Abstract
Purpose
Although not as life-threatening as anastomotic leakage, anastomotic stricture reduces the quality of life. The risk factors for such an important life complication have not been revealed. This article examines the risk factors affecting anastomotic strictures due to colorectal cancers.
Methods
Patients who underwent anterior and low anterior resection for colorectal cancer under elective conditions between 2015 and 2021 were included in the study. The patients were divided into two groups, those who developed anastomotic stricture and those who did not. The parameters determined between the two groups were compared, and multivariate analysis of statistically significant parameters was performed.
Results
A total of 375 patients were included in the study. The anastomotic stricture was detected in 36 (9.6%) patients. In the multivariate analysis, non-mobilization of the splenic flexure and a proximal clean surgical margin of < 10 cm and a distal surgical margin of < 2 cm were identified as risk factors affecting anastomotic stricture. The risk factor with the highest odds ratio in the development of anastomotic stricture is the non-mobilization of the splenic flexure (p = 0.001, OR 11.375).
Conclusion
It is recommended that the mobilization of the splenic flexure to reduce the development of strictures. In addition, a clean surgical margin of 10 cm proximally and 2 cm distally and high ligation of the inferior mesenteric artery may reduce the development of stricture.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer (CRC) is the third most common malignancy worldwide and the second leading cause of cancer-related death in 2018 [1]. Surgery is the gold standard treatment for colorectal cancer. In addition to surgical treatment, neoadjuvant CRT and cytotoxic chemotherapy have also improved survival rates [2,3,4].
Some complications related to anastomosis may develop in the surgical treatment of colorectal cancer. Anastomotic leakage is the most important life-threatening complication and occurs in 1–30% of patients [4, 5]. Many studies have been conducted to reveal the causes of anastomotic leaks [6,7,8].
The second important complication related to anastomosis is anastomosis strictures. These complications occur in 3–30% of patients [9, 10]. Although not as life-threatening as anastomotic leakage, anastomotic stricture reduces the quality of life and creates additional costs for treatment. Anastomotic stricture may cause urgent defecation, incontinence, and obstructions in advanced cases [11]. The risk factors for such an important life complication have not been revealed yet. By revealing the risk factors that affect the development of stricture, these rates may decrease, which may positively affect the patients’ quality of life.
The current study aims to investigate the risk factors that are effective in forming benign anastomosis strictures in patients with colorectal cancer who underwent anterior and low anterior resection.
Materials and methods
The files of patients who underwent anterior and low anterior resection for colorectal cancer under elective conditions in the general surgery department of Bakırkoy Training and Research Hospital between 2015 and 2021 were reviewed. Although the study was designed retrospectively, data were collected prospectively.
Patients who were diagnosed with colorectal cancer, stage 1–4 according to American Joint Committee on Cancer (AJCC) (8th edition), underwent anterior or low anterior resection and colorectal anastomosis, those with intact anastomosis and had moderate clinical status with post-operative radiological anastomotic leakage with anastomotic continuity (including patients who performed secondary diverting ileostomy), and patients over 18 years of age were included in the study. Patients under 18 underwent abdominoperineal resection, and in the second surgery, performed Hartman colostomy for uncontrollable anastomotic leakage, patients who did not attend follow-up controls, and patients with local recurrence in colonoscopies were not included in the study.
Anastomosis performed above the peritoneal reflection was accepted as anterior resection, and anastomosis performed below the peritoneal reflection was considered low anterior resection.
Anastomotic leaks included in the study describe patients without generalized peritonitis who were treated with drains and/or antibiotic therapy (Intravenous Ciprofloxacin + Metronidazole) alone or underwent secondary ileostomy and endoscopic clips. Patients who underwent Hartman colostomy due to anastomotic leakage were excluded from the study.
All surgeries were performed by experienced colorectal surgeons working in the gastrointestinal surgery department of our clinic.
All anastomosis procedures of the patients were performed mechanically, and a circular stapler (EEA 31 mm DST circular stapler) was used during these procedures. All anastomoses were performed using the end-to-end technique.
The patients were divided into two groups: those with and without anastomotic stricture. The group with anastomotic stricture included patients with stenosis that did not allow the passage of colonoscopy. All patients who underwent colonoscopy through the anastomosis without difficulty defined the group that did not develop anastomotic stricture.
All colonoscopies were performed and followed up by experienced colorectal surgeons. All colonoscopy procedures were performed with 12.8 mm diameter adult colonoscopies.
The formation of stenosis was detected in the colonoscopies performed for complaints, such as constipation and incontinence of the patients, and in the control colonoscopies performed for annual control time or ileostomy closure.
A colonoscopic evaluation was performed on all patients included in the study. It was pathologically confirmed that the developing strictures were benign. Local recurrence or malignant strictures were excluded from the study.
Patients’ ages, genders, body mass index (BMI) values, comorbidities, American Society of Anesthesiology (ASA) scores, neoadjuvant treatments, types of surgery, post-operative complications, post-operative pathologies, presence of distant metastases, and post-operative chemotherapy were recorded. The obtained data were compared between the two groups, and multivariate analysis was performed for the parameters with statistically significant differences between the two groups.
Ethical approval
All procedures in this study involving human participants were performed following the standard ethical guidelines, including the 1964 Declaration of Helsinki and all later amendments. The ethics committee approval for the study was obtained from the local ethics committee (Ethics committee approval date: 07.06.2021 Decision no: 2011-11-18).
Statistical analysis
Statistical analyses were performed using the SPSS (Statistical Package for the Social Sciences) 24.0 program (Armonk, NY). While descriptive statistical methods (Mean, Standard Deviation, Median, Frequency, Ratio, Minimum, and Maximum) were used to evaluate the study data, the independent sample t-test was used to compare the parameters with normal distribution between the two groups. The Pearson Chi-Square test was used in the analysis of qualitative data. Multivariate regression analysis was used to determine the effect levels. Statistical significance was evaluated at p < 0.01 and p < 0.05 levels.
Results
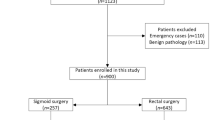
Between January 2015 and January 2021, 394 patients underwent anterior and low anterior resection for colorectal cancer under elective conditions in our clinic. Seven of these patients were excluded from the study because uncontrollable leakage developed in their anastomoses, and the Hartman colostomy procedure was performed, four of them had local recurrence in control colonoscopies, and eight of them did not come to the follow-up controls after surgery. After the exclusion of patients who did not meet the study criteria, a total of 375 patients were included in the study.
One-hundred-twenty-six patients had sigmoid colon, 72 had rectosigmoid, and 177 had rectal tumors. According to AJCC, 82 patients were stage 1, 128 were stage 2, 126 were stage 3, and 39 were stage 4.
Low anterior resection was performed in 177 patients, and anterior resection was performed in 198 patients. Diverting ileostomy was performed in 165 patients at the first operation. A secondary ileostomy was performed in four patients due to anastomotic leakage.
Forty-eight patients had moderate anastomotic leakage (fistula-like contrast leakage from the anastomosis and/or local abscess developing adjacent to the anastomosis). Twenty-eight of these patients had a diverting ostomy. Endoscopic clips were placed in three of these patients, and the other patients were followed up with only drains. Anastomotic leakage was observed in 20 patients without diverting ileostomy in the first operation. Secondary ileostomy was performed in four of them, and these patients were included in the ileostomy-positive group. The endoscopic clip procedure was applied to two patients. Interventional drainage was applied to five patients. The other nine patients were followed up with only drains.
Neoadjuvant chemoradiotherapy was performed on a total of 82 patients. Our clinical approach is to perform standard long-course neoadjuvant chemoradiotherapy, but short-course chemoradiotherapy was performed on three patients included in the study. Diverting ileostomy was performed in 75 (91.5%) of the patients who received neoadjuvant chemoradiotherapy.
Post-operative chemotherapy was performed in patients with neoadjuvant chemoradiotherapy, with lymph node and/or distant metastases, with pT4 and pT3 with low-level microsatellite instability (MSI-L). A total of 239 patients received post-operative chemotherapy.
In the colonoscopies performed on these patients, anastomotic stricture was not detected in 339 patients, while 36 patients had anastomotic stricture, and the rate of benign anastomotic stricture in this study was 9.6% (Fig. 1). Anastomotic stenosis was detected in 20 patients during preparations for ileostomy closure. Stenosis was found in the control colonoscopies performed with the complaints of difficult defecation and frequent defecation in 12 patients and the 1st year control colonoscopy in four patients. Although there were no clinical complaints in these four patients, colonoscopy could not be advanced proximally from the anastomosis line.
While 34 patients who developed stenosis were treated with balloon dilatation, anastomosis was performed again in one patient. One patient did not want to be treated by choosing to live with an ileostomy due to his advanced age and comorbidities.
In the univariant analysis, genders, ASA Scores, Charlson comorbidity index (CCI), diabetes mellitus (DM), heart failure (HF), hypertension (HT), chronic obstructive pulmonary disease (COPD), smoking status of patients, stages of tumor (according to AJCC), presence of distant metastases, neoadjuvant chemoradiotherapy intakes, surgery time and surgery types (open, laparoscopic, robotic), operation times after neoadjuvant treatment, pathology results (stages of tumors according to AJCC, T stage, presence of lymph node metastases, number of lymph nodes removed, histological type of tumor), clinically moderate anastomotic leakages, and the rates of post-operative chemotherapy did not differ statistically between the two groups (Tables 1, 2, 3).
While a higher mean BMI was an effective risk factor for the development of anastomotic stricture in the univariate analysis, it was found to have no effect in the multivariate analysis (p = 0.029, p = 0.136, respectively) (Tables 1 and 4).
Diverting ileostomy was performed in 169 (45%) patients. Four of them had a secondary ileostomy. Although diverting loop ileostomy was detected as a risk factor affecting anastomotic stricture in the univariate analysis, it was determined that it had no effect in the multivariate analysis (p = 0.006, p = 0.121, respectively) (Table 3 and 4).
The distal surgical margin being at a distance of 0–2 cm from the mass and the proximal surgical margin being less than 10 cm were found to be risk factors affecting anastomotic stricture in both the univariant (p = 0.001) (Table 3) and multivariate analyses (p = 0.021, OR = 1.964, p = 0.046, OR 2.141, respectively) (Table 4).
Low ligation of IMA was performed in 44 (11.74%) patients, and high ligation was performed in 331 (88.26%) patients. Although low ligation of the inferior mesenteric artery was identified as an anastomotic stricture risk factor in the univariate analysis, it was found to have no effect in the multivariate analysis (p = 0.040, p = 0.434, respectively) (Tables 3 and 4).
The splenic flexure was mobilized in 364 (97%) patients and not in 11 (3%) patients. Non-mobilization of the splenic flexure was determined as a risk factor affecting anastomotic stricture in both univariant and multivariate analyses. Non-mobilization of the splenic flexure was the most probable risk factor according to the odds ratio (p = 0.001, p = 0.001, OR 11.375, respectively) (Tables 3 and 4).
Discussion
Anastomotic strictures are one of the most common complications of colorectal surgery, and anastomotic stricture is detected in up to 30% of cases by colonoscopic evaluation [12, 13]. Although it is a fairly common complication, little attention has been paid to identifying risk factors for stricture formation. Ischemia is accepted to be the most common cause of stricture formation [14]. Predisposing factors have been identified, including anastomotic leaks, diverting loop ileostomies, pelvic sepsis, obesity, bleeding, and neoadjuvant radiotherapy, but these have not always been confirmed [13, 14].
In the study by Picazo-Ferrera et al. [14], being less than 52 years old was identified as a risk factor affecting anastomotic stricture in both the univariant and the multivariate analysis. The current study concluded that age is not a factor in the formation of anastomotic stricture. In the study by Polese et al. [13], however, it has been determined that age does not affect the formation of anastomotic stricture, similar to the result obtained in this study.
Gender has an impact on anastomotic stricture. In the study by Bannura et al. [15], the male gender was determined to be an effective risk factor in the formation of stricture in both univariant and multivariate analyzes. In the study by Polese et al. [13], although female gender was identified as a risk factor in the univariant analysis, it was found that it was ineffective in forming stricture in the multivariate analysis. In the current study, it was determined that gender was not a risk factor for stricture formation.
The study by Bertocchi et al. [16] stated that BMI is not a risk factor for the formation of anastomotic stricture in colorectal anastomoses. In the current study, due to its higher average BMI value, it was found to be an effective risk factor in the formation of anastomotic stricture in the univariant analysis, but it was determined that it had no effect in the multivariate analysis. In the study by Silva-Velazco et al. [17], it was stated that anastomotic leaks are more common in patients with high BMI values. In the current study, the reason for the higher incidence of anastomotic stricture in patients with high BMI values may be small anastomotic leaks, which remain clinically invisible.
Previous studies determined that the presence of comorbidity in patients is not an effective risk factor for the formation of anastomotic stricture [12,13,14]. In the current study, comorbidity and high ASA score did not affect anastomotic stricture formation.
There is no study examining the relationship between the presence of distant metastases and anastomotic stricture. In the current study, however, it was determined that distant metastases did not affect anastomotic stricture.
In a randomized controlled study by Qin et al. [18], neoadjuvant radiotherapy was reported to increase the formation of anastomotic stricture. However, in the related study, the rate of opening a diverting loop ileostomy in patients who received radiotherapy was statistically more significant than in patients who did not receive radiotherapy, and this was the biggest limitation of this study. Two studies investigating anastomotic stricture-independent risk factors found no effect of neoadjuvant radiotherapy on anastomotic stricture [13, 14]. Similarly, the current study determined that neoadjuvant radiotherapy was not an effective risk factor for the formation of anastomotic stricture.
The present study concluded that the timing of surgery after neoadjuvant radiotherapy did not affect the formation of anastomotic stricture. There is no study in which this result can be compared, but in a study by Terzi et al. [19], it was reported that there was no difference in terms of anastomotic leakage, other ileus, and infections that received surgical treatment in operations performed at the 8th week and 12th week after radiotherapy.
Another result of this study is that open, laparoscopic, and robotic surgery options are not risk factors that increase anastomotic stricture. Studies in the literature have shown that the laparoscopic and open approach does not affect anastomotic stricture [13, 14].
In a recently published meta-analysis, it was stated that the mobilization of the splenic flexure could be ignored, and it was reported that its release did not increase anastomotic leakage and stricture. The rates of wound infection and reoperation were found to be increased in the laparoscopy subgroup [20]. In another study, it was reported that splenic flexure mobilization had no effect on anastomotic stricture [13]. The current study claims the opposite of these results, and non-mobilization of the splenic flexure was found to be an effective risk factor in the formation of anastomotic stricture in both univariant and multivariate analysis. The results of the study by Hiranyakas et al. [21] are consistent with the results obtained in the current study, and it has been reported that non-mobilization of the splenic flexure increases both the anastomotic stricture formation and the rate of reoperation. Due to the lack of mobilization of the splenic flexure, tension occurs in the anastomosis, and this causes ischemia, anastomotic leaks, and strictures due to intense inflammation in the anastomosis. The finding of splenic flexure mobilization among the techniques that should be routinely performed to reduce colorectal anastomotic leakage in a study also supports our conclusion [22].
It has been reported that high or low ligation of the inferior mesenteric artery does not make a difference to the risk of developing complications and oncologic outcomes [23]. Polese et al. [13] reported that the attachment site of the inferior mesenteric artery did not affect the anastomotic stricture. In the current study, unlike other studies, anastomotic stricture was ineffective in multivariate analysis, although it was more common in patients who underwent low ligation. The study by Hiranyakas et al. [21] recommends high ligation of the inferior mesenteric artery to reduce anastomotic stricture formation. The findings that low ligation increases anastomotic stricture in univariant analysis can be explained by the following theory: low ligation of the inferior mesenteric artery may cause less mobilization of the colon compared to high ligation, which may create more tension in the anastomotic region, resulting in more inflammation and stricture.
The distance of the anastomosis to the anal verge is one of the factors affecting anastomotic stricture. In the study by Bannura et al. [15], distal rectal anastomosis did not affect anastomotic stricture. In the study by Polese et al. [13], however, it was stated that although more anastomotic stricture was observed in anastomoses between 8 and 12 cm, it had no effect in the multivariate analysis. Although more stricture was seen in anastomoses less than 5 cm from the anal verge in the current study, this was an ineffective risk factor in the multivariate analysis. In the current study, this result may be associated with more ileostomy performed in the distal rectal anastomoses.
No previous study has examined the relationship between distal and proximal surgical margins and anastomotic stricture. In the current study, a clean proximal surgical margin of less than 10 cm and a distal surgical margin of less than 2 cm were risk factors for stricture in both univariate and multivariate analyses. In distal rectal cancers, the distal intact margin is considered to be 2 cm, but in recent years it has been stated that intact distal surgical margins of 1 cm or < 1 cm do not indicate a poor prognosis [24,25,26]. However, in the study by Cong et al. [27], it was stated that the distal margin being < 1 cm increased the risk of anastomotic leakage. In a study by Qin et al. it was stated that extending the proximal surgical margin due to radiation-induced injury reduces the risk of anastomotic leakage in patients receiving neoadjuvant radiotherapy [28]. The results of these two studies and the present study reveal that the distal and proximal surgical margins should be expanded as much as possible to reduce anastomotic complications.
Performed a diverting loop ileostomy is one of the factors affecting anastomotic stricture, but this has not always been confirmed. Some studies have reported that it does not affect anastomotic stricture [13, 15]. In other studies, performing the diverting ileostomy was a risk factor for anastomotic stricture in the univariant analysis, while it was found to be ineffective in the multivariate analysis [14, 29]. In the current study, it was found to be an effective risk factor in the univariant analysis but not in the multivariate analysis.
In the study by Lee et al. [29], it was reported that mean operative time and the pathological stage did not affect anastomotic stricture. Another study found that the stage of the disease did not affect anastomotic stricture [14]. The current study determined that the mean operation time, pathological stage of the disease, and histology type did not affect anastomotic stricture.
In the current study, anastomotic leakage did not increase the risk of anastomotic stricture, similar to the results of previous studies [13, 15, 29].
This study has some limitations. The most important limitation is that it is a retrospective study, and follow-up information on some patients is unavailable. The number of patients in the stricture group was relatively small (n = 36). However, this is one of the rare studies investigating risk factors for anastomotic stricture. More prospective randomized controlled studies are needed in this area.
Conclusion
The results of this study reveal that it is in the hands of us surgeons to reduce anastomotic stricture. The risk factor most likely to affect anastomotic stricture was the non-mobilization of the splenic flexure. This reveals that anastomotic tension should be minimized to reduce the risk of anastomotic stricture. To reduce the risk of anastomotic stricture, it is recommended to mobilize the splenic flexure, to be 10 cm above the clean proximal surgical margin, be at least 2 cm above the distal surgical margin, and to perform high ligation of the inferior mesenteric artery in resections following the principles of total meso-colic excision.
Data availability
The data are stored locally.
Code availability
Not applicable.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM (2007) Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC trial group. J Clin Oncol 25(21):3061–3068. https://doi.org/10.1200/JCO.2006.09.7758
Pramateftakis MG, Kanellos D, Vrakas G, Tsachalis T, Raptis D, Makrantonakis A, Koukouritaki Z, Kanellos I (2010) Progress in rectal cancer staging and treatment. Tech Coloproctol 14(1):29–31. https://doi.org/10.1007/s10151-010-0619-7
Somashekhar SP, Reddy GRK, Deshpande AY, Ashwin KR, Kumar R (2021) A prospective study of real-time identification of line of transection in robotic colorectal cancer surgery by ICG. J Robotic Surg 15:369–374. https://doi.org/10.1007/s11701-020-01095-2
Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P (2011) Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 253(5):890–899. https://doi.org/10.1097/SLA.0b013e3182128929
Arron MNN, Greijdanus NG, Ten Broek RPG, Dekker JWT, van Workum F, van Goor H, Tanis PJ, de Wilt JHW (2021) Trends in risk factors of anastomotic leakage after colorectal cancer surgery (2011–2019): a dutch population-based study. Colorectal Dis 23(12):3251–3261. https://doi.org/10.1111/codi.15911. (Epub 2021 Oct 7 PMID: 34536987)
Sparreboom CL, van Groningen JT, Lingsma HF, Wouters MWJM, Menon AG, Kleinrensink GJ, Jeekel J, Lange JF, Dutch ColoRectal Audit Group (2018) Different risk factors for early and late colorectal anastomotic leakage in a nationwide audit. Dis Colon Rectum 61(11):1258–1266. https://doi.org/10.1097/DCR.0000000000001202. (PMID: 30239395)
Qu H, Liu Y, Bi DS (2015) Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc 29(12):3608–3617. https://doi.org/10.1007/s00464-015-4117-x. (Epub 2015 Mar 6 PMID: 25743996)
Biraima M, Adamina M, Jost R, Breitenstein S, Soll C (2016) Long-term results of endoscopic balloon dilation for treatment of colorectal anastomotic stenosis. Surg Endosc 30(10):4432–4437. https://doi.org/10.1007/s00464-016-4762-8
Chan RH, Lin SC, Chen PC, Lin WT, Wu CH, Lee JC, Lin BW (2020) Management of colorectal anastomotic stricture with multidiameter balloon dilation: long-term results. Tech Coloproctol 24(12):1271–1276. https://doi.org/10.1007/s10151-020-02318-2. (Epub 2020 Aug 5. PMID: 32757156; PMCID: PMC7661393)
Kraenzler A, Maggiori L, Pittet O, Alyami MS, la Denise PÀJ, Panis Y, (2017) Anastomotic stenosis after coloanal, colorectal, and ileoanal anastomosis: what is the best management? Colorectal Dis 19(2):O90–O96. https://doi.org/10.1111/codi.13587. (PMID: 27996184)
Schlegel RD, Dehni N, Parc R, Caplin S, Tiret E (2001) Results of reoperations in colorectal anastomotic strictures. Dis Colon Rectum 44(10):1464–1468. https://doi.org/10.1007/BF02234598. (PMID: 11598475)
Polese L, Vecchiato M, Frigo AC, Sarzo G, Cadrobbi R, Rizzato R, Bressan A, Merigliano S (2012) Risk factors for colorectal anastomotic stenoses and their impact on quality of life: what are the lessons to learn? Colorectal Dis 14(3):e124–e128. https://doi.org/10.1111/j.1463-1318.2011.02819.x. (PMID: 21910814)
Picazo-Ferrera K, Jaurrieta-Rico C, Manzano-Robleda M, Alonso-Lárraga J, de la Mora-Levy J, Hernández-Guerrero A, Ramírez-Solis M (2021) Risk factors and endoscopic treatment for anastomotic stricture after resection in patients with colorectal cancer. Rev Gastroenterol Mex (Engl Ed). 86(1):44–50. https://doi.org/10.1016/j.rgmx.2020.03.001. (Epub 2020 May 6. PMID: 32386994)
Bannura GC, Cumsille MA, Barrera AE, Contreras JP, Melo CL, Soto DC (2004) Predictive factors of stenosis after stapled colorectal anastomosis: prospective analysis of 179 consecutive patients. World J Surg 28(9):921–925. https://doi.org/10.1007/s00268-004-7375-7
Bertocchi E, Barugola G, Benini M, Bocus P, Rossini R, Ceccaroni M, Ruffo G (2019) Colorectal anastomotic stenosis: lessons learned after 1643 colorectal resections for deep infiltrating endometriosis. J Minim Invasive Gynecol 26(1):100–104. https://doi.org/10.1016/j.jmig.2018.03.033. (Epub 2018 Apr 17 PMID: 29678755)
Silva-Velazco J, Stocchi L, Costedio M, Gorgun E, Kessler H, Remzi FH (2016) Is there anything we can modify among factors associated with morbidity following elective laparoscopic sigmoidectomy for diverticulitis? Surg Endosc 30:3541–3551. https://doi.org/10.1007/s00464-015-4651-6
Qin Q, Ma T, Deng Y, Zheng J, Zhou Z, Wang H, Wang L, Wang J (2016) Impact of preoperative radiotherapy on anastomotic leakage and stenosis after rectal cancer resection: post hoc analysis of a randomized controlled trial. Dis Colon Rectum 59(10):934–942. https://doi.org/10.1097/DCR.0000000000000665. (PMID: 27602924)
Terzi C, Bingul M, Arslan NC, Ozturk E, Canda AE, Isik O, Yilmazlar T, Obuz F, Birkay Gorken I, Kurt M, Unlu M, Ugras N, Kanat O, Oztop I (2020) Randomized controlled trial of 8 weeks’ vs 12 weeks’ interval between neoadjuvant chemoradiotherapy and surgery for locally advanced rectal cancer. Colorectal Dis 22(3):279–288. https://doi.org/10.1111/codi.14867. (Epub 2019 Oct 20 PMID: 31566843)
Rondelli F, Pasculli A, De Rosa M, Avenia S, Bugiantella W (2021) Is routine splenic flexure mobilization always necessary in laparotomic or laparoscopic anterior rectal resection? A systematic review and comprehensive meta-analysis. Updates Surg 73(5):1643–1661. https://doi.org/10.1007/s13304-021-01135-y
Hiranyakas A, Da Silva G, Denoya P, Shawki S, Wexner SD (2013) Colorectal anastomotic stricture: is it associated with inadequate colonic mobilization? Tech Coloproctol 17(4):371–375. https://doi.org/10.1007/s10151-012-0929-z
Rink AD, Kienle P, Aigner F, Ulrich A (2020) How to reduce anastomotic leakage in colorectal surgery-report from German expert meeting. Langenbecks Arch Surg 405(2):223–232. https://doi.org/10.1007/s00423-020-01864-5. (Epub 2020 Mar 18 PMID: 32189067)
Yasuda K, Kawai K, Ishihara S, Murono K, Otani K, Nishikawa T, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, Yamaguchi H, Aoki S, Mishima H, Maruyama T, Sako A, Watanabe T (2016) Level of arterial ligation in sigmoid colon and rectal cancer surgery. World J Surg Oncol 14:99. https://doi.org/10.1186/s12957-016-0819-3.PMID:27036117;PMCID:PMC4818479
Khatri VP, Rodrigues-Bigas MA, Flewell R, Petrelli NJ (2018) Operative approach to rectal cancer: an anatomical and technical description. Surg Oncol 27(2):A5–A15. https://doi.org/10.1016/j.suronc.2018.05.033. (PMID: 29937190)
Park IJ, Kim JC (2010) Adequate length of the distal resection margin in rectal cancer: from the oncological point of view. J Gastrointest Surg 14(8):1331–1337. https://doi.org/10.1007/s11605-010-1165-3
Manegold P, Taukert J, Neeff H, Fichtner-Feigl S, Thomusch O (2019) The minimum distal resection margin in rectal cancer surgery and its impact on local recurrence—a retrospective cohort analysis. Int J Surg 69:77–83. https://doi.org/10.1016/j.ijsu.2019.07.029. (Epub 2019 Jul 27 PMID: 31362126)
Cong ZJ, Fu CG, Wang HT, Liu LJ, Zhang W, Wang H (2009) Influencing factors of symptomatic anastomotic leakage after anterior resection of the rectum for cancer. World J Surg 33(6):1292–1297. https://doi.org/10.1007/s00268-009-0008-4. (PMID: 19363687)
Qin Q, Zhu Y, Wu P, Fan X, Huang Y, Huang B, Wang J, Wang L (2019) Radiation-induced injury on surgical margins: a clue to anastomotic leakage after rectal-cancer resection with neoadjuvant chemoradiotherapy? Gastroenterol Rep 7(2):98–106. https://doi.org/10.1093/gastro/goy042. (Epub 2018 Dec 11 PMID: 30976422; PMCID: PMC6454846)
Lee SY, Kim CH, Kim YJ, Kim HR (2018) Anastomotic stricture after ultralow anterior resection or intersphincteric resection for very low-lying rectal cancer. Surg Endosc 32(2):660–666. https://doi.org/10.1007/s00464-017-5718-3. (Epub 2017 Jul 19 PMID: 28726144)
Funding
The authors also declare that they have no competing financial interests.
Author information
Authors and Affiliations
Contributions
AS contributed to design and writing of the study. All authors have contributed in data collection, analysis and critical revision.
Corresponding author
Ethics declarations
Disclosures
Ahmet Surek, Turgut Donmez, Eyup Gemici, Ahmet Cem Dural, Cevher Akarsu, Arif Kaya, Sina Ferahman, Mehmet Abdussamet Bozkurt, Mehmet Karabulut and Halil Alis have no conflict of interest or financial ties to disclose.
Ethical approval
The ethics committee approval for the study was obtained from the local ethics committee (Ethics committee approval date: 07.06.2021 Decision no: 2011–11-18).
Informed consent
For this study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Surek, A., Donmez, T., Gemici, E. et al. Risk factors affecting benign anastomotic stricture in anterior and low anterior resections for colorectal cancer: a single-center retrospective cohort study. Surg Endosc 37, 5246–5255 (2023). https://doi.org/10.1007/s00464-023-10002-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10002-3