Abstract
Background
Using conventional endoscope to perform endoscopic submucosal dissection (ESD) is difficult because of the one-handed operation and blind dissection caused by gravity. Poor visualization of the submucosal plane causes ESD to be associated with a high risk of bleeding and perforation. This study aimed to develop a novel ESD-assistive robot system and to evaluate its efficacy.
Methods
A novel flexible auxiliary single-arm transluminal endoscopic robot (FASTER) was developed. A total of 36 artificial lesions in ex vivo porcine stomachs were removed using the FASTER-assisted ESD method (n = 18) and the conventional ESD method (n = 18). Lesions were 2 cm or 4 cm in diameter, located on the anterior and posterior walls of the antrum. Primary outcome measurements were dissection time and dissection speed.
Results
The dissection time in FASTER-assisted ESD was significantly shorter than that in conventional ESD (7 min vs 13 min, p = 0.012), mainly because of the faster dissection speed (148.6 vs 97.0 mm2/min, p = 0.002). The total procedure time in FASTER-assisted ESD was shorter than that in conventional ESD, but the difference was not significant (16 min vs 24 min, p = 0.252). Complete en bloc resection was achieved in all lesions. No perforations were detected. The FASTER exhibited the ability of regrasp, multidirectional traction, and proper tension control during ESD.
Conclusion
FASTER significantly increased the dissection speed by providing proper traction and achieving good submucosal vision. This new device is expected to facilitate ESD in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Endoscopic submucosal dissection (ESD) is a widely accepted therapeutic option for early gastrointestinal cancer that achieves en bloc resection regardless of lesion size [1, 2]. Despite being a new technique, ESD is usually performed using a conventional endoscope. The construction of the conventional single-channel endoscope limits the deployment of instruments and fails to provide sufficient visibility of the dissection area [3]. As a result, ESD is technically demanding, time-consuming, and associated with a high risk of perforation and bleeding [4,5,6,7,8].
To overcome this limitation, various traction methods have been developed in recent years [9,10,11,12,13,14,15]. Position change is a simple method to provide traction by gravity, but it is only suitable for lesions on specific sites [10]. The clip-with-line method is useful for gastric, esophageal, and colonic lesions [11]. However, the direction of traction is immutable and the traction power may decrease over time. Endoscope-assisted ESD and magnetic-assisted ESD may provide traction in multiple directions, but they are complicated to use and expensive [12, 14, 15].
Robot-assisted ESD is another attempt to mitigate this difficulty. Different types of endoscopic robot systems are currently under development [16,17,18,19]. Using them, bimanual manipulation of dual arms is allowed, which is analogous to the surgical Da Vinci robot. Robotic systems had demonstrated their efficacy in endoscopic operation; however, exclusively designed endoscopes and instruments slowed down their large-scale clinical application.
We developed a flexible auxiliary single-arm transluminal endoscopic robot (FASTER) that is designed to be attached to a conventional endoscope and retains endoscopists’ original operation habits. In the present study, we evaluated the efficacy, safety, and maneuverability of ex vivo porcine ESD.
Materials and methods
FASTER
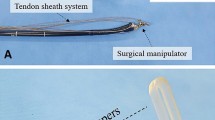
In this study, FASTER, an endoscopic, flexible, detachable, single-arm, master–slave robot was developed (Fig. 1A). FASTER consists of three major components: a robot arm, a driven housing, and a console with a user interface. The robotic arm is designed to be attached to a general-use endoscope using an elastic white hood. To accommodate the long, narrow digestive tract, the diameter of the robotic arm is 4 mm, and the diameter of end clip is 2.3 mm. The overall diameter of the endoscope (EG29-i10, Pentax Medical, Shanghai, China) with the attached FASTER is 14–17 mm, and the length of the robotic arm is 1.8 m. For safe introduction of the robotic arm during esophageal insertion, the end clip of the arm was designed to be smooth and retractable (Online Video), and no overtube was used. In addition to retractable, another three degrees of freedom (DOFs), including down bending, right/left bending, and open/close, have been designed to ensure multidirectional traction and regrasping.
The movement of the arm was controlled by a master on the console interface. The operation is intuitive for bending down, left, and right based on hand-eye feedback, and the opening and closure of the gripper are controlled by two buttons on the touch bar handle (Online Video). The motion of the end clip is designed within the endoscopic field of view for safe and easy operation. The motion signal is detected, processed, and converted into a force signal by the driven housing to drive the end-effector via a tendon driven. An “emergency stop” button is designed to ensure safety during the operation.
In vitro model
Fresh porcine stomachs with the esophagus and duodenum were used for this ex vivo study. After careful washing, the stomach was turned over into an inside-out position and a circular artificial lesion (Fig. 2A) with a diameter of 2 cm or 4 cm was created on the anterior and posterior walls of the antrum (Table 1). The stomach was everted to a normal anatomic position and the duodenal end was sealed. A specially designed dissection platform was used to mount the model (Fig. 1B). For the model simulated in the supine position, the posterior wall was easy to collect water due to gravity, which made it difficult to visualize the dissection plane. To maintain the vitality, all stomachs were flushed with normal saline solution and were not refrigerated until the beginning of the operation. After the operation, the stomach was opened for direct observation of the dissection plane.
ESD procedure
All procedures were performed by an ESD endoscopist who had performed gastric ESD for 3 years, conducted aproximately 200 conventional ESDs and ten FASTER-assisted ESDs. All ESDs were conducted using a conventional single-channel upper gastrointestinal endoscope (EG29-i10, Pentax Medical, Shanghai, China). The FASTER system was operated by an endoscopy nurse after a week of training. The robotic arm was attached to the tip of the endoscope in the FASTER-assisted ESD group, and a transparent attachment cap was used in the conventional ESD group. The ESDs were performed following the same steps. First, the lesion was lifted by submucosal injection of normal saline and indigo carmine mixture. A circumferential mucosal incision was performed using a dual knife (KD-650 L, Olympus Inc.). The robotic gripper was then used to grasp the edge of the mucosa, which was lifted for a good vision in the FASTER-assisted ESD group. Submucosal dissection was performed using a dual knife. Finally, the resected tissue was extracted with the endoscope using FASTER or by suction for the conventional group.
All experiments were conducted at the Qilu Hospital of Shandong University with approval from the Medical Ethics Committee of Qilu Hospital of Shandong University.
Outcome measurements and definitions
Primary outcome measurements were submucosal dissection time (minutes) and submucosal dissection speed (mm2/min), which was calculated as the area of the resected specimen divided by the submucosal dissection time. Secondary outcome measurements were other factors that were used to evaluate the ESD efficacy, including total procedure time (minutes), incision time (minutes), number of lens cleanings, number of accessory tool replacements, complete resection rate, and en bloc resection rate. The total procedure time was measured from the beginning of the injection to the end of the submucosal dissection. Complete resection was defined as the removal of mucosa inside the markers, and all markers could be identified on the resected specimen. In addition to the efficacy, maneuverability, safety, and operator satisfaction with the robot system were also recorded. The indicators used to evaluate the maneuverability of the robot included the number of attempts for one successful grasp and the number of unexpected falls. “Successful grasp” was defined as when the gripper grasped the tissue with proper counter traction and provided a good submucosal vision. The perforation rate was used to evaluate safety. A graded structured scale of 1 to 3 that was analogous to a unipolar Likert Scale was used to estimate operators’ satisfaction, 1 indicated “somewhat satisfied”, 2 indicated “satisfied”, and 3 indicated “very satisfied”.
Statistical analysis
Data are presented as the mean ± standard deviation (SD) or median (interquartile range) according to normality. Independent t-tests and Wilcoxon rank-sum tests were used to analyze the continuous data. A p < 0.05 was considered significant. All analyses were performed using SPSS version 19 (IBM Corp., Armonk, NY, USA).
Results
Efficacy and safety
A total of 36 lesions were successfully removed using either the FASTER-assisted (n = 18) or conventional ESD method (n = 18). The submucosal dissection time of FASTER-assisted ESD was 7 (4–13) min, which was significantly shorter than that of conventional ESD [13 (8–20) min, p = 0.012]. The submucosal dissection speed of FASTER-assisted ESD was significantly faster than that of conventional ESD (p = 0.002). A lower number of lens cleanings were recorded in the FASTER-assisted ESD group (p = 0.009). No significant differences were observed in the total procedure time (p = 0.252), incision time (p = 0.055), or the number of tool replacements (p = 0.126) between the two groups. Complete en bloc resection was achieved in all lesions (Fig. 2B and C). No perforations were detected. Table 2 summarizes the outcomes of the study.
For lesions with different sizes, FASTER significantly increased the submucosal dissection speed and decreased the submucosal dissection time for both small (p = 0.023 and 0.001) and large lesions (p = 0.039 and 0.026). For lesions with different locations, FASTER significantly increased the submucosal dissection speed (p = 0.001) and reduced the submucosal dissection time (p = 0.011) of lesions on the posterior wall, but did not significantly improve the submucosal dissection speed (p = 0.480) or shorten the submucosal dissection time (p = 0.645) of lesions on the anterior wall. Table 3 summarizes these outcomes.
Maneuverability
During FASTER-assisted ESD, 1–3 attempts were needed to achieve a successful grasp; moreover, no incidence of unexpected gripper falls, and tissue damage were observed. FASTER was easy to operate for multidirectional traction, proper tension, and regrasping (Fig. 3). The device operator felt “very satisfied” (grade 3) with the robot’s maneuverability in all ESD operations. The endoscopist felt “very satisfied” (grade 3) with the robot’s performance in the operations for small lesions and a part of the large lesions, but “satisfied” (grade 2) in the operation for the other part of large lesions.
The flexible auxiliary single-arm transluminal endoscopic robot (FASTER) exhibits multidirectional motion and multidirectional traction. A (1), translational movement. A (2), down bending. A (3), right bending. A (4), left bending. B Upward traction. C Leftward traction. D Rightward traction. E Forward pushing
Discussion
A novel robot system named FASTER was developed. Compared with the existing ESD-assistive devices, FASTER has several advantages: (1) it can be simply installed on a general-use single-channel endoscope, convenient for widespread clinical application; (2) it has four DOFs to ensure multidirectional traction and regrasping. In addition, it is not restricted by the joint construction and moves flexibly because of the structure of a pair of soft steel wires with steel coils, (3) although the arm of the robot is attached to the outside of the endoscope, the spring structure balances its flexibility and rigidity so that it does not affect the control of the endoscope during the ESD operation. Hwang et al. had recently reported a novel portable endoscopic tool handler (PETH) [20]. Although PETH is similar to FASTER, they have two main differences: (1) FASTER has the capability of translational movement. The robotic arm can be retracted during endoscope insertion to present mucosal injury. In addition, the forward and backward movements allow FASTER to optimize the viewing distance during operation. (2) in FASTER, the motion of the clip is designed within the endoscopic view, which is beneficial for safe hand-eye feedback control, especially in human applications.
In this comparative ex vivo study, FASTER-assisted ESDs were successfully performed in the antrum. The median dissection time was 7 min in the FASTER-assisted group, which was significantly shorter than that in the conventional group. This result was mainly attributed to the faster dissection speed, which was increased by 53% with the help of FASTER. The endoscopic application of the flexible robotic arm enabled the endoscopist to maintain visualization of the submucosal layer more easily, assisting them in overcoming the technical difficulty of ESD. The median total procedure time was 16 min in the FASTER-assisted group; however, it was not significantly different compared with 24 min in the conventional group. As the total procedure time consisted of individual steps, the FASTER was only used in submucosal dissection, but not in submucosal injection and circumferential incision, which may be the main reason for the insignificant difference in our results.
The lesion size was associated with incomplete dissection. ESD for larger lesions is challenging. In this study, the dissection speed in FASTER-assisted ESD increased by 1.5 times compared with that of the conventional ESD where the cap was used to stake the flap, and the improvement was similar between small and large lesions. The traction pattern was an important factor affecting the procedure efficiency and endoscopists’ satisfaction. For most small lesions, upward traction once or twice was efficient. However, in terms of large lesions, the traction pattern become more complicated. The traction site needed to be replaced from the edge of the mucosal flap to the inner side, and multiple multidirectional traction was necessary.
Another factor that made ESD difficult was the site of the lesion [21]. In this study, the lesions on the posterior wall required more difficult operations because water was easily collected by gravity. The dissection time of posterior wall ESD was significantly reduced using FASTER. For lesions on the anterior wall where the traction force can be achieved through gravity, no significant difference in dissection time was observed. However, FASTER was still very helpful in the early stage of the dissection before the flap was sufficiently prepared, and in case where the flap was contracted inward.
Coaxial motion was the main limitation of FASTER because it was attached to the outside of the endoscope. Although this attachment was beneficial in the clinical application, coaxial motion was inevitable. In addition, endoscopists should be careful to avoid mucosal injury during esophageal insertion. Due to its smooth appearance, mucosal injury never occurred during ex vivo and in vivo animal studies. However, this risk can not be ignored. This study had some limitations. First, all procedures were performed in the antrum, the results were not generalizable for lesions in more challenging locations. Since this was a pilot ex vivo study, further in vivo studies with multiple locations and a larger sample would be scheduled. Second, all ESDs were performed by a single endoscopist; although this was good for controlling for bias, results were less representative. Finally, novices were not included, and their learning effects may be more obvious. The learning curve in novices should be evaluated in future studies.
In conclusion, we developed a novel ESD assistant robot system named FASTER and evaluated its efficacy, safety, and maneuverability in ex vivo porcine stomach ESD. Our result verified that FASTER facilitated ESD by providing various directional tractions and enhancing the operation visualization. FASTER is expected to promote the clinical application of the ESD procedure.
References
Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH (2015) Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 47(9):829–854. https://doi.org/10.1055/s-0034-1392882
ASGE Standards of Practice Committee, Evans JA, Early DS, Chandraskhara V, Chathadi KV, Fanelli RD, Fisher DA, Foley KQ, Hwang JH, Jue TL, Pasha SF, Sharaf R, Shergill AK, Dominitz JA, Cash BD, American Society for Gastrointestinal Endoscopy (2013) The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 77(3):328–334. https://doi.org/10.1016/j.gie.2012.10.001
Fukami N (2013) What we want for ESD is a second hand! Traction method. Gastrointest Endosc 78(2):274–286. https://doi.org/10.1016/j.gie.2013.04.192
Suzuki H, Takizawa K, Hirasawa T, Takeuchi Y, Ishido K, Hoteya S, Yano T, Tanaka S, Endo M, Nakagawa M, Toyonaga T, Doyama H, Hirasawa K, Matsuda M, Yamamoto H, Fujishiro M, Hashimoto S, Maeda Y, Oyama T, Takenaka R, Yamamoto Y, Naito Y, Michida T, Kobayashi N, Kawahara Y, Hirano M, Jin M, Hori S, Niwa Y, Hikichi T, Shimazu T, Ono H, Tanabe S, Kondo H, Iishi H, Ninomiya M, Ichiro Oda for J-WEB/EGC group (2019) Short-term outcomes of multicenter prospective cohort study of gastric endoscopic resection: “Real-world evidence” in Japan. Dig Endosc 31(1):30–39. https://doi.org/10.1111/den.13246
Ma MX, Bourke MJ (2018) Endoscopic submucosal dissection in the West: current status and future directions. Dig Endosc 30(3):310–320. https://doi.org/10.1111/den.12960
Draganov PV, Wang AY, Othman MO, Fukami N (2019) AGA Institute clinical practice update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol 17(1):16–25. https://doi.org/10.1016/j.cgh.2018.07.041
Ebigbo A, Probst A, Römmele C, Messmann H (2018) Step-up training for colorectal and gastric ESD and the challenge of ESD training in the proximal colon: results from a German Center. Endosc Int Open 6(5):E524–E530. https://doi.org/10.1055/a-0584-6457
Yang DH, Jeong GH, Song Y, Park SH, Park SK, Kim JW, Jung KW, Kim KJ, Ye BD, Myung SJ, Yang SK, Kim JH, Park YS, Byeon JS (2015) The feasibility of performing colorectal endoscopic submucosal dissection without previous experience in performing gastric endoscopic submucosal dissection. Dig Dis Sci 60(11):3431–3441. https://doi.org/10.1007/s10620-015-3755-0
Yoshida M, Takizawa K, Nonaka S, Shichijo S, Suzuki S, Sato C, Komori H, Minagawa T, Oda I, Uedo N, Hirasawa K, Matsumoto K, Sumiyoshi T, Mori K, Gotoda T, Ono H, CONNECT-E Study Group (2020) Conventional versus traction-assisted endoscopic submucosal dissection for large esophageal cancers: a multicenter, randomized controlled trial (with video). Gastrointest Endosc 91(1):55–65. https://doi.org/10.1016/j.gie.2019.08.014
Lee BI (2013) Debates on colorectal endoscopic submucosal dissection - traction for effective dissection: gravity is enough. Clin Endosc 46(5):467–471
Oyama T, Kikuchi Y, Shimaya S (2002) Endoscopic mucosal resection using a hooking knife (hooking EMR). Stomach Intest 37:1155–1161
Ahn JY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim DH, Song HJ, Lee GH, Jung HY, Kim JH (2011) Transnasal endoscope-assisted endoscopic submucosal dissection for gastric adenoma and early gastric cancer in the pyloric area: a case series. Endoscopy 43(3):233–235. https://doi.org/10.1055/s-0030-1256037
Kobayashi T, Gotohda T, Tamakawa K, Ueda H, Kakizoe T (2004) Magnetic anchor for more effective endoscopic mucosal resection. Jpn J Clin Oncol 34(3):118–123. https://doi.org/10.1093/jjco/hyh025
Matsuzaki I, Miyahara R, Hirooka Y, Funasaka K, Furukawa K, Ohno E, Nakamura M, Kawashima H, Maeda O, Watanabe O, Ando T, Kobayashi M, Goto H (2014) Simplified magnetic anchor-guided endoscopic submucosal dissection in dogs (with videos). Gastrointest Endosc 80(4):712–716. https://doi.org/10.1016/j.gie.2014.05.334
Jeon WJ, You IY, Chae HB, Park SM, Youn SJ (2009) A new technique for gastric endoscopic submucosal dissection: peroral traction-assisted endoscopic submucosal dissection. Gastrointest Endosc 69(1):29–33. https://doi.org/10.1016/j.gie.2008.03.1126
Ho KY, Phee SJ, Shabbir A, Low SC, Huynh VA, Kencana AP, Yang K, Lomanto D, So BY, Wong YY, Chung SC (2010) Endoscopic submucosal dissection of gastric lesions by using a Master and Slave Transluminal Endoscopic Robot (MASTER). Gastrointest Endosc 72(3):593–599. https://doi.org/10.1016/j.gie.2010.04.009
Hourneaux T, de Moura D, Aihara H, Jirapinyo P, Farias G, Hathorn KE, Bazarbashi A, Sachdev A, Thompson CC (2019) Robot-assisted endoscopic submucosal dissection versus conventional ESD for colorectal lesions: outcomes of a randomized pilot study in endoscopists without prior ESD experience (with video). Gastrointest Endosc 90(2):290–298. https://doi.org/10.1016/j.gie.2019.03.016
Yeung BP, Chiu PW (2016) Application of robotics in gastrointestinal endoscopy: a review. World J Gastroenterol 22(5):1811–1825. https://doi.org/10.3748/wjg.v22.i5.1811
Zorn L, Nageotte F, Zanne P, Legner A, Dallemagne B, Marescaux J, de Mathelin M (2018) A novel telemanipulated robotic assistant for surgical endoscopy: preclinical application to ESD. IEEE Trans Biomed Eng 65(4):797–808. https://doi.org/10.1109/TBME.2017.2720739
Hwang M, Lee SW, Park KC, Sul HJ, Kwon DS (2020) Evaluation of a robotic arm-assisted endoscope to facilitate endoscopic submucosal dissection (with video). Gastrointest Endosc 91(3):699–706. https://doi.org/10.1016/j.gie.2019.11.014
Kim JH, Nam HS, Choi CW, Kang DH, Kim HW, Park SB, Kim SJ, Hwang SH, Lee SH (2017) Risk factors associated with difficult gastric endoscopic submucosal dissection: predicting difficult ESD. Surg Endosc 31(4):1617–1626. https://doi.org/10.1007/s00464-016-5149-6
Acknowledgements
Thanks to our colleaguesYuxiang Zhang, Zhenjun Wang, Xue Wei, Jingyi Li, Tao Cong, Qihui Geng, Jing Xie, Chen Lu, Xiaoli Jia, Huimu Chen, Yingying Qi, Wenwen Fu, Mei Xu, Donghui Zhai, Wenxue Qi, Fujia Liu, Min Zhang, Cheng Peng, Qian Li, Dawei Shen, Minjuan Lin, Juan Wang, Wenlin Zhang, and Chen Qiao for assistance during the study.
Funding
This study was supported by National Key R&D Program of China (2018YFB1307700), Key Research and Development Program of Shandong Province (2018CXGC1209), Taishan Scholars Program of Shandong Province and National Clinical Research Center for Digestive Diseases supporting technology project (2015BAI13B07), and Natural Science Foundation of Shandong Province, China (ZR2020LZL003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Xiao-Xiao Yang, Shi-Chen Fu, Rui Ji, Li-Xiang Li, Xiu-Li Zuo, and Yan-Qing Li have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Video exhibits the composition and movement of the flexible auxiliary single-arm transluminal endoscopic robot (FASTER), and uses the FASTER in an ex vivo porcine gastric endoscopic submucosal dissection (MP4 153043 kb)
Rights and permissions
About this article
Cite this article
Yang, XX., Fu, SC., Ji, R. et al. A novel flexible auxiliary single-arm transluminal endoscopic robot facilitates endoscopic submucosal dissection of gastric lesions (with video). Surg Endosc 36, 5510–5517 (2022). https://doi.org/10.1007/s00464-022-09194-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09194-x