection of the surrounding tissue [9]. ICG visualization is not only useful for the identification of PGs but also for a more reliable determination of gland viability. This study has demonstrated that intraoperative angiography with ICG is simple and reproducible. Although the laparoscopic imaging camera system is currently expensive, it produces high-quality images and can be used in other surgical procedures, making the equipment more cost-effective [28].
In analyzing the postoperative results from our study, we observed that calcium levels during the immediate postoperative phase (day 1) did not showcase any statistically significant differences, as initially anticipated. By day 30, while still not achieving statistical significance, we noticed a growing divergence in calcium and PTH levels.
However, of paramount importance to us was not solely the alteration in calcium levels but the noticeable decrease in the incidence of persistent hypoparathyroidism. Within the control group, persistent hypoparathyroidism was evident in 2 out of 48 cases at the 3-month mark, whereas in the main group, no instances were reported. While this difference was not statistically significant (p = 0.225), it hints at a trend that may become evident with a larger study cohort. Our hypothesis is that expanding the sample size could potentially yield statistically significant differences.
Conclusion
NIR fluorescent imaging with intraoperative PG ICG angiography is a safe and an easily repeatable method. This technique provides improved detecting and assessment of the perfusion of the PG. The need for autotransplantation of the PG can be determined more objectively using ICG imaging than simple visualization.
References
Almquist M, Hallgrimsson P, Nordenström E, Bergenfelz A (2014) Prediction of permanent hypoparathyroidism after total thyroidectomy. World J Surg 38(10):2613–2620. https://doi.org/10.1007/s00268-014-2622-z
Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian SP (2014) Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 101(4):307–320. https://doi.org/10.1002/bjs.9384
Rao SS, Rao H, Moinuddin Z, Rozario AP, Augustine T (2023) Preservation of parathyroid glands during thyroid and neck surgery. Front Endocrinol 14:1173950. https://doi.org/10.3389/fendo.2023.1173950
Vidal Fortuny J, Belfontali V, Sadowski SM, Karenovics W, Guigard S, Triponez F (2016) Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 103(5):537–543. https://doi.org/10.1002/bjs.10101
Spartalis E, Ntokos G, Georgiou K, Zografos G, Tsourouflis G, Dimitroulis D, Nikiteas NI (2020) Intraoperative indocyanine green (ICG) angiography for the identification of the parathyroid glands: current evidence and future perspectives. In vivo (Athens, Greece) 34(1):23–32. https://doi.org/10.21873/invivo.11741
Jin H, Dong Q, He Z, Fan J, Liao K, Cui M (2018) Application of a fluorescence imaging system with indocyanine green to protect the parathyroid gland intraoperatively and to predict postoperative parathyroidism. Adv Ther 35(12):2167–2175. https://doi.org/10.1007/s12325-018-0834-6
van den Bos J, van Kooten L, Engelen SME, Lubbers T, Stassen LPS, Bouvy ND (2019) Feasibility of indocyanine green fluorescence imaging for intraoperative identification of parathyroid glands during thyroid surgery. Head Neck 41(2):340–348. https://doi.org/10.1002/hed.25451
Jin H, Fan J, Yang J, Liao K, He Z, Cui M (2019) Application of indocyanine green in the parathyroid detection and protection: report of 3 cases. Am J Otolaryngol 40(2):323–330. https://doi.org/10.1016/j.amjoto.2018.11.003
Alesina PF, Meier B, Hinrichs J, Mohmand W, Walz MK (2018) Enhanced visualization of parathyroid glands during video-assisted neck surgery. Langenbecks Arch Surg 403(3):395–401. https://doi.org/10.1007/s00423-018-1665-2
Zaidi N, Bucak E, Yazici P, Soundararajan S, Okoh A, Yigitbas H, Dural C, Berber E (2016) The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 113(7):775–778. https://doi.org/10.1002/jso.24237
Suh YJ, Choi JY, Chai YJ, Kwon H, Woo JW, Kim SJ, Kim KH, Lee KE, Lim YT, Youn YK (2015) Indocyanine green as a near-infrared fluorescent agent for identifying parathyroid glands during thyroid surgery in dogs. Surg Endosc 29(9):2811–2817. https://doi.org/10.1007/s00464-014-3971-2
Chang YK, Lang BHH (2017) To identify or not to identify parathyroid glands during total thyroidectomy. Gland Surg 6(Suppl 1):S20–S29. https://doi.org/10.21037/gs.2017.06.13
Cui Q, Li Z, Kong D, Wang K, Wu G (2016) A prospective cohort study of novel functional types of parathyroid glands in thyroidectomy: In situ preservation or auto-transplantation? Medicine 95(52):e5810. https://doi.org/10.1097/MD.0000000000005810
Reeve TS, Curtin A, Fingleton L, Kennedy P, Mackie W, Porter T, Simons D, Townend D, Delbridge L (1994) Can total thyroidectomy be performed as safely by general surgeons in provincial centers as by surgeons in specialized endocrine surgical units? Making the case for surgical training. Arch Surg (Chicago, IL: 1960) 129(8):834–836. https://doi.org/10.1001/archsurg.1994.01420320060011
Algethami RF, Algarni F, Fallatah S, Almehmadi RA, Aljuaid H, Alsalem AS, Mahfouz MEM, Alosaimi M (2022) Prevalence and risk factors for hypoparathyroidism following total thyroidectomy in Taif City. Cureus 14(12):e32460. https://doi.org/10.7759/cureus.32460
Zhu JQ, Tian W, Xu ZG, Jiang KW, Sun H, Wang P et al (2015) The expert consensus of parathyroid glands protection during thyroidectomy. Chin J Pract Surg 35:731–736. https://doi.org/10.3978/j.issn.2305-5839.2015.08.20
Lorente-Poch L, Sancho J, Muñoz JL, Gallego-Otaegui L, Martínez-Ruiz C, Sitges-Serra A (2017) Failure of fragmented parathyroid gland autotransplantation to prevent permanent hypoparathyroidism after total thyroidectomy. Langenbecks Arch Surg 402(2):281–287. https://doi.org/10.1007/s00423-016-1548-3
Tartaglia F, Blasi S, Giuliani A, Merola R, Livadoti G, Krizzuk D, Tortorelli G, Tromba L (2016) Parathyroid autotransplantation during total thyroidectomy. Results of a retrospective study. Int J Surg (London England) 28(Suppl 1):S79–S83. https://doi.org/10.1016/j.ijsu.2015.05.059
Promberger R, Ott J, Kober F, Mikola B, Karik M, Freissmuth M, Hermann M (2010) Intra- and postoperative parathyroid hormone-kinetics do not advocate for autotransplantation of discolored parathyroid glands during thyroidectomy. Thyroid 20(12):1371–1375. https://doi.org/10.1089/thy.2010.0157
Kuhel WI, Carew JF (1999) Parathyroid biopsy to facilitate the preservation of functional parathyroid tissue during thyroidectomy. Head Neck 21(5):442–446. https://doi.org/10.1002/(sici)1097-0347(199908)21:5%3c442::aid-hed10%3e3.0.co;2-z
Delbridge L (2003) Total thyroidectomy: the evolution of surgical technique. ANZ J Surg 73(9):761–768. https://doi.org/10.1046/j.1445-2197.2003.02756.x
Promberger R, Ott J, Bures C, Kober F, Freissmuth M, Seemann R, Hermann M (2014) Can a surgeon predict the risk of postoperative hypoparathyroidism during thyroid surgery? A prospective study on self-assessment by experts. Am J Surg 208(1):13–20. https://doi.org/10.1016/j.amjsurg.2013.11.007
Fanaropoulou NM, Chorti A, Markakis M, Papaioannou M, Michalopoulos A, Papavramidis T (2019) The use of Indocyanine green in endocrine surgery of the neck: a systematic review. Medicine 98(10):e14765. https://doi.org/10.1097/MD.0000000000014765
Sanchez EQ, Chinnakotla S, Khan T, Nikitin D, Vasani S, Randall HB, McKenna GJ, Ruiz R, Onaca N, Levy MF, Goldstein RM, Docherty JC, Hurd DK, Klintmalm GB (2008) Intraoperative imaging of pancreas transplant allografts using indocyanine green with laser fluorescence. Proceedings (Baylor Univ Med Center) 21(3):258–260. https://doi.org/10.1080/08998280.2008.11928406
Tobis S, Knopf JK, Silvers CR, Marshall J, Cardin A, Wood RW, Reeder JE, Erturk E, Madeb R, Yao J, Singer EA, Rashid H, Wu G, Messing E, Golijanin D (2012) Near infrared fluorescence imaging after intravenous indocyanine green: initial clinical experience with open partial nephrectomy for renal cortical tumors. Urology 79(4):958–964. https://doi.org/10.1016/j.urology.2011.10.016
DeLong JC, Ward EP, Lwin TM, Brumund KT, Kelly KJ, Horgan S, Bouvet M (2018) Indocyanine green fluorescence-guided parathyroidectomy for primary hyperparathyroidism. Surgery 163(2):388–392. https://doi.org/10.1016/j.surg.2017.08.018
Boni L, David G, Mangano A, Dionigi G, Rausei S, Spampatti S, Cassinotti E, Fingerhut A (2015) Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc 29(7):2046–2055. https://doi.org/10.1007/s00464-014-3895-x
Perry D, Bharara M, Armstrong DG, Mills J (2012) Intraoperative fluorescence vascular angiography: during tibial bypass. J Diabetes Sci Technol 6(1):204–208. https://doi.org/10.1177/193229681200600125
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Viktor V. Grubnyk, Volodymyr V. Grubnik, Yurii V. Grubnik, and Roman S. Parfentiev have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grubnik, V.V., Parfentiev, R.S., Grubnik, Y.V. et al. Intraoperative indocyanine green angiography for predicting postoperative hypoparathyroidism. Surg Endosc 37, 9540–9545 (2023). https://doi.org/10.1007/s00464-023-10466-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10466-3






