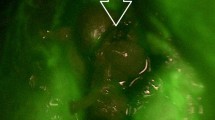
Abstract
Introduction
Hypoparathyroidism is the most frequent complication after total thyroidectomy and, when permanent, it becomes a severe chronic disease. We assessed the usefulness of indocyanine green (ICG) angiography-guided thyroidectomy to reduce the postoperative hypocalcemia.
Methods
Prospective study with two consecutive cohorts of patients who underwent total thyroidectomy: historical control group (CG) and angiography-guided thyroidectomy group (AG). In all patients, ICG-angiography was performed at the end of the surgery to predict immediate parathyroid gland (PG) function. In the AG, ICG-angiography was also done after PG identification to show their vascular supply. We compared the rate of postoperative hypocalcemia (calcium supplementation needed due to hypocalcemia symptoms or calcium levels < 1.8 mmol/L on the first postoperative day) and permanent hypocalcemia (need of calcium ± vitamin D supplementation 12 months after thyroidectomy).
Results
We included 120 consecutive patients (84 CG; 36 AG). Thyroid cancer was the most common diagnostic (63.1% CG–69.4% AG; p = 0.646) and central neck dissection was also frequent (54.8% CG–64.3% AG; p = 0.468). The AG developed a lower rate of postoperative (26.2–5.6%; p = 0.011) and permanent hypocalcemia (11.9–0%; p = 0.032). The OR for permanent hypocalcemia was 0.673 (95% CI 0.591–0.766). A significant higher rate of well vascularized PG at the end of the surgery (score 2) in the AG (39.2–52.9%; p = 0.018) was also seen.
Conclusion
ICG angiography-guided thyroidectomy is a useful tool to identify PG vascularization, allowing a better PG preservation and a significant decrease in hypocalcemia rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraoperative real-time indocyanine green (ICG) angiography has gained popularity for the localization and assessment of vascularization of the parathyroid glands during thyroid surgery [1,2,3,4,5,6,7,8,9,10,11], although estimation of the gland perfusion with this ICG technique still lacks standardization [1,2,3,4]. Also, a limited number of studies have examined the correlation of intraoperative ICG angiography findings and postoperative parathyroid function with different conclusions [5,6,7,8]. Recently, a new application of the ICG angiography technique is based on the possibility to show those vessels feeding the parathyroid glands before performing thyroidectomy, allowing the surgeons to perform an accurate dissection to preserve these vessels and hence parathyroid gland function [12, 13]. However, whether intraoperative ICG mapping angiograms will further reduce hypoparathyroidism has not been previously evaluated.
We assessed whether intraoperative identification of the feeding vessels of the parathyroid glands using ICG angiography before total thyroidectomy could reduce the occurrence of postoperative hypocalcemia.
Methods
Design and patients
This was a prospective cohort study of all consecutive patients with thyroid diseases in which elective total thyroidectomy, with or without neck dissection was the treatment of choice. The study was performed at the Unit of Endocrine Surgery of an acute-care teaching hospital between October 2018 and February 2022. Eligible patients were included in two consecutive cohorts. The first cohort included patients who underwent post-thyroidectomy ICG angiography to predict immediate parathyroid gland function by scoring the degree of fluorescence of the parathyroid glands (from October 2018 to October 2020) (control group). These patients belonged to a previous study in which all 4 parathyroid glands had to be identified to define the value of a well perfused PG (score 2) in post-thyroidectomy calcium levels [14, 15]. The second cohort included patients undergoing initially ICG angiography guided thyroidectomy to identify the vessels feeding the parathyroid glands and then, similarly to control group, post-thyroidectomy ICG angiography to predict immediate parathyroid function (from November 2020 to February 2022) (angiography-guided thyroidectomy group). Exclusion criteria were history of previous parathyroid surgery, severe hepatic or renal dysfunction and allergy or intolerance to ICG or iodine dyes. All included patients were controlled at 1 month after surgery, and since then until resolution of hypoparathyroidism when necessary.
The primary endpoint was a comparison of post-thyroidectomy hypocalcemia between the two cohorts. Secondary objectives were as follows: (a) to compare the occurrence of severe and permanent hypocalcemia after total thyroidectomy between the two cohorts, (b) to compare the number of parathyroid glands identified, left in situ and with an ICG score of 2 after total thyroidectomy.
The study protocol was approved by the local Clinical Research Ethical Committee (codes PR054/19 and PR004/22), and written informed consent was obtained from all patients.
Procedures
All patients were managed similarly in the perioperative period throughout the study period. In patients with a preoperative 25-OH vitamin D deficit (< 20 ng/mL), supplementation was recommended. None of the patients received preoperative calcium supplementation.
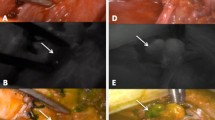
All patients were operated on by the same senior endocrine surgeon (P.M.Ll.) using a standard protocol for thyroidectomy, starting with luxation of each thyroid lobe and performing careful dissection to minimize damage to the parathyroid glands, followed by search and visual identification of the glands in each lobe. A reasonable effort was made to identify all parathyroid glands in orthotopic localizations. Then, in the angiography-guided thyroidectomy group an ICG angiography of the parathyroid glands was performed. Briefly, 1 mL of the contrast material was administered through a peripheral vein after dilution of a powdered vial of 25 mg (Verdye®, Diagnostic Green GmbH, Aschheim-Dornach, Germany) of ICG in 10 mL of sterile water. After a few seconds, ICG-enhanced fluorescence imaging of the vascular pattern of the parathyroid glands previously identified was acquired using a near-infrared camera (SPY-PHI, Stryker Endoscopy, San Jose, CA, USA) (Fig. 1). The vascular pattern included visualization of a clear feeding vessel with its pedicle or a vascular network around the parathyroid gland without a clear feeding vessel (Figs. 2 and 3). In these patients, once the vessel supplying each parathyroid gland was identified, the dissection was performed in a guided manner, when possible, to minimize its damage.
In both groups (angiography-guided thyroidectomy and control), after each lobectomy, 3 mL of the contrast material was administered, with repeated doses when needed allowed until a maximum of 5 mg/kg was reached, and a black and white near-infrared view was obtained as previously described [14,15,16]. The degree of ICG fluorescence on parathyroids was classified as 0, black (non-vascularized), 1, gray/heterogeneous (partially vascularized) and 2, white similar to an artery (well vascularized) (Fig. 4). The same procedures were followed in the contralateral lobe. In patients undergoing central neck dissection, angiography was performed after each procedure, lymphadenectomy and thyroidectomy. The surgical procedure is shown in an supplementary video (https://youtu.be/nZR8wJ7b3iE).
Measurement of intact PTH (iPTH) levels was determined preoperatively, 10 min after completion of the thyroidectomy, 10 min after central neck dissection (when performed) and at 24 h postoperatively, using a chemiluminescence immunometric assay (reference limit 1.6–6.9 pmol/L). Corrected serum calcium levels were measured 24 h after operation using a colorimetric assay, and results were expressed as mmol/L (reference 2.20–2.54 mmol/L). Calcium was administered (1500 mg every 8 h) in the presence of symptoms of hypocalcemia or serum calcium levels < 1.8 mmol/L in asymptomatic patients. Hypocalcemia was defined as the need of calcium supplementation, and severe hypocalcemia as the need of calcium and vitamin D supplementation. Vitamin D supplementation was introduced on the second postoperative day when the patient failed to normalize calcium levels after oral calcium administration. Permanent hypocalcemia was defined functionally as the need of calcium and/or vitamin D supplementation for 12 months after thyroid surgery to maintain calcium levels within the normal limit and free of hypocalcemic symptoms.
For each patient the following data were recorded: demographics (age, gender); clinical diagnosis of thyroid disease; central/lateral neck dissection (yes/no); number of parathyroid glands identified; arteriographic pattern in all parathyroid glands identified at the beginning of the surgical procedure and showing ICG uptake; number of parathyroid glands preserved in situ and autotransplanted; quantitative ICG fluorescence of the parathyroid glands (score 0, 1 and 2); histological diagnosis; number of glands inadvertently resected or included in the surgical specimen; serum iPTH levels before thyroid gland removal, 10 min after completion of thyroidectomy and 24 h postoperatively; serum corrected calcium levels 24 h after surgery; and requirements of calcium and/or vitamin D supplementation and its duration.
Statistical analysis
Categorical variables are expressed as frequencies and percentages, and continuous variables as mean and standard deviation (SD) or median and interquartile range (IQR) (25th-75th percentile). The Student’s t test or the Mann–Whitney U test were used for the comparison of continuous variables, and the chi-square test or the Fisher’s exact test for categorical variables. The odds ratio (OR) and 95% confidence intervals (CI) were also calculated. Statistical significance was set at P < 0.05. The Statistical Package for the Social Sciences (SPSS) version 26 for Windows was used for data analysis.
Results
A total of 120 patients undergoing elective total thyroidectomy met the inclusion criteria and were included in the study. There were 37 men and 83 women, with a mean (SD) age of 51.3 (13.8) years. Eighty-four patients were included in the historical control group and 36 in the angiography guided group. Salient clinical characteristics are shown in Table 1. Thyroid cancer and multinodular goiter were the most frequent clinical diagnosis in 65% and 25.8% of patients, respectively, whereas papillary cancer was the most frequent final histological diagnosis (63%). Central and lateral neck dissection were performed in 57.5% and 31.3% of the patients (88.5% and 44.9% in those with cancer), respectively. Statistically significant differences in preoperative and surgery-related variables between the two study groups were not found (Table 1).
Post-thyroidectomy hypocalcemia
Calcium supplementation was needed by 24 patients (20.0%) postoperatively, 2 (5.6%) from the arteriography-guided group and 22 (26.2%) from the control group (p = 0.011). Severe hypocalcemia was diagnosed in 1 (2.8%) patient in the arteriography-guided group and in 12 (14.3%) control patients (p = 0.105). Permanent hypocalcemia occurred in 10 (11.9%) patients from the control group compared to no patients in the angiography group (p = 0.032). The OR for permanent hypocalcemia was 0.673 (95% CI 0.591–0.766).
Patients in the angiography and control groups showed similar levels of iPTH before thyroid surgery but 10 min and 24 h after completion of total thyroidectomy, iPTH levels were significantly higher in the angiography group. Corrected calcium levels 24 h after surgery were significantly higher in the angiography group than in controls (Table 2).
Table 3 displays those factors related to the development of postoperative hypocalcemia. The performance of lateral neck dissection was associated with a greater risk of hypocalcemia, while the main protector factor against hypocalcemia was the preservation of at least one gland with an ICG score 2.
Preservation of the parathyroid glands
As shown in Table 4, of the total potential number of parathyroid glands in the study population, the percentage of glands identified in the angiography group (88.2%) was higher than in the control group (80.4%), being this difference almost significant (P = 0.051). The percentage of glands left in situ was also slightly higher in the angiography group than in controls (95.3% vs. 92.6%), but differences were not significant (p = 0.429). Seventeen glands had been autotransplanted, 2 in the arteriography group and 15 in the control group (p = 0.118). Also, 29 parathyroid glands were identified in the histopathological examination, 7 of them corresponded to the angiography group and 22 in the control group.
In the 127 parathyroid glands identified in the arteriography-guided group, there was one feeding vessels with a clear vascular pedicle in 66 (52.0%) and a visible vascular network around the gland without a dominant vessel in 45 (44.1%). In 5 parathyroid glands the vascular pattern could not be evaluated. When a clear vascular pedicle was observed, the final ICG score was 2 in 37 glands preserved in situ (60.7%), while when no dominant vessel was found, we only could preserve in situ with an ICG score 2, 25 glands (45.5%). Although a tendency was observed, the difference did not reach statistical significance (p = 0.146). Also, the number of parathyroid glands with an ICG score 2 was significantly higher in the arteriography-guided group than in the control group (52.9% vs. 39.2%; p = 0.018) (Table 4).
Discussion
The use of ICG angiography at the beginning of thyroidectomy and once the parathyroid glands have been identified, has been a feasible technique to reveal the vascular map of the glands. This finding is consistent with the prospective study of Benmiloud et al. [13]. However, the novelty of our study is the evaluation of the influence of this technique on transient and permanent hypocalcemia. Identification of the vascular pattern and feeding vessels of the parathyroids favored not only a higher number of glands left “in situ” but especially well vascularized, as shown by a higher number of glands with an ICG score of 2, resulting in a fewer number of patients with hypocalcemia.
Routine identification of the parathyroid glands during thyroid surgery is controversial, as systematic identification could be associated to transient hypoparathyroidism [17]. Moreover, a pitfall with this strategy is that not all parathyroid glands could be found in their usual or orthotopic positions. In contrast, selective identification of fewer in situ well vascularized glands may further lower the risk of hypoparathyroidism, since identification of parathyroids is not equivalent to preserve functioning glands [17, 18]. However, assessment of the vascular pattern by means of intraoperative ICG angiography may be a useful tool for preserving visually identified parathyroid glands. In our study, all patients were consecutively operated on by the same endocrine surgeon, which may prevent surgery-related performance bias, although worse results could be obtained in the historical control group if the surgeon improves over time. Also, the decreasing rate of hypocalcemia could be the result of a natural evolution in the quality of care of the surgeon.
Interestingly, the vascular pattern was characterized by the presence of one feeding vessel with clear vascular pedicle or a poorly defined pattern with a visible vascular network around the gland. In 5 cases, one case in which the parathyroid gland was retracted by the thyroid tumor, the vascular pattern could not be assessed.
When considering the degree of angiographic perfusion after initial angiography, it could be argued that after the initial dose of ICG, some dye could remain in the operative field and could alter the ability to evaluate the glowing of the glands after consecutive ICG doses and therefore their score. However, we have observed that after every repeated dye (ICG) injection the remaining fluorescence disappear immediately after the new dose of ICG shows the vessels and perfuses the gland. In fact, intraoperative ICG angiography was useful because it allowed guiding thyroidectomy according to the vascular map and, in a second step, to predict the parathyroid function based on score system 0-1-2. However, a scoring system based on quantification of ICG fluorescence should be necessary for a more objective and easy assessment of parathyroid gland perfusion and viability status.
It should be noted that there was a high representation of patients with cancer and patients treated with central neck dissection, which may account for the relatively high rate of permanent hypoparathyroidism in the first cohort. This means that although on the one hand it would allow finding differences more easily, in a certain way it constitutes a selection bias because this was a cohort of special risk. In this regard, we found that although central neck dissection did not significantly increase the rate of hypoparathyroidism, this was increased in patients with a lateral neck dissection. This is probably due to the presence of patients with a greater tumor burden, who require more aggressive dissections, with a higher risk of hypoparathyroidism.
Besides, patients in the control group belonged to a study in which it was necessary to find all 4 parathyroid glands. This “intensive search” could have been contributed to a higher rate of permanent hypoparathyroidism in this group. However, although in the second cohort of patients the representation of cancer patients was similar, the use of arteriography-guided thyroidectomy allowed us to drastically reduce the rate of hypoparathyroidism, even in these high-risk patients. Although some patients in the second cohort have not completed one-year follow-up, none of them remained with hypoparathyroidism at the time this study was completed.
Intraoperative identification of vessels feeding parathyroids implies a change in the way thyroidectomy is performed especially when the supplying vessels are intimately attached to the thyroid, come from the thyroid or have an anomalous course, which happens very often. Thus, conventional surgery aims at resecting the thyroid leaving the parathyroids in situ, sometimes intuitively. However, when performing angiography-guided thyroidectomy the main objective is twofold and in this order: firstly, to dissect and preserve both the vessels supplying the glands and secondly, to complete the thyroidectomy.
Conclusion
This study reports the evolution of surgical results of one thyroid senior surgeon using angiography-guided thyroidectomy in a large endocrine clinic. In the present study, identification of the parathyroid vessels before conducting the thyroidectomy allowed to preserve “in situ” more well-perfused parathyroid glands and consequently contributing to prevent the development of postoperative hypoparathyroidism.
However, these findings should be confirmed in a multicenter randomized controlled trial.
References
Vidal Fortuny J, Sadowski SM, Belfontali V, Karenovics W, Guigard S, Triponez F (2016) Indocyanine green angiography in subtotal parathyroidectomy: technique for the function of the parathyroid remnant. J Am Coll Surg 223(5):e43–e49. https://doi.org/10.1016/j.jamcollsurg.2016.08.540
Spartalis E, Ntokos G, Georgiou K, Zografos G, Tsourouflis G, Dimitroulis D, et al (2020) Nikiteas NI. Intraoperative indocyanine green (ICG) angiography for the identification of the parathyroid glands: current evidence and future perspectives. In Vivo 34(1):23–32. https://doi.org/10.21873/invivo.11741.
Demarchi MS, Seeliger B, Lifante JC, Alesina PF, Triponez F (2021) Fluorescence image-guided surgery for thyroid cancer: utility for preventing hypoparathyroidism. Cancers (Basel) 13(15):3792. https://doi.org/10.3390/cancers13153792
Demarchi MS, Karenovics W, Bédat B, Triponez F (2020) Intraoperative autofluorescence and indocyanine green angiography for the detection and preservation of parathyroid glands. J Clin Med 9(3):830. https://doi.org/10.3390/jcm9030830
Lang BH, Wong CK, Hung HT, Wong KP, Mak KL, Au KB (2017) Indocyanine green fluorescence angiography for quantitative evaluation of in situ parathyroid gland perfusion and function after total thyroidectomy. Surgery 161(1):87–95. https://doi.org/10.1016/j.surg.2016.03.037
Rudin AV, McKenzie TJ, Thompson GB, Farley DR, Lyden ML (2019) Evaluation of parathyroid glands with indocyanine green fluorescence angiography after thyroidectomy. World J Surg 43(6):1538–1543. https://doi.org/10.1007/s00268-019-04909-z
Liang TJ, Wang KC, Wang NY, Chen IS, Liu SI (2021) Indocyanine green angiography for parathyroid gland evaluation during transoral endoscopic thyroidectomy. J Pers Med 11(9):843. https://doi.org/10.3390/jpm11090843
Yavuz E, Biricik A, Karagulle OO, Ercetin C, Arici S, Yigitbas H et al (2020) A comparison of the quantitative evaluation of in situ parathyroid gland perfusion by indocyanine green fluorescence angiography and by visual examination in thyroid surgery. Arch Endocrinol Metab 64(4):427–435. https://doi.org/10.20945/2359-3997000000219
Gálvez-Pastor S, Torregrosa NM, Ríos A, Febrero B, González-Costea R, García-López MA (2019) Prediction of hypocalcemia after total thyroidectomy using indocyanine green angiography of parathyroid glands: a simple quantitative scoring system. Am J Surg 218(5):993–999. https://doi.org/10.1016/j.amjsurg.2018.12.074
Vidal Fortuny J, Belfontali V, Sadowski SM, Karenovics W, Guigard S, Triponez F (2016) Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 103(5):537–543. https://doi.org/10.1002/bjs.10101
Vidal Fortuny J, Sadowski SM, Belfontali V, Guigard S, Poncet A, Ris F, Karenovics W et al (2018) Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine green fluorescence predicting parathyroid function after thyroid surgery. Br J Surg 105(4):350–357. https://doi.org/10.1002/bjs.10783
Sadowski SM, Vidal Fortuny J, Triponez F (2017) A reappraisal of vascular anatomy of the parathyroid gland based on fluorescence techniques. Gland Surg 6(Suppl 1):S30–S37. https://doi.org/10.21037/gs.2017.07.10
Benmiloud F, Penaranda G, Chiche L, Rebaudet S (2022) Intraoperative mapping angiograms of the parathyroid glands using indocyanine green during thyroid surgery: results of the Fluogreen study. World J Surg 46(2):416–424. https://doi.org/10.1007/s00268-021-06353-4
Moreno Llorente P, Francos Martínez JM, García Barrasa A (2020) Intraoperative parathyroid hormone measurement vs indocyanine green angiography of parathyroid glands in prediction of early postthyroidectomy hypocalcemia. JAMA Surg 155(1):84–85. https://doi.org/10.1001/jamasurg.2019.3652
Moreno Llorente P, García Barrasa A, Francos Martínez JM, Alberich Prats M, Pascua Solé M (2022) Intraoperative indocyanine green angiography of parathyroid glands and the prevention of post-thyroidectomy hypocalcemia. World J Surg 46(1):121–127. https://doi.org/10.1007/s00268-021-06322-x.ç
Moreno Llorente P, García Barrasa A, Francos Martínez JM, Alberich Prats M, Pascual Solé M (2021) Intraoperative indocianine green (ICG) angiography of the parathyroid glands in prediction of post-thyoidectomy hypocalcemia: diagnostic accuracy of ICG score 2 versus the 4-ICG score. Cir Esp (Engl Ed) Apr 27:S0009–739X(21)00130–5. https://doi.org/10.1016/j.ciresp.2021.03.017.
Chang YK, Lang BHH (2017) To identify or not to identify parathyroid glands during total thyroidectomy. Gland Surg 6(Suppl 1):S20–S29. https://doi.org/10.21037/gs.2017.06.13
Lang BH, Chan DT, Chow FC (2016) Visualizing fewer parathyroid glands may be associated with lower hypoparathyroidism following total thyroidectomy. Langenbecks Arch Surg 401(2):231–238. https://doi.org/10.1007/s00423-016-1386-3
Acknowledgements
We thank Marta Pulido, MD, PhD, for editing the manuscript and editorial assistance.
Funding
No funding was received for this clinical study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
Ethical approval was obtained by the Clinical Research Ethical Committee of Hospital Universitari de Bellvitge (codes PR054/19 and PR004/22).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moreno-Llorente, P., García-Barrasa, A., Pascua-Solé, M. et al. Usefulness of ICG Angiography-Guided Thyroidectomy for Preserving Parathyroid Function. World J Surg 47, 421–428 (2023). https://doi.org/10.1007/s00268-022-06683-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-022-06683-x